Abstract
Photocatalytic degradation of methyl orange (MO) in water was examined using TiO2 nanopowders under solar irradiation. These photocatalysts were successfully synthesized by hydrolysis of titanium tetra chloride (TiCl4) in the temperature range of 70-95 °C and calcined at higher temperatures of between 400 and 900 °C. The samples prepared were characterized using x-ray powder diffraction, scanning electron microscope (SEM) and Fourier transform infrared spectrophotometer (FTIR). UV-Vis spectrometer was used for analyzing the concentration of MO in solution at different time intervals during the photodegradation experiment. Parameters affecting the photodegradation rate such as catalyst crystallinity, concentration of the catalyst, MO concentration, and pH of the solution have been investigated. The results indicate that TiO2 nanopowder was antase at low calcination temperatures in the range of 400-500 °C. The sample calcined at 600 °C is composed of both anatase and rutile phase. Further increase in the temperature enhanced the intensities of diffraction peaks of the rutile phase. The size of the crystallites for all the samples prepared were found to be in the 6-13 nm range and from SEM micrographs it was in the range of 19-43 nm. The mixture of both phases exhibited a higher photoactivity in comparison with pure anatase or rutile catalysts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) and titanium-based materials have attracted considerable ongoing interest from researchers due to their high chemical stability, nontoxicity, and low cost (Ref 1, 2). They have strong semiconductor photocatalytic properties for environmental protection, water purification, and their ability to decompose harmful organic compounds. They may also be used in the generation of hydrogen gas. Titanium dioxide can exist in three crystallographic structures, anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic); among these, anatase phase shows higher photoactivity than rutile or brookite crystal phases due to low recombination rate of its photogenerated electrons and holes on the surface of TiO2. Additionally, the mixture of both the phases shows higher photocatalytic activity and degradation compared to pure anatase or rutile crystals, their absorption spectra differ from the mixture of anatase and rutile mixed mechanically (Ref 3, 4).
One of the major problems is the low value of overall quantum efficiencies combined with use of UV radiation in photocatalysis. Numerous efforts are under way to make use of sunlight for environmental protection and water purification. Sunlight consists of about 5-7% UV light, 46% visible light, and 47% infrared radiation (Ref 5). Photocatalytic oxidation of various inorganic pollutants and organic dyes in industrial waste water has been carried over TiO2 semiconductor under UV light irradiation. Some successes have been achieved through the ultrafine TiO2 crystallites instead of bulk TiO2 materials. The photocatalytic activity is dependent on a number of parameters including particle size, surface area, anatase-rutile ratio, light intensity, and material to be degraded (Ref 6). However, the precise roles of the other parameters and details of the mechanisms involved on the photocatalytic activity are still unclear and require further investigations.
Many methods for the production of TiO2 nanopowder have been reported and include sol gel process, hydrolysis, hydrothermal, and microemulsion technique (Ref 7). However, these methods are all complex, highly costly, and not easily scaled up. Among these, the most common method of producing the TiO2 nanopowder is hydrolysis of alkoxides. The major disadvantage of using this method is the control of particle size and size distribution due to rapid precipitation, which often give larger particle sizes. The controlled hydrolysis produces anatase even though sulphates or chlorides are used. Calcination is a common treatment that can be used to increase the photocatalytic activity. Even though the effects of calcination on crystallinity, anatase-rutile ratio, and photocatalytic activity has been investigated (Ref 6-10) extensively, it is still far from clear how these impact the physicochemical parameters that control the photoactivity. However, very little work has been carried out on the effects of calcination on the photoactivity of ultrafine (<10 nm) nanocrystalline TiO2 powders under solar light.
In this study, nanocrystalline TiO2 powders were prepared by the hydrolysis of TiCl4 in aqueous solution in the hydrolysis within the temperature range of 70-95 °C. The effects of calcination temperature on the crystallinity, particle size, and photocatalytic activity were investigated. The photocatalytic activities were evaluated by the degradation of methyl orange (MO) under UV irradiation. Parameters affecting the photodegradation process such as catalyst crystallinity, concentration of catalyst, MO concentration, and pH of the solution have been investigated.
Experimental Method
TiO2 catalysts were prepared by the hydrolysis of TiCl4 (98%, Sigma Aldrich) as the main starting material without any further purification. TiCl4 was dissolved in distilled water in an ice-water bath by adjusting the concentration of TiCl4 to 3 M. The mixture was then treated with 0.5 M HCl and 2.5 M dilute NH4OH solutions maintaining a neutral pH value. This mixture was stirred at high speed in hydrolysis temperature range of 70-95 °C for 5 h and then the solution was aged for 48 h in dark light. Subsequently, the precipitated titanium hydroxide/hydrous titanium oxide (TiO2·nH2O) was repeatedly washed with distilled water to make TiO2·nH2O free of chloride ions. The hydrous oxides were dried at 120 °C for 6 h. The TiO2 powders were calcined at 400, 500, 600, 700, 800, 900 °C for 2 h to improve its crystallinity (Ref 6).
Powder XRD was used for crystal phase identification and estimation of the particle size. The XRD measurements were carried out using Shimadzu x-ray diffractometer equipped with Cu Kα radiation (λ = 1.5406 Ǻ). The morphology of the samples was inspected with scanning electron microscope (SEM). Fourier transform infrared spectrophotometer (FTIR) Perkin-Elmer System 2000 was used to determine the specific functional groups.
Photocatalytic activities were measured by the decomposition of MO in an aqueous solution at ambient temperature. In each experiment, a desired amount of TiO2 photocatalyst was added into beaker (Pyrex glass) containing 50 mL solution of varying concentrations of MO. Prior to irradiation, the mixed solution was stirred in the dark for 30 min to obtain the saturated absorption of MO onto the catalysts. The solution was then exposed to sunlight along while stirring. Experimental place was Multan (30°15′N, 71°36′) during the summer season (May 10-29). After every 1 h of irradiation the concentration of the MO was monitored using a UV-Vis spectrophotometer (Hitachi U-2001) by checking the absorbance at 510 nm. The degradation rate of MO was calculated based on the following formula:
where C 0 is the initial concentration of MO mg/L, C is the concentration at each interval of time in mg/L.
Experimental Results and Discussion
Figure 1 shows the FTIR spectrum of the nanocrystalline TiO2 powder calcined at temperatures of 400, 600, 700, and 900 °C in the range of 400-4000 cm−1. The spectrum exhibit absorption bands at 3400, 2900, 2350, 1640, and 400-650 cm−1 respectively, corresponding to hydroxyl (-OH) group, (C-H) mode, atmospheric (CO2), primary amine (NH3-CH2), (Ti-O-Ti) bonds in TiO2 stretching modes, respectively. The C-H vibrations can be attributed to the organic part of the surfactants that remain incorporated even after the calcination at 400 °C. After calcination at higher temperatures, all these bands decrease in intensity as a result of thermal treatment the spectra show the Ti-O-Ti absorption peaks at 400-650 cm−1 (Ref 4, 7-10).
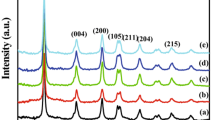
Figure 2 represents the XRD patterns of the samples calcined at different temperatures ranging from 400 to 900 °C. Calcination is a common treatment used to improve the crystallinity of TiO2 nanopowder. The XRD pattern of nanocrystalline TiO2 powder calcined at 400 °C showed the presence of broad peaks of anatase structure. Anatase TiO2 structure was confirmed by the XRD peaks at angles, 25.3°, 38.0°, 48.0°, 54.7°, and 63.1° (JCPDS, 21-1272). The anatase structure was maintained even after calcinations at 500 °C but showing weaker intensities of the diffraction peaks. The sample calcined at 600 °C is composed of crystallites of both anatase and rutile phases. Anatase to rutile transformation has been reported to occur in the different temperature range from 600 to 1100 °C (Ref 7), depending on the preparation method. As the calcination temperature was raised to 700 °C the anatase phase totally disappears and only rutile phase remained. Intensities of diffraction peaks become stronger by increasing calcinations temperature to 900 °C (Ref 7-10). XRD results of all the samples are summarized in Table 1.
The crystallite size was estimated using the full-width at half-maximum (FWHM) of the intense diffraction peaks of anatase and rutile crystal phase at 2θ = 25.3° (101) and 2θ = 27.4° (110), respectively, according to the Scherrer formula (Ref 11)
where D is the crystallite size in nm, k = 0.89 which is a constant, λ is the wavelength of x-ray radiation in nm, θ is the Bragg angle in radians, and β is the FWHM in radians. As seen from Table 1, the smallest crystallite is obtained from the sample calcined at 400 °C. At higher calcination temperatures, the crystallites formed are larger in size, which can be attributed to the thermally promoted crystallite growth. The crystallite sizes of the samples estimated after calcination at 400, 500, 600, 700, 800, and 900 °C, respectively, was found to be in the ~6-13 nm range.
Figures 3 to 6 show SEM images of the powders obtained at different calcinations temperatures 400 600, 700, and 900 °C. The SEM images show surface and crystallite morphologies of the TiO2 powders. These images show heavily aggregated particles of very small crystallites with loose appearance. The morphologies of all samples were similar but their aggregate particle sizes were different from one another. The SEM micrographs show that the average crystallite size of TiO2 nanopowders increases from ~19 to 43 nm after calcination at higher temperatures (Table 1)
The Scherrer formula was used to calculate the crystallite size. Compared to the SEM crystallite values the Scherrer formula gave lower crystallite sizes. This may be attributed to the nonuniformity in the interplanar spacing due to stress in the crystallites can also produce the broadening of the x-ray diffraction peaks. This fact suggests that the broadening of the diffraction peaks in sample is due to existence of significant number of structural defects with in the crystallites. These defects have a dominant effect on the broadening of diffraction peaks. Scherrer formula, therefore, gives an indirect estimate of their density rather than the true crystallite size (Ref 12).
To study the effect of crystal structure on the photodegradation of MO, samples calcined at different temperatures were used. Figure 7 shows that the highest photodegradation of MO for the samples calcined at a temperature of 600 °C have an anatase and rutile mixed structure. This enhanced photocatalytic activity is because of suppressed recombination of photogenerated electrons and holes (Ref 13). The calcined samples at 800 and 900 °C have rutile structure and gave lower degradation efficiency compared with the samples calcined at lower temperatures. Our results are in good agreement with those obtained in a previous study (Ref 13).
Figure 8 illustrates the influence of catalyst dosage on the MO degradation behavior. As expected, it can be seen that in the absence of TiO2 catalyst the degradation of MO after 4 h of solar irradiation is very low. This is mainly photolysis process. As the photocatalyst amount was increased from 5 to 25 mg/L the degradation efficiency of MO enhanced, and reached a maximum when catalyst amount was 10 mg/L, this is due to the increase in the amount of catalyst, which contributed to the number of photons and dye molecules adsorbed (Ref 14). Further increase in catalyst dosage beyond 10 mg/L had a negative effect on degradation efficiency of MO. It has been suggested that light penetration is blocked by the photocatalyst suspension may account for the decreased degradation efficiency of MO (Ref 15).
The effect of varying concentration of MO on its degradation rate is shown in Fig. 9. These results demonstrate that lower the concentration of MO the higher the efficiency of MO decomposition. Hence, it appears that the photocatalytic oxidation process has more promise at lower concentrations of the pollutants. The rate of degradation is higher at 5 ppm, but beyond that degradation efficiency decreases due to the saturation of surface sites for decomposition of MO. The pH of the MO solution has strong effect on the photocatalytic activity of the samples, which controls the production rate of hydroxyl radical. It is an important parameter in the treatment of waste-water. The results of degradation of MO solution at different values of pH are shown in Fig. 10. Degradation rate of MO has not been found significant at low pH values, attaining a maximum efficiency at pH 9. Further increase in the pH showed a small increase in degradation rate of MO. The surface charge development is affected by the pH because protons are potential determining ions for TiO2 (Ref 15). Upon hydration, the surface hydroxyl groups can undergo proton association or dissociation reactions, thereby bringing about surface charge depending on pH.
TiOH, TiO−, and TiOH2 + are neutral, negative, and positive hydroxyl groups, respectively. Positively charged surface at low value of pH cannot produce hydroxyl groups that are needed for hydroxyl radical formation (OH●). Consequently, the rate of MO degradation may decrease. On the other hand, higher pH value can provide higher concentration of hydroxyls ions (OH−), thereby enhanced degradation of MO (Ref 14, 16, 17).
Conclusions
TiO2 nanopowders were produced by hydrolysis of TiCl4 in the temperature range of 70-95 °C. The XRD patterns revealed that samples obtained were anatase at between 400 and 500 °C and became rutile after calcination at higher temperatures. The crystallite size increased from ~6 to 13 nm and showed a dependence on the calcination temperature. This data are also supported by evidence from the SEM micrographs which gave crystallite sizes in the range of ~19-43 nm. The photoactivity of the sample of TiO2 nanopowder calcined at 600 °C for degradation of MO was obviously higher than those calcined at other temperatures because mixed phase of anatase and rutile exhibited higher photoactivity.
References
X. Li, R. Xiong, and G. Wei, Preparation of MnOx/TiO2 Ultrafine Nanocomposite with Large Surface Area and its Enhanced Toluene Oxidation at Low Temperature, J. Hazard. Mater., 2009, 164, p 587–591
U. Diebold, The Surface Science of Titanium Dioxide, Surf. Sci. Rep., 2003, 48, p 53–229
S. Ardizzone, C.L. Bianchi, G. Cappelletti, S. Gialanella, C. Pirola, and V. Ragaini, Tailored Anatase/Brookite Nanocrystalline TiO2—The Optimal Particle Features for Liquid- and Gas-Phase Photocatalytic Reactions, Phys. Chem. C, 2007, 111, p 13222–13231
K.M. Reddy, C.V.G. Reddy, and S.V. Manorama, Physiochemical and Structural Properties of TiO2 Precipitated in an Emulsion System, J. Solid State Chem., 2001, 58, p 180–186
S. Rehman, R. Ullah, A.M. Butt, and N.D. Gohar, Strategies of Making TiO2 and ZnO Visible Light Active, J. Hazard. Mater., 2009, 170, p 560–569
Q. Zhang, L. Gao, and J. Guo, Preparation of Plasma Sprayed Titania/Hydroxyapatite Photocatalytic Coatings with Nanostructured Powder, Appl. Catal. B, 2000, 26, p 207–215
Y.F. Chen, C.Y. Lee, M.Y. Yeng, and H.T. Chiu, The Effect of Calcination Temperature on the Crystallinity of TiO2 Nanopowder, J. Cryst. Growth, 2003, 247, p 363–370
M. Ocana, V. Fornes, and C.J. Serna, A Simple Procedure for the Preparation of Spherical Oxide Particles by Hydrolysis of Aerosols, Ceram. Int., 1992, 18, p 99–106
S. Pavasupree, Y. Suzuki, S. Yoshikawa, and R. Kawahata, Synthesis of Titanate, TiO2 (B), and Anatase TiO2 Nanofibers from Natural Rutile Sand, J. Solid State Chem., 2005, 178, p 3110–3116
Y. Li and G.P. Demopoulos, Precipitation of nanosized titanium dioxide from aqueous titanium (IV) chloride solutions by neutralization with MgO, Hydrometallurgy, 2008, 90, p 26–33
B.D. Cullity, Elements of X-ray Diffraction, 3rd ed., Addison Wesley, Boston, 1978, p 102
E. Ahmed, M.S. Abid, and W. Ahmed, Growth of Nano Sized Grains in CuInSe2 Thin Films Using E-Beam Deposition Technique, Nanosci. Nanotechnol., 2009, 9, p 4233–4239
M.A. Barakat, H. Schaeffer, G. Hayes, and S. Ismat-Shah, Photocatalytic Degradation of Methylene Blue by CNT/TiO2 Composites Prepared from MWCNT and Titanium n-Butoxide with Benzene, Appl. Catal. B, 2004, 57, p 23–30
M. Muruganadham and M. Awaminathan, Photocatalytic Decolourisation and Degradation of Reactive Orange 4 by TiO2-UV Process, Dyes Pigments, 2005, 68, p 133–142
J.M. Tseng and C.P. Haung, Removal of Chlorophenols from Water by Photocatalytic Oxidation, Water Sci. Technol., 1991, 23, p 377
R.A. Doong, C.H. Chen, R.A. Maithreepala, and S.M. Chang, The Influence of PH on CdS on Photodegradation of 2-Chlorophenol in TiO2 Suspensions, Water Res., 2001, 35, p 2873–2880
J. Sun, X. Wang, J. Sun, R. Sun, S. Sun, and L. Qiao, Photocatalytic Degradation and Kinetics of Orange G Using Nano-Sized Sn(IV)/TiO2/AC Photocatalyst, J. Mol. Catal. A, 2006, 260, p 241–246
Acknowledgments
N. R. Khalid and M. Ahmad are thankful to Higher Education Commission of Pakistan for their financial support during this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalid, N.R., Ahmed, E., Ikram, M. et al. Effects of Calcination on Structural, Photocatalytic Properties of TiO2 Nanopowders Via TiCl4 Hydrolysis. J. of Materi Eng and Perform 22, 371–375 (2013). https://doi.org/10.1007/s11665-012-0272-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0272-6