Abstract
Cf/SiC ceramic composites have been brazed to Nimonic alloys using TiCuAg filler metal. In order to improve wettability and to provide compatibility between ceramic and metal, the Cf/SiC surface was metallized through the deposition of a chromium layer. Subsequent heat treatments were carried out to develop intermediate layers of chromium carbides. Excellent wetting of both the composite ceramic and the metal from the filler metal is observed in the fabricated joints. Shear tests show that failure occurs always within the ceramic material and not at the joint. In the filler region depletion of Ti and formation of Ag and Cu rich regions are observed. At the Cf/SiC-filler interface a layered structure of the filler metallic elements is observed. Titanium interacts with the SiC matrix to form carbides and silicides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced ceramic matrix composite (CMC) materials have the potential for being used at high temperatures as thermo-structural materials and in different fields including heat exchangers used in severe environments such as rocket and jet engines (Ref 1), gas turbines for power plants, heat shields for space vehicles, inner walls of the plasma chamber of nuclear fusion reaction (Ref 2, 3) aircraft brakes, heat treatment furnaces, etc. The main advantages of CMCs lie in the fact that they present superior mechanical properties and resistance against high temperatures and at the same time they are lightweight and cost-effective (Ref 4, 5).
Carbon fiber-reinforced SiC ceramic matrix composites (Cf/SiC) are lightweight, hard, wear resistant and stable in oxidizing environment up to a high temperature. Owing to the embedded carbon fibers, they have an excellent combination of mechanical properties. Therefore, Cf/SiC composites are promising new structural materials for a variety of high-temperature aerospace applications (Ref 6).
Most such applications in aerospace and nuclear industry require joining CMCs to metals, ceramics or composites. Thus, advances in joining science and technology are important in order the benefits of these advanced materials to be realized (Ref 7). Generally the requirements for CMC-metal joints are high mechanical strength at high temperatures and in aggressive environments, high oxidation resistance, high stability under service and low production cost. A general requirement for aerospace applications is the joining of the CMC to a high temperature alloy. In the literature there is limited information on the joining of Cf/SiC composites to metallic alloys and this mainly refers to joining of Cf/SiC to Ti based alloys.
Cu-Ti or Ag-Cu-Ti alloys have been the base of brazing Cf/SiC to Ti alloys and in some cases carbon or carbon fibers have been introduced in the braze. Lin G. et al. used carbon fiber-reinforced brazing material (67.6Ag-26.4Cu-6Ti, wt.%) to braze Cf/SiC to Ti alloy (Ref 8). The volume fraction of the carbon fibers in the braze defines the strength of the joint, controls the reaction between the Ti element and the brazed composite and it is associated with the brazing parameters. Successful joining of Cf/SiC to TC4 alloys (Ti-26Al-4V (wt.%)) was realized using 94(72Ag-28Cu)-6Ti (wt.%) alloy powder with particle size of 320 mesh (Ref 9). The joint interfaces were microstructurally sound, well bonded, and without cracks and voids. Also mixed powders of Cu, Ti, and graphite were used for brazing Cf/SiC composite to Ti alloys (Ref 10, 11). Formation of TiC around surplus graphite and TiC particles in the bonding layer reduced the thermal stress significantly. The reaction rate was controlled by the diffusion rate of C from graphite particles to the liquid bonding layer. Cf/SiC composites were vacuum brazed to Ti and a Ni-base superalloy using Ni-base 28 metallic glass braze foils (MBF-20 and MBF-30) (Ref 12). For the case of the Ni-base superalloy the joints for both braze foils were sound, but in the case of MBF-20 shrinkage cavities had been formed. Compositional changes due to substrate dissolution led to secondary-phase precipitation which aided interfacial bonding although interlaminar shear failure occurred within some composites. Residual thermal stresses in the joint led to hardness gradients; however, stress accommodation by the braze prevented interfacial cracking.
Also interlayers have been used to achieve bonding and relieve strain mismatch between the composite and steel or Ti or Ni alloys (Ref 13). Li et al. used Cu/W/Cu/W/Cu multiple interlayers to bond Cf/SiC to Ni-based superalloy (Ref 14).
Whatever the joining process is, chemical equilibrium at the joint interface is needed to form a stable ceramic/metal bond for operation at high temperature (Ref 15). Interfacial reactions can affect every stage of the formation of a joint from the onset of bonding through the development of equilibrated microstructure and to the optimization of the mechanical properties (Ref 16). It has been demonstrated that an adequate surface modification of the CMC could lead to improvements of the composite wettability by metals (Ref 17, 18).
In this work, Cf/SiC ceramic composites have been brazed to Nimonic alloys using TiCuAg as filler metal. Ιn order to accommodate the different linear coefficients of expansion between ceramic and metal as well as to provide compatibility between the surfaces to be joined, the Cf/SiC surface was metallized through the deposition of a chromium layer. Subsequent heat treatment was carried out to develop intermediate layers of chromium carbides. The distribution of the different elements at the cross section is discussed.
Experimental
The Cf/SiC (SICARBON) ceramic composites were supplied by EADS Innovation Works (Ref 19). They consist of carbon fibers embedded in a silicon carbon matrix. The production process of this material is based on the Polymer Infiltration Pyrolysis (PIP) process. The infiltration of the carbon fibers with a pre-ceramic polymer-based and powder-filled slurry system is performed by Liquid Polymer Infiltration (LPI) via filament winding. From the supplied material samples of 12 × 12 × 3 mm3 were cut and used for all the experiments.
Chromium was deposited by magnetron sputtering at nearly ambient temperature (70 °C). A 2″-diameter Cr target of 99.95% purity was used. The base pressure before introducing the Ar gas was 1 × 10−6 mbar and the pressure during deposition was 4 × 10−3 mbar. Depositions were performed using a DC power of 120 W, resulting in a deposition rate of 17 Å/s. The Cr layer thickness was about 2 μm. For the post-deposition thermal annealing the SICARBON/Cr samples were enclosed in a quartz tube and they were annealed in a tubular furnace for 1 h at 700 °C under high vacuum (10−6 mbar).
X-ray diffraction (XRD) patterns were measured using a Bruker D8 diffractometer equipped with a Cu Kα radiation. The microstructure of the brazed joints were examined using JEOL JSM 5910 LV scanning electron microscopy (SEM) coupled with INCA 300 energy-dispersive X-ray spectroscopy (EDS).
The ceramic samples were brazed to nimonic superalloys using an IPSEN VFCK-124 high vacuum furnace. For the metallographic analysis of the cross sections of the brazed samples the Nimonic 105 superalloy was used which is a wrought nickel-chromium-cobalt based alloy (Ni, 18-22 Co, 14-15.5 Cr wt.%) strengthened by additions of titanium and aluminum. It combines high strength and creep resistance for service up to 920 °C. The brazing was performed using as a filler metal a commercial TiCuAg alloy (Ag-26.7Cu-4.6Ti, wt.%) in paste form containing Ti as the active metal. The heating was initiated at room temperature and follows a ramp with rates of 10-15 °C/min. Finally, the temperature of brazing was 980 °C. A slow cooling rate of 1 °C/min was selected to provide a sufficient relief of stresses due to different CTEs.
For the shear strength mechanical tests composite Nimonic joints of the geometry depicted in Fig. 1(a) and a mechanical device (Fig. 1b) was specially fabricated. For the mechanical tests the Nimonic 90 (Ni-18Co-20Cr wt.%) superalloy was used in elongated sheets of 100 × 15 × 3 mm3 and the brazing parameters were kept the same as described above.
Results and Discussion
Metallized SICARBON Substrate
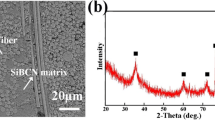
The Cf/SiC surface has been modified by the deposition of a Cr layer, of 2 μm thickness, on the Cf/SiC substrate. The resulted structure was annealed at 700 °C for 1 h in order to achieve the formation of a chromium carbide layer at the interface with the substrate. Cr as a carbide former improves wettability and as it has been found in the case of graphite and Cf/C joining to Nimonic (Ref 17, 18) the filling of the pores and the in-depth formation of different carbides resulted in a mechanical type of joining i.e., in which the joint strength does not rely only on interface adhesion but also on substrate/braze interlocking. The as-deposited and annealed structures have been examined using XRD measurements. Figure 2 presents the XRD pattern of the Cf/SiC substrate, the as-deposited Cr on Cf/SiC and the annealed at 700 °C for 1 h Cr/Cf-SiC. The as-deposited Cr layer presents the strong (110) and (200) Bragg peaks (Fig. 2b), on top of the substrate structure (Fig.2a), characteristic of the bcc crystallographic structure. Heat treatment at 700 °C for 1 h results in the formation of Cr7C3 (orthorhombic Pmcm) at the interface with the Cf/SiC substrate (Fig. 2c). In Fig. 2(c) it is observed that after annealing the Bragg peaks of Cr are still observable, showing that not all of the 2 μm as-deposited Cr layer is consumed in the formation of the chromium carbide.
Consequently, the structure used for the brazing experiments consists of a Cr7C3 layer adjacent to the Cf/SiC surface followed by a Cr layer.
SICARBON Brazing to Nimonic
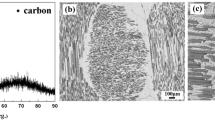
The Cf-SiC/Cr7C3/Cr structures were brazed to the nimonic 90 alloy using TiCuAg filler metal in paste form. The optical microscopy image of the cross section of the joint is depicted in Fig. 3. This shows that in the composite ceramic a number of microcracks exist and close to the filler/ceramic interface a crack propagates parallel to the interface. This crack propagation parallel to the interface may have been produced during the brazing process, and a weak region in the composite with a high density of microcracks acted as the initiation point.
Figure 4(a) shows the SEM micrograph of the cross section of the Cf-SiC/Cr7C3/Cr/filler/Nimonic joint of sample in Fig. 3. It is observed that the filler metal wets well both the metallic and the ceramic parts and the interfaces are sound with a very good physical contact. The chemical mapping of the various elements at the joint area shows a layered structure along the interface (Fig. 4b-g). At the filler/Cf-SiC interface and adjacent to the interface a Ti-rich layer of about 15 μm thickness is formed. This Ti layer bonds the Cf-SiC/Cr7C3/Cr structure to the nimonic alloy. Adjacent to the Ti layer there is a Ag-rich layer followed by a second Ti-rich layer with an average thickness of about 50 μm. In this second Ti layer a significant amount of Ni from the nimonic alloy has diffused, indicating that some dissolution of the metal in the molten braze has occurred. Cu and Ag do not interact with the ceramic substrate, they remain in the filler and they are separated. A similar layered structure with the same characteristics was observed in the joint of Cf/C to nimonic alloy using the same filler metal (Ref 18). Part of the Cu and Ag from the filler metal has diffused in the nimonic alloy in a depth of about 50 μm. Also, in Fig. 4(a) a certain degree of protrusion of carbon fibers on the interface between the Cf/SiC composite and interlayer is observed. This indicates that the reaction of Ti with the carbon from the SiC matrix is more intense than that with the carbon fibers. This kind of reaction is beneficial for increased interfacial bonding strength. A similar and even more intense reaction of Ti with the carbon of the matrix had been observed in the brazing of Cf/SiC to a Ti alloy using the TiCuAg filler metal (Ref 9).
(a) SEM micrograph of the cross section of the SICARBON/Cr/Nimonic joint of sample in Fig. 3 and (b)-(g) the corresponding chemical elements mapping
Figure 5(a) depicts the cross section of the SICARBON-joint, in the case of TiCuAg filler metal, at the ceramic-filler interface, with the fibers along the direction of the interface, and the corresponding elements mapping (Fig. 5b-e). The reactive zone comprises a Cr thin layer on top of the ceramic composite, followed by a thicker layer of Ti. The light gray area in the filler zone corresponds to Ag and the darker one to Cu islands as it has been observed in the joint of Cf/C to nimonic alloy (Ref 18). From the chemical analysis it is concluded that Ti from the filler metal interacts with the carbon of the SiC matrix surface and as result its concentration in the TiCuAg melt adjacent to the surface decreases. Ti from the bulk TiCuAg melt diffuses to the depleted zone and interacts further with the carbon of the SiC increasing the carbide layer thickness and leading to the good physical contact shown in Fig. 5(a). It is noted that Ti diffusion, in this study, is not observed in depth in the composite as it was observed in the case of Cf/C joining to nimonic alloy (Ref 18). XRD measurements performed on the cross section of the joint confirm the formation of stoichiometric TiC and TiSi. Stoichiometric titanium carbide is thermodynamically more favorable as the Gibb’s free energy for the reaction Ti + C → TiC is −172 kJ. Thermodynamic calculations, also, show that sub-stoichiometric carbides TiC x could form from Cu-Ti alloys (Ref 20).
Mechanical Tests
For the mechanical tests, a new series of brazed samples were fabricated, with and without the Cr deposition on the ceramic part, employing the geometry shown in Fig. 1(a) and the brazing parameters as described in Sect. 2. Subsequently, the joint structures were subjected to mechanical shear tests performed at room temperature, as depicted in Fig. 1(b). Figure 6(a) depicts the fractured surfaces of SICARBON/Cr to nimonic alloy joints using TiCuAg filler. In the case of Cr deposition followed by heat treatment at 700 °C for 1 h the fracture has been produced extensively through the ceramic part (Fig. 6b). In the case of no Cr, areas exist in the fractured surface where the fracture is mixed between the carbon fibers and the filler metal (Fig. 6c). This shows the key role of Cr deposition for the fabrication of sound joints. Delamination of the ceramic occurs parallel to the ceramic/metal interface. This type of failure has been, also, observed in the bonding of Ti-tubes to C/C composite plates using TiCuAg filler (Ref 21) and to the bonding of a nimonic alloy to Cf/C ceramic composites (Ref 18). The interlaminar shear strength of the Cf/SiC composites depends strongly on the test method (Ref 22), and these tests only show that the joining is stronger than the composite under the method of examination. Also, it is possible the brazing process to have modified the mechanical properties of the composite as the interfacial shear strength of Cf/SiC composite may change drastically by heat treatment (Ref 23, 24).
Conclusions
Cf/SiC composite was brazed to metallic nimonic alloy using TiCuAg filler metal. Prior to brazing a Cr layer is deposited on the ceramic composite and it is subsequently heat treated leading to the formation of the Cr7C3 compound which promotes the good wetting and bonding of the Cf/SiC. It is suggested that the presence of Cr may act as a barrier to excessive reactions of Ti with the SiC from the matrix of the composite and it can be used as a control to these reactions. Ti from the filer metal reacts mainly with the matrix of the Cf/SiC and the formation of TiC and TiSi is confirmed by XRD measurements of the cross section. The shear tests show a fracture within the Cf/SiC material near the joint region, producing an interlaminar failure. The crucial role of Cr deposition on the ceramic is evident from the examination of the fractured surfaces. When Cr is deposited on the ceramic and Cr7C3 is formed through heat treatment fracture occurs within the ceramic. On the contrary, in the case of no Cr metallization the facture surface is mixed lying in both the filler and the ceramic composite.
References
S. Schmidt, S. Beyer, H. Immich, H. Knabe, R. Meistring, and A. Gessler, Ceramic Matrix Composites: A Challenge in Space-Propulsion Technology Applications, Int. J. Appl. Ceram. Technol., 2005, 2(2), p 85–96
J. Schlosser, F. Escourbiac, M. Merola, S. Fouquet, P. Bayetti, J.J. Cordier, A. Grosman, M. Missirlian, R. Tivey, and M. Rödig, Technologies for ITER Divertor Vertical Target Plasma Facing Components, Nucl. Fusion, 2005, 45(6), p 512–518
T. Nozawa, T. Hinoki, A. Hasegawa, A. Kohyama, Y. Katoh, L.L. Snead, Jr., C.H. Henager, and J.B.J. Hegeman, Recent Advances and Issues in Development of Silicon Carbide Composites for Fusion Applications, J. Nucl. Mater., 2009, 386–388(C), p 622–627
F. Lamouroux, S. Bertrand, R. Pailler, R. Naslain, and M. Cataldi, Oxidation-Resistant Carbon-Fiber-Reinforced Ceramic-Matrix Composites, Compos. Sci. Technol., 1999, 59(2), p 1073–1085
R. Naslain, Design, Preparation and Properties of Non-oxide CMCs for Application in Engines and Nuclear Reactors: An Overview, Compos. Sci. Technol., 2004, 64(2), p 155–170
T. Ishikawa, S. Kajii, K. Matsanaga, T. Hogani, Y. Kohtoku, and T. Nagasawa, A Tough, Thermally Conductive Silicon Carbide Composite with High Strength up to 1600°C in Air, Science, 1998, 282, p 1295–1297
A. Passerone and M.L. Muolo, Joining Technology in Metal-Ceramic Systems, Mater. Manuf. Process., 2000, 15(5), p 631–648
G. Lin, J. Huang, and H. Zhang, Joints of Carbon Fiber-Reinforced SiC Composites to Ti-Alloy Brazed by Ag-Cu-Ti Short Carbon Fibers, J. Mater. Process. Technol., 2007, 189(1–3), p 256–261
J.H. Xiong, J.H. Huang, H. Zhang, and X.K. Zhao, Brazing of Carbon Fiber Reinforced SiC Composite and TC4 Using Ag-Cu-Ti Active Brazing Alloy, Mater. Sci. Eng. A, 2010, 527(4–5), p 1096–1101
Y.H. Ban, J.H. Huang, H. Zhang, X.K. Zhao, and Z.Y. Zhang, Microstructure of Reactive Composite Brazing Joints of Cf/SiC Composite to Ti-6Al-4V Alloy with Cu-Ti-C Filler Material, Rare Met. Mater. Eng., 2009, 38(4), p 713–716
J.H. Xiong, J.H. Huang, Z.P. Wang, Y.H. Ban, H. Zhang, and X.K. Zhao, Brazing of Carbon Fibre Reinforced SiC Composite and Ti Alloy Using Cu-Ti-C Filler Materials, Mater. Sci. Technol., 2010, 26(3), p 356–360
M. Singh, R. Asthana, and T.P. Shpargel, Brazing of Ceramic-Matrix Composites to Ti and Hastealloy Using Ni-Base Metallic Glass Interlayers, Mater. Sci. Eng. A, 2008, 498(1–2), p 19–30
D.G. Dixon, Ceramic Matrix Composite-Metal Brazed Joints, J. Mater. Sci., 1995, 30(6), p 1539–1544
S. Li, J. Zhang, X. Liang, H. Duan, and Y. Zhang, Joining of Carbon Fibre Reinforced SiC (Cf/SiC) to Ni-Based Superalloy with Multiple Interlayers, Int. J. Mod Phys. B, 2003, 17(8–9), p 1777–1781
M.G. Nicholas and S.D. Peteves, Reactive Joining: Chemical Effects on the Formation and Properties of Brazed and Diffusion Bonded Interfaces, Scr. Metall. Mater., 1994, 31(8), p 1091–1096
S.D. Peteves, M. Paulasto, G. Ceccone, and V. Stamos, The Reactive Route to Ceramic Joining: Fabrication, Interfacial Chemistry and Joint Properties, Acta Mater., 1998, 46(7), p 2407–2414
N.V. Moutis, C. Jiménez, Th Speliotis, X.A. Azpiroz, and K. Mergia, Graphite-Nimonic Alloy Brazing, Adv. Mater. Res., 2009, 59, p 209–213
N.V. Moutis, C. Jiménez, X. Azpiroz, Th Speliotis, C. Wilhelmi, S. Messoloras, and K. Mergia, Brazing of Carbon-Carbon Composites to Nimonic Alloys, J. Mater. Sci., 2010, 45(1), p 74–81
G. Motz, S. Schmidt, and S. Beyer, The PIP-Process: Precursor Properties and Applications, Ceramic Matrix Composites, W. Krenkel, Ed., Wiley-VCH, Germany, 2008, p 165–186
R. Standing and M. Nicholas, The Wetting of Alumina and Vitreous Carbon by Copper-Tin-Titanium Alloy, J. Mater. Sci., 1978, 13(7), p p1509–p1514
G.N. Morscher, M. Singh, T. Shpargel, and R. Asthana, A Simple Test to Determine the Effectiveness of Different Braze Compositions for Joining Ti-Tubes to C/C Composite Plates, Mater. Sci. Eng. A, 2006, 418(1–2), p 19–24
M. Li, R. Matsuyama, and M. Sakai, Interlaminar Shear Strength of C/C-Composites: The Dependence on Test Methods, Carbon, 1999, 37(11), p 1749–1757
N. Iwashita, Y. Sawada, K. Shimizu, S. Shinke, and H. Shioyama, Effect of Matrix Texture on Tensile Strength and Oxidation Behavior of Carbon Fiber Reinforced Carbon Composites, Carbon, 1995, 33(4), p 405–413
K. Fujita, H. Sakai, N. Iwashita, and Y. Sawada, Influence of Heat Treatment Temperature on Interfacial Shear Strength of C/C, Compos. Part A, 1999, 30(4), p 497–501
Acknowledgments
This work has been carried out within the framework of the Integrated European Project “ExtreMat” (contract NMP-CT-2004-500253) with financial support by the European Community. The paper only reflects the views of the authors and the European Community is not liable for any use of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the Symposia “Wetting, soldering and brazing” and “Diffusion bonding and characterization” belonging to the Topic “Joining” at the European Congress and Exhibition on Advanced Materials and Processes (EUROMAT 2011), held September 12-15, 2011, in Montpellier, France, and has been expanded from the original presentation.
Rights and permissions
About this article
Cite this article
Jiménez, C., Mergia, K., Moutis, N.V. et al. Joining of Cf/SiC Ceramics to Nimonic Alloys. J. of Materi Eng and Perform 21, 683–689 (2012). https://doi.org/10.1007/s11665-012-0183-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0183-6