Abstract
Wettability and interfacial interaction of the Ta2O5/Cu-Al system were studied. Pure Cu does not wet the Ta2O5 substrate, and improved spreading is achieved when relatively a high fraction of the active element (~40 at.% Al) was added. The Al2O3 and AlTaO4 phases were observed at the Ta2O5/Cu-Al interface. A thermodynamic evaluation allowed us to suggest that the lack of wetting bellow 40 at.% Al is due to the presence of a native oxide, which covers the drop. The conditions of the native oxide decomposition and the formation of the volatile Al2O suboxide strongly depend on the vacuum level during sessile drop experiments and the composition of the Cu-Al alloy. In our case, Al contents greater than 40% provides thermodynamic conditions for the formation of Al2O (as a result of Al reaction with Al2O3) and the drop spreading. It was suggested that the final contact angle in the Ta2O5/Cu-Al system (50°) is determined by Ta adsorption on the newly formed alumina interlayer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tantalum pentoxide (Ta2O5) displays transparency in a wide wavelength range, low losses, and high stability in the near-UV and -IR regions and has very high reflection index. Due to its high dielectric permittivity, Ta2O5 gained attention in the integrated circuit technology as a gate insulator and as a capacitor (Ref 1-5), and it is of technological interest to develop brazing alloys for joining tantalum pentoxide with metals and other ceramics. No wettability properties of Ta2O5 in contact with liquid metals as well as information about interfacial interaction were reported in the literature. In the present study, interfacial interaction of Ta2O5 with molten Cu alloyed with an active element (Al), and the wetting behaviors in these systems were investigated.
Experimental
Ta2O5 (99.8 purity) samples were prepared using cold press followed by sintering in air at 1673 K for 3 h. The measured density of the samples by the Archimedes method was 94% of the theoretical density. For wetting experiments, the surface of the samples was polished down to 1 μm diamond paste (the measured substrate roughness (Ra) was 0.16 μm) and cleaned ultrasonically in acetone and ethanol.
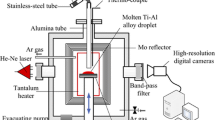
The metals used for these experiments were 99.99% pure, and the alloys were prepared in situ by co-melting of the appropriate amounts of metals. The sessile drop wetting experiments were performed at 1423 K, in a dynamic vacuum furnace (10−3 Pa) with a dwell time of 30 min for each experiment.
The contact angles were measured using a Nikon 990 Coolpix digital camera and derived from the magnified images using the “Image Pro 4” software. After solidification, the samples were cross sectioned, and the interfacial structure and composition were studied by scanning electron microscope (SEM; Jeol GSM 5600, HRSEM-Jeol 7400F) equipped with energy dispersive spectrometer analyzer (EDS).
XRD analysis of the Ta2O5 surface after the removal of the Al drop was carried out using RIGAKU Dmax 2100 apparatus (Cu Kα).
Experimental Results
Spreading and Final Apparent Contact Angles
The drop spreadings in the Ta2O5/Cu-Al system for various aluminum concentrations are presented in Fig. 1. At low aluminum contents (up to ~20 at.%), the spreading is limited, and the final contact angle is about 130°. As the aluminum content increases greater than 25 at.%, the spreading leads to the final apparent contact angle after 25-30 min. This angle (Fig. 2) decreases slightly for aluminum content of up to 40 at.% Al (from 130 to 120°), and subsequently drops significantly to 60° for aluminum content of 60 at.%. The contact angle for pure Al is about 50°.
Interfacial Characterization
For the Ta2O5/Cu-Al systems, the formation of a thin (~1 μm) continuous wavy shape Al2O3 layer beneath the metallic drop and a noncontinuous AlTaO4 phase between the newly formed Al2O3 and the Ta2O5 substrates were detected by EDS analysis (Al2O3 and Ta2O5 were used as standards). Typical BSE-SEM images of the cross-sectioned samples after the sessile drop experiment are presented in Fig. 3. It has to be pointed out that Al3Ta particles attached to the alumina layer (10-20 μm) were randomly found (Fig. 3c) and were probably formed during cooling. In order to clarify the results of the EDS analysis, the metal drops were removed from the substrate (by etching with 30% NaOH solution) and the later was characterized by XRD analysis. The existence of Al2O3 and AlTaO4 (in addition to Ta2O5) was confirmed (Fig. 4). The intermetallic particles in the Ta2O5/Al system were removed together with the metal drop and its peaks are absent.
BSE images of the cross-sectioned (a) Ta2O5/Cu-40at.%Al; (b) Ta2O5/Al samples. A continuous wavy (~1 μm) Al2O3 layer was formed adjacent the drop, and a noncontinuous AlTaO4 layer between the Ta2O5 substrate and the newly formed substrate of Al2O3 layer was observed; and (c) Al3Ta precipitates, probably formed during cooling, were randomly distributed close to the interface
Discussion
Interfacial Interaction
The moderate thermodynamic stability of oxides of elements from V group compared with stable oxides, such as Al2O3 or MgO, is presented in Fig. 5.
The standard free Gibbs energy for formation of various oxides (per 1/2 mole of O2) (Ref 6)
We assume that the observed noncontinuous layer of the ternary oxide phase is formed by a solid-state reaction of the initially formed Al2O3 layer with the substrate and does not affect the spreading and the final contact angle.
It is reasonable to assume that Al dissolved in liquid Cu reacts with Ta2O5 according to the reaction (1):
where (S) and (L) denote solid and liquid standard states of the oxide phases and metallic components in a liquid Cu-Al-Ta solution, respectively.
It has to be pointed out that Ta solubility in molten Cu is extremely low (0.0088 at.% at 1473 K), and no stable intermetallic compounds exist in this system (Ref 7). According to the binary Ta-Al phase diagram, the tantalum solubility in liquid Al at 1423 K is also limited (to about 1.5 at.%), and at greater tantalum content, the Al3Ta intermetallic phase is stable (Ref 8).
At 1423 K, ∆G° and the equilibrium constant, \(K = {\raise0.7ex\hbox{${a_{\text{Ta}}^{6} }$} \!\mathord{\left/ {\vphantom {{a_{\text{Ta}}^{6} } {a_{\text{Al}}^{10} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${a_{\text{Al}}^{10} }$}}\), for the reaction (1) are −1.69 × 106 J and 7.22 × 1061, respectively (Ref 6). According to these thermodynamic data, extremely low fraction of Al has to be added to the molten Cu to form an alumina layer and effect tantalum transfer to the Cu-Al melt. As a result of the interaction, a thin continuous alumina layer covers the substrate and prevents its direct contact with the melt. Thus, the Ta fraction in the melt is limited, and the Al3Ta intermetallic phase is formed only during cooling. Nevertheless, even a small amount of Ta fraction is sufficient to lower the contact angle in comparison with the Al2O3/Al system because of its adsorption at the Al2O3/melt interface. The effect of an adsorbed element on wetting angle was widely discussed in the literature (Ref 9-13).
The results of the thermodynamic analysis are in a good agreement the observations of the metal/ceramic interface.
Wetting Mechanism
The above analysis shows that interfacial interaction between the Cu-Al melts and the substrate occurs at even low fraction of aluminum, and an alumina layer is formed. However, its presence affects the wetting behavior only for aluminum concentration greater than ~40 at.% (Fig. 2). We suggest that this feature is attributed to the formation of volatile aluminum sub-oxide (Al2O) as a result of alumina reaction with liquid Al as was reported previously (Ref 14, 15). It is clear that the decomposition of the aluminum native oxide and the formation of the sub-oxide, according to reaction (2), depend on aluminum activity in the melt, the vacuum level in the experimental chamber, and the temperature.
where (G) denotes the gaseous phase. According to (Ref 16), at 1423 K, ∆G° for reaction (2) is 491 kJ.
Using thermodynamic data for reaction (2), the thermodynamic properties of the Cu-Al liquid solution (Ref 17), and equilibrium parameters of the Al-O system were calculated [as was done in (Ref 18)]. We assumed that reaction (2) takes place if the partial pressure of Al2O is greater than the pressure in the experimental chamber (10−3 Pa). According to the calculated parameters at 1423 K, presented in Fig. 6, this condition is fulfilled if the Al content in the Cu-Al melt is greater than ~30 at.%.
Furthermore, examination of our previous results (Ref 18-20) on the wetting behaviors of various oxides in contact with Cu-Al shows that the nonwetting-to-wetting transition occurs at almost the same aluminum content, regardless of the substrate and the nature of the interfacial interaction (Fig. 7). Thus, wetting behaviors of Al-containing melts in contact with oxide substrate is determined by the possibility of the dissolved aluminum to react with a native aluminum oxide layer and to form the volatile sub-oxide.
Summary
The wetting behavior and interfacial interaction in the Ta2O5/Cu-Al system were studied. The moderate thermodynamic stability of Ta2O5 compared with Al2O3 results in the formation of a thin continuous alumina layer at the interface even for Cu-Al melts with low Al contents. Nevertheless, the improved wetting was achieved for melts for Al contents greater than 40 at.% Al. It was shown that for Ta2O5/Cu-Al as well as for other oxide/Cu-Al systems, wetting behavior is determined by the possibility of the dissolved aluminum to react with a native aluminum oxide layer and to form the volatile sub-oxide.
We suggest that even small Ta fraction transferred from the substrate to the melt could be adsorbed to the Al2O3/melt interface and lead to a lower contact angle compared with the Al2O3/Al system.
References
Y. Nishioka, H. Shinriki, and K. Mukai, Influence of SiO2 at the Ta2O5/Si interface on dielectric characteristics of Ta2O5 capacitors, J. Appl. Phys., 1987, 61(6), p 2335–2338
H. Shinriki, T. Kisu, S.I. Kimura, Y. Nishioka, Y. Kawamoto, and K. Mukai, Promising storage capacitor structures with thin Ta2O5 film for low-power high density DRAM’s, IEEE Trans. Electron. Dev., 1990, 37(9), p 1937–1947
E. Atanassova, T. Dimitrova, and J. Koprinarova, AES and XPS study of thin RF-sputtered Ta2O5 layers, Appl. Surf. Sci., 1995, 84(2), p 193–202
K.W. Kwon, C.S. Kang, S.O. Park, H.K. Kang, and S.T. Ahn, Thermally robust Ta2O5 capacitor for the 256 Mbit DRAM, IEEE Trans. Electron. Dev., 1996, 43(6), p 919–923
C. Chaneliere, J.L. Autran, R.A.B. Devine, and B. Balland, Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications, Mater. Sci. Eng. R, 1998, 22(6), p 269–322
Scientific Group Thermo-data Europe, Thermodynamic Data-Base SSUB3, version 3.1, Produced by Scientific Group Thermo-data Europe, 2001
P.R. Subramanian and D.E. Laughiln, The Cu-Ta (Copper-Tantalum) system, Bull. Alloy Phase Diagr., 1989, 10(6), p 652–686
Y. Du and R. Schmid-Fetzer, Thermodynamic modeling of the Al-Ta system, J. Phase Equilib., 1996, 17(4), p 311–324
W.D. Kaplan, D. Chatain, P. Wynblatt, and W.C. Carter, A review of wetting versus adsorption, complexions, and related phenomena: the rosetta stone of wetting, J. Mater. Sci., 2013, 48(17), p 5681–5717
P. Wynblatt, The effects of interfacial segregation on wetting in solid metal-on-metal and metal-on-ceramic systems, Acta Mater., 2000, 48(18–19), p 4439–4447
Z. Wang and P. Wynblatt, Wetting and energetics of solid Au and Au-Ge/SiC interfaces, Acta Mater., 1998, 46(14), p 4853–4859
N. Froumin, S. Barzilai, M. Aizenshtein, M. Lomberg, and N. Frage, Wetting induced by near-surface Ti-enrichment in the CaF2/In–Ti and CaF2/Cu–Ti systems, Mater. Sci. Eng. A, 2008, 495(1–2), p 181–186
N. Froumin, M. Piness, S. Barzilai, M. Aizenshtein, and N. Frage, Interfacial interaction between quasi-binary oxides (MgAl2O4 and Y3Al5O12) and liquid aluminum, J. Mater. Sci., 2012, 47(24), p 8450–8453
V. Laurent, D. Chatain, C. Chatillon, and N. Eustathopoulos, Wettability of monocrystalline. Alumina by aluminium between its melting point and 1273 K, Acta Master, 1988, 36(7), p 1797
N. Sobczak, R. Asthana, W. Radziwill, R. Nowak, and A. Kudyba, The role of aluminum oxidation in the wetting-bonding relationship of Al/oxide couples, Arch. Metall. Mater., 2007, 52(1), p 55–61
N. Froumin, N. Frage, M. Polak, and M.P. Dariel, Wetting phenomena in the TiC/(Cu-Al) system, Acta Mater., 2000, 48(7), p 1435–1441
I. Ansara, A.T. Dinsdale, and M.H. Rand, Cost 507: Thermochemical Database for Light Metal Alloys, Vol 2, European Communities, Belgium, 1998
M. Aizenshtein, S. Barzilai, N. Froumin, and N. Frage, Interface interaction and wetting in the Er2O3/(Cu-Al) and Er2O3/(Cu-Ti) systems, J. Mater. Sci., 2008, 43(4), p 1259–1264
S. Barzilai, M. Aizenshtein, N. Froumin, and N. Frage, Interface phenomena in the Y2O3/(Al-Cu) system, Mater. Sci. Eng. A, 2006, 420(1–2), p 291–295
S. Barzilai, H. Nagar, M. Aizenshtein, N. Froumin, and N. Frage, Interface interaction and wetting of Sc2O3 exposed to Cu-Al and Cu-Ti melts, Appl. Phys. A, 2009, 95(2), p 507–512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kish, O., Froumin, N., Aizenshtein, M. et al. Interfacial Interaction and Wetting in the Ta2O5/Cu-Al System. J. of Materi Eng and Perform 23, 1551–1554 (2014). https://doi.org/10.1007/s11665-014-0894-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-014-0894-y