Abstract
The effect of austenitizing on the microstructure and hardness of two martensitic stainless steels was examined with the aim of supplying heat-treatment guidelines to the user that will ensure a martensitic structure with minimal retained austenite, evenly dispersed carbides and a hardness of between 610 and 740 HV (Vickers hardness) after quenching and tempering. The steels examined during the course of this examination conform in composition to medium-carbon AISI 420 martensitic stainless steel, except for the addition of 0.13% vanadium and 0.62% molybdenum to one of the alloys. Steel samples were austenitized at temperatures between 1000 and 1200 °C, followed by oil quenching. The as-quenched microstructures were found to range from almost fully martensitic structures to martensite with up to 35% retained austenite after quenching, with varying amounts of carbides. Optical and scanning electron microscopy was used to characterize the microstructures, and X-ray diffraction was employed to identify the carbide present in the as-quenched structures and to quantify the retained austenite contents. Hardness tests were performed to determine the effect of heat treatment on mechanical properties. As-quenched hardness values ranged from 700 to 270 HV, depending on the amount of retained austenite. Thermodynamic predictions (using the CALPHAD™ model) were employed to explain these microstructures based on the solubility of the carbide particles at various austenitizing temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Martensitic stainless steels were developed to satisfy a need in industry for corrosion resistant alloys that respond to hardening through heat treatment. These steels are alloyed with between 11.5 and 18.0% chromium and up to 0.6% carbon, and are designed to be fully austenitic at elevated temperatures. This austenite can subsequently be hardened by quenching or cooling to room temperature from the austenitizing temperature (Ref 1), which enables transformation to martensite (Ref 1, 2). Owing to their high alloying element content, martensitic stainless steels demonstrate excellent hardenability.
For applications involving wear or that which require retention of sharp cutting surfaces in finished products, steels containing 11-14% chromium and between 0.3 and 0.4% carbon are preferred (Ref 3). The medium-to-high carbon contents of these steels ensure the high as-quenched hardness values required in these applications. AISI 420 is a low-chromium member of the martensitic family of stainless steels and is commercially available in a low-carbon version (with a specified carbon content of less than 0.15%), and a medium-carbon version (with a maximum carbon content of 0.5%). In the hardened and tempered condition, medium-carbon AISI 420 has high strength and excellent wear resistance, which makes it the ideal choice for applications, such as cutlery, hand tools, dental and surgical instruments, valve trim and parts, shafts, and plastic moulding. The typical chemical composition range specified for medium-carbon AISI 420 is shown in Table 1 (Ref 4).
The typical heat-treatment sequence for martensitic stainless steels includes annealing to soften the steel in preparation for subsequent cold work or machining, austenitizing to form an austenitic structure and fully or partially dissolve carbides, cooling or quenching to transform the austenite to martensite, followed by tempering of the martensitic structure to improve toughness and ductility. The final microstructure of AISI 420 is very dependent on the prior heat treatment that the steel receives, and typically consists of martensite, undissolved, and/or re-precipitated carbides and retained austenite. The volume fraction and size of the carbide particles present in the steel and the amount of retained austenite play a major role in determining the hardness, strength, toughness, corrosion resistance, and wear resistance of the steel (Ref 5).
The austenitizing temperature employed during heat treatment determines the partitioning of carbon and alloying elements between the austenite and carbide phases, with an increase in temperature leading to increased carbide dissolution, higher dissolved alloying element contents, and unwanted grain growth. When in solid solution at temperatures above the carbide dissolution temperature, carbon and carbide-forming elements affect the transformation to martensite by depressing the martensite transformation range and reducing the martensite start (M s) and martensite finish (M f) temperatures. If the M f temperature is depressed below room temperature or even below 0 °C, then the retained austenite may be present in the as-quenched microstructure (Ref 2). The effect of austenitizing temperature on the microstructure and properties of martensitic stainless steels has been the subject of several investigations. Calliari et al. (Ref 6) reported that the maximum as-quenched hardness in AISI 420 martensitic stainless steel develops after austenitizing at a temperature of 1050 °C, as complete carbide dissolution is assumed to have occurred at this temperature. Tavares et al. (Ref 7) proposed austenitizing temperatures in the range of 980-1100 °C for medium-carbon AISI 420 martensitic steel. This range is considered to be too wide to guarantee consistent as-quenched hardness values, particularly in view of the strict requirements specified for the steels examined in the current investigation. Latrobe (Ref 8) reported in their data sheet for LSS 420 HC stainless steel that the steel is fully austenitic after heating to above 860 °C, with a hardness peak of 660 HV (hardness on the Vickers scale) and minimally retained austenite after air cooling from 1025 °C.
Once the steels had been austenitized, quenching or cooling to below the martensite transformation range facilitates the formation of martensite (Ref 9). Most martensitic stainless steels are air-hardening, but larger sections are routinely oil quenched to ensure full transformation to martensite. The M s temperature of AISI 420 martensitic stainless steel is reported to be in the range of 70-, whereas the M f temperature is estimated to be approximately 150-200 °C lower than the M s temperature.
In its as-quenched martensitic condition, the steel is hard and brittle and may contain pockets of retained austenite. Quenching is therefore followed by tempering to reduce brittleness, increase ductility and toughness, and reduce residual stress. Owing to its high temper resistance, AISI 420 martensitic stainless steel is usually tempered at temperatures higher than approximately 550 °C. Secondary hardening due to the precipitation of alloy carbides may increase the hardness during the tempering of martensitic stainless steels.
The preceding discussion emphasized the importance of heat treatment in developing the preferred properties in martensitic stainless steels. Since these steels are often supplied in the annealed condition to facilitate machining or cold work, the consumer or fabricator is required to perform the final hardening heat treatments to ensure high hardness and wear resistance in the final product. Well-defined heat treatment guidelines are therefore required to assist the consumer or fabricator in performing the correct hardening heat treatments to develop optimal properties. Although such guidelines are available in the literature, the published heat-treatment parameters are often contradictory, and the recommended temperature ranges too wide to ensure consistent results.
The medium-carbon AISI 420 stainless steel examined in this investigation is earmarked for the production of razor blades. For optimal wear resistance and good edge retention in this application, the steel is required to have a fully martensitic structure with minimal retained austenite, a final hardness of between 610 and 740 HV after hardening, and evenly dispersed spherical carbides. This investigation therefore examined the influence of austenitizing temperature on the as-quenched microstructure and properties of two heats of medium-carbon AISI 420 martensitic stainless steel.
Experimental Procedure
Two medium-carbon heats of AISI 420 martensitic stainless steel (containing approximately 0.47% carbon) were supplied for the purpose of this investigation. As shown in Table 2, the steels contain 13.48 and 14.33% chromium, respectively, with small additions of copper, nickel, and vanadium. The primary difference between the two heats is the deliberate addition of molybdenum to HEAT 1. Molybdenum is expected to increase the hardenability, raise the temper resistance, and improve the high-temperature strength of the alloy.
The two steels were received in the spheroidize-annealed condition. This plant treatment involved soaking at 860 °C (1133 K) for 20 h, followed by slow cooling to 770 °C (1043 K), to facilitate the formation of globular carbides and to obtain maximum softness for cold rolling. The steels were supplied in the form of 5-mm-thick plate material.
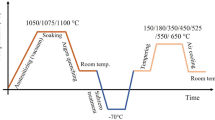
Samples with dimensions of 5 mm × 10 mm × 5 mm were sectioned from the as-supplied plate material. These samples were austenitized in a muffle furnace at various temperatures between 1000 °C (1273 K) and 1200 °C (1273 K). An average heating rate of approximately 0.2 °C (0.2 K) per second was used. As small samples were used and enough time was allowed for the sample temperature to equalize in the furnace, a 15-min soaking time was found to be sufficiently long to attain equilibrium.
Each heat-treated sample was sectioned, mounted in resin, and polished to a mirror finish. The polished samples were etched using Vilella’s reagent (consisting of 1 g picric acid, 10 mL hydrochloric acid, and 100 mL ethanol) to reveal the general microstructure. The etched samples were examined microscopically using an optical microscope and a scanning electron microscope (SEM), and photomicrographs were taken of each specimen.
The mean lineal intercept method (Ref 10) was employed to estimate the ASTM grain size (G) of the metallographic samples. Five randomly distributed test lines were drawn across a printed micrograph, and the number of times a given line intersected grain boundaries was recorded. Equation 1 was adopted to calculate the mean lineal intercept length, L L .
where L L is the mean lineal intercept length, L T is the total length of the test lines, P is the total number of grain boundary intersections, and M is the magnification.
The ASTM grain size, G, was then determined using Eq 2.
The average diameter of the carbide particles observed in each heat-treated sample was measured using image analysis techniques. To determine the carbide density, scanning electron micrographs were divided into squares with a total area of 2300 μm2. The number of carbides in each square was determined, and the carbide density was reported as the number of carbides per mm2.
Calibrated Vickers hardness measurements with an applied load of 10 kg were performed on all the heat-treated samples. The results were reported as the average of five tests per sample. In all cases, the 95% confidence interval is quoted along with the average measured hardness.
To quantify the volume fraction of retained austenite present in various heat-treated samples, X-ray diffraction (XRD) analyses were performed. XRD was also employed to identify the carbide particles observed in the samples. Since the carbide particles were too small to be identified using Energy Dispersive X-ray Spectroscopy (SEM-EDS) analysis, and too large for transmission electron microscope (TEM) analysis, the carbides were extracted by dissolving the martensitic matrix in hydrochloric acid. The sediment was filtered through glass-fiber micropaper, washed in distilled water, and rinsed in acetone. The carbide residue was collected and subjected to XRD analysis.
Computational simulations were performed using CALPHAD™ software, and phase diagrams of the two heats of AISI 420 martensitic stainless steel were compiled at four austenitizing temperatures [1075 °C (1348 K), 1100 °C (1373 K), 1130 °C (1403 K), and 1175 °C (1448 K)] to determine the phase stability and the equilibrium dissolution temperatures of the carbides.
Results and Discussion
As-Received Samples
The two heats of medium-carbon AISI 420 material in the as-supplied spheroidize annealed condition had measured hardness values of 209 ± 7 HV for HEAT 1 and 195 ± 4 HV for HEAT 2. The microstructures of both steels consist of coarse, globular carbides in a ferrite matrix, as demonstrated in Fig. 1 for HEAT 2.
De Andrés et al. (Ref 11) reported that the only carbide present in the spheroidize-annealed microstructure of AISI 420 is M23C6; however, according to Bjarbo and Hatterstrand (Ref 12), steels with more than 0.2% carbon and 12-13% chromium contain M3C, M7C3, and M23C6 carbides. The precipitation of the carbides is reported to be dependent on time with M3C precipitating first, followed by M7C3, and then M23C6. In this investigation, only M23C6 carbides were identified, with M consisting mainly of iron and chromium.
The higher chromium and molybdenum contents of HEAT 1 result in a higher volume fraction of carbides as opposed to HEAT 2. The presence of a high volume fraction of carbides is likely to affect the austenitizing treatment of both heats. According to the available literature (Ref 3), chromium-rich M23C6 carbides dissolve in the 950-1050 °C temperature range, whereas M7C3 carbides dissolve in the 1050-1150 °C temperature range. A higher austenitizing temperature causes more carbides to dissolve, which raises the alloy content of the austenite and depresses the martensite transformation range, increasing the likelihood of retained austenite after quenching.
The Effect of Austenitizing Temperature on As-Quenched Microstructure and Properties
When alloying elements dissolve in the steel at high temperatures, the martensite transformation temperatures are depressed. This is illustrated by Eq 3, which shows the effect of various alloying elements on the M s temperature of 12% chromium steels (all alloy contents in weight percentage, wt.%) (Ref 13).
Equation 3 yields predicted martensite start temperatures of 21 °C for HEAT 1 and 46 °C for HEAT 2. These temperatures are close to ambient, suggesting that the martensite transformation is unlikely to go to completion (unless sub-zero treated) if all the alloying elements are in solution. Since the carbides in AISI 420 increasingly dissolve with an increase in austenitizing temperature, the martensite start temperature is expected to decrease with higher austenitizing temperatures, which increases the risk of retained austenite. The presence of molybdenum in HEAT 1 depresses the martensite start temperature to well below that of HEAT 2, predicting a higher risk of retained austenite after quenching to room temperature. A summary of the results obtained during the course of this investigation is shown in the Appendix.
In order to verify the results reported in literature, HEATS 1 and 2 were initially austenitized at 1000 °C for 15 min, a temperature too low to dissolve significant amounts of M23C6. As only a small percentage of carbides goes into solution during heat treatment, very little retained austenite is expected after quenching. This was confirmed by XRD analysis which shows that 4% retained austenite is present in both HEATS 1 and 2 after austenitizing at 1000 °C. The as-quenched microstructure, shown in Fig. 2, consists of coarse, globular carbides in a fine martensitic matrix. The shape and distribution of the carbide particles suggest that they are undissolved precipitates (from the spheroidize-annealing treatment), rather than re-precipitated carbides. The carbide densities in HEATS 1 and 2, austenitized at 1000 °C, were determined as 181 and 227 carbides per mm2, respectively. The carbides have an average diameter of 1.28 μm in HEAT 1 and 0.75 μm in HEAT 2. The measured hardness values are 664 ± 12 HV for HEAT 1 and 639 ± 10 HV for HEAT 2. These high hardness values can be attributed to the fine martensitic matrix and low levels of retained austenite.
According to Pickering (Ref 2), the equilibrium carbide dissolution temperature in AISI 420 (without added molybdenum) is 1050 °C. The heating rate used by Pickering was, however, not reported. According to De Andres et al. (Ref 11), the total carbide dissolution temperature is, to a certain degree, dependent on the heating rate. A carbide dissolution temperature of 1110 °C was reported for a heating rate of 0.5 °C per second. Owing to furnace constraints, a heating rate of approximately 0.2 °C per second was used for the purpose of this investigation, and a relatively higher percentage of carbides is therefore expected in the as-quenched microstructure after austenitizing at 1050 °C. Based on published literature (Ref 11), the carbide dissolution temperature, using a 0.2 °C per second heating rate, is expected to be approximately 1090 °C.
To determine the extent of carbide dissolution at 1050 °C, samples from HEAT 1 and HEAT 2 were austenitized at 1050 °C for 15 min, followed by quenching in oil. A representative optical photomicrograph of HEAT 1 in the as-quenched condition is shown in Fig. 3. The microstructure consists of well-defined carbide particles in a martensitic matrix. This confirms that an austenitizing temperature of 1050 °C is still below the temperature required to completely dissolve the M23C6 precipitates in the two heats examined. Carbide densities of 131 and 184 carbides per mm2 were obtained for HEAT 1 and HEAT 2, respectively. These densities are lower than those observed in samples quenched from 1000 °C, implying that partial dissolution of carbides had occurred. The measured hardness values are 678 ± 9 HV for HEAT 1 and 665 ± 9 HV for HEAT 2. These hardness values are somewhat higher than those measured after quenching from 1000 °C. This increase in hardness is attributed to an increase in the carbon content of the martensite phase due to the partial dissolution of carbides. The martensite is therefore harder due to its higher carbon content, but the amount of carbon and alloying elements in solid solution was not high enough to depress the martensite transformation range much below room temperature. The retained austenite contents of the two heats therefore remain low at 5 and 6%, respectively.
Austenitizing at a temperature of 1075 °C yields as-quenched hardness values of 684 ± 10 HV for HEAT 1 and 674 ± 12 HV for HEAT 2. The microstructures of the two heats are predominantly martensitic, with well-defined carbide particles (as shown in Fig. 4 for HEAT 1). Carbide densities of 87 and 117 carbides per mm2 were determined for HEATS 1 and 2, respectively. Although these densities are somewhat lower than those measured after austenitizing at 1050 °C, suggesting that partial dissolution of carbides had occurred, the results suggest that 1075 °C is still below the complete carbide dissolution temperature. The average carbide particle diameters of 0.93 μm (HEAT 1) and 0.58 μm (HEAT 2) are very similar to those observed at lower austenitizing temperatures. Partial dissolution of carbides increased the carbon and alloying element contents of the austenite, resulting in slightly higher as-quenched hardness values (compared to those recorded after quenching from 1050 °C) and higher retained austenite levels of 15 and 10% in HEATS 1 and 2, respectively.
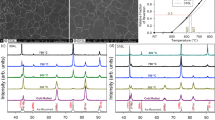
The published literature (Ref 9, 14) suggests that extensive carbide dissolution should occur in both heats during austenitizing at 1075 °C. In order to explain the observed discrepancy between the published data of dissolution temperatures and the microstructures observed after austenitizing at 1075 °C, thermodynamic predictions (using the CALPHAD™ model) of the austenite and carbide stabilities in the two heats during austenitizing were used. Figure 5 shows the predicted equilibrium phase diagram for HEATS 1 and 2 at 1075 °C. In this figure, the # symbol denotes the position of HEAT 1 in terms of percentage chromium and percentage carbon, whereas * denotes the position of HEAT 2. The solid lines represent the calculated phase boundaries for HEAT 1 and the broken lines the boundaries for HEAT 2. It is evident from Fig. 5 that the higher molybdenum and chromium contents of HEAT 1 restrict the austenite phase field (both elements are strong ferrite-formers).
Figure 5 predicts that both heats contain austenite and M7C3 carbides during austenitizing at 1075 °C, with all M23C6 carbides in solution. This is in agreement with the observations of Salem (Ref 15) who reported that M23C6 chromium carbides dissolve in the 950-1050 °C temperature range, whereas M7C3 carbides dissolve in the 1050-1150 °C temperature range. Partial dissolution of M7C3 carbides is therefore likely at 1075 °C. Information derived from the CALPHAD model predicts 1.23% M7C3 in HEAT 1 at 1075 °C, and 0.91% M7C3 in HEAT 2. No M23C6 is expected in these steels at 1075 °C, but M23C6 forms during cooling on conversion of the M7C3 carbides. The presence of M23C6 carbide in the as-quenched microstructures of the steels was confirmed by XRD analysis. No M7C3 was observed in any of the steels after cooling from the austenitizing temperature.
The existing literature predicts that increasing the austenitizing temperature to 1100 °C should result in a large percentage of carbides going into solution (Ref 9, 11, 12). The critical temperature for complete carbide solution in AISI 420 (without molybdenum) was recorded as 1110 °C at a heating rate of 0.5 °C per second (Ref 4, 12). At the 0.2 °C per second heating rate used in the current investigation, a carbide dissolution temperature of approximately 1090 °C is predicted (Ref 11). Figure 6 and 7 confirm, however, that carbides remain present in the as-quenched microstructures of HEAT 1 and HEAT 2. The carbide densities were estimated as 84 carbides per mm2 for HEAT 1 and 63 carbides per mm2 for HEAT 2. XRD analysis reported 23% retained austenite in HEAT 1, and 12% retained austenite in HEAT 2. The increase in retained austenite content accounts for the lower as-quenched hardness values of 653 ± 8 HV for HEAT 1 and 639 ± 8 HV for HEAT 2 (compared to those measured at 1075 °C).
This inconsistency between the reported carbide dissolution temperatures and those determined during the course of the current investigation was investigated further by examining the predicted phase diagram at 1100 °C (shown in Fig. 8). At this temperature, both steels are located in the dual-phase region where austenite and M7C3 carbides are stable, suggesting that the temperature is not high enough to completely dissolve all carbides. Extensive carbide dissolution does, however, take place at this temperature, with the CALPHAD model predicting 0.62% M7C3 in HEAT 1 and 0.27% in HEAT 2 at 1100 °C. The increased dissolution of carbides depresses the martensite transformation range, resulting in the formation of higher levels of retained austenite.
Scanning electron micrographs of HEATS 1 and 2 after austenitizing at 1130 °C are shown in Fig. 9 and 10. Contrary to the published predictions, carbides remain visible in the as-quenched microstructures, with measured carbide densities of 81 carbides per mm2 for HEAT 1 and 32 carbides per mm2 for HEAT 2. The hardness of HEAT 1 is, however, significantly lower than that of HEAT 2 at 474 ± 7 HV. This can be attributed to the presence of a significant volume fraction of retained austenite (25%) after austenitizing at 1130 °C. The hardness of HEAT 2 remains high at 620 ± 4 HV, which is in agreement with the measured retained austenite content of 15%. A significant increase in ASTM grain size was observed at this austenitizing temperature, with ASTM grain size numbers of 8.6 for HEAT 1 and 6.9 for HEAT 2. This increase in grain size can be attributed to the higher austenitizing temperature and the increased dissolution of grain-pinning carbides.
The predicted phase diagrams for HEATS 1 and 2 at 1130 °C are shown in Fig. 11. HEAT 1 is located on the boundary between the austenite and (austenite + M7C3) phase fields, whereas HEAT 2 is located well within the single-phase austenite region. The CALPHAD model therefore predicts complete dissolution of carbides during austenitizing at 1130 °C. This suggests that equilibrium was not reached during heat treatment, resulting in the presence of retained carbides, or that the model does not predict the phase boundaries to the desired level of accuracy.
Austenitizing at a temperature of 1150 °C results in a significant increase in retained austenite after quenching. Both heats are expected to be located well within the austenite phase field at this temperature. HEAT 1 contains 27% retained austenite (see Fig. 12), resulting in a low as-quenched hardness of 308 ± 6 HV. HEAT 2 contains approximately 17% retained austenite and displays a higher hardness of 609 ± 10 HV. The carbides have almost completely dissolved, with residual carbide densities of 43 and 14 carbides per mm2 for HEATS 1 and 2, respectively. Owing to the higher austenitizing temperature and the dissolution of grain-pinning carbides, considerable grain growth is observed, and the average ASTM grain size number decreases to 6.3 for HEAT 1 and 5.1 for HEAT 2.
Scanning electron micrographs of HEATS 1 and 2 after austenitizing at 1175 °C are shown in Fig. 13 and 14. Both heats contain martensite and retained austenite (29% retained austenite in HEAT 1 and 21% in HEAT 2). No carbide particles are visible, suggesting that this temperature is above the temperature for complete carbide dissolution in both alloys. Excessive grain growth is evident in both heats, with the average ASTM grain size numbers of 4 and 3.4 for HEATS 1 and 2, respectively. The high percentage retained austenite resulted in low hardness values of 279 ± 4 HV for HEAT 1 and 488 ± 3 HV for HEAT 2.
The CALPHAD model predicts that no carbides are present in either heat at an austenitizing temperature of 1175 °C. As shown in Fig. 15, both alloys are located well within the austenite phase field. The complete dissolution of carbides during heat treatment depresses the martensite transformation range and results in high levels of retained austenite.
At 1200 °C, all carbides are in solution in the austenite, resulting in as-quenched microstructures containing martensite and retained austenite. HEAT 1 contains 33% retained austenite, whereas HEAT 2 contains 24%. Hardness values of 270 ± 12 HV for HEAT 1 and 459 ± 2 HV for HEAT 2 were measured. The higher hardness of HEAT 2 can be attributed to the higher martensite content after quenching. Excessive grain growth occurs at the austenitizing temperature, yielding the average as-quenched ASTM grain size numbers of 2.8 and 3.2 for HEATS 1 and 2, respectively.
The results considered above confirm that an increase in austenitizing temperature is associated with a decrease in carbide density and an increase in the percentage retained austenite. Figure 16 shows the effect of austenitizing temperature on the carbide density in HEATS 1 and 2. The carbide densities in both steels decrease with an increase in austenitizing temperature. At austenitizing temperatures between 1000 and 1050 °C, the carbide densities of both heats decrease at similar rates, suggesting the progressive dissolution of M23C6 carbides (M23C6 is reported to dissolve at temperatures between 950 and 1050 °C). HEAT 2 has a higher initial carbide density, but at temperatures higher than 1050 °C, the carbide density decreases at a faster rate than that of HEAT 1. This suggests that the higher molybdenum content of HEAT 1 retards carbide dissolution at higher austenitizing temperatures, possibly because of the increased stability of M7C3 in the molybdenum-containing alloy (as confirmed by the CALPHAD phase diagrams). Molybdenum appears to stabilize and promote the equilibrium M7C3 carbide at temperatures higher than 1050 °C, retarding carbide dissolution. Complete carbide dissolution takes place at temperatures higher than 1175 °C in both heats.
The gradual dissolution of carbides up to austenitizing temperatures of 1175 °C (where complete carbide dissolution is observed in both heats) affects the measured retained austenite content, as-quenched hardness, and grain size of the steels. The effect of austenitizing temperature on the as-quenched hardness is shown in Fig. 17. The measured hardness values of both heats increase slightly with an increase in austenitizing temperature up to 1075 °C. This can be attributed to the gradual dissolution of M23C6 carbides, which raises the carbon content of the austenite at elevated temperatures. On quenching, a higher-carbon martensite forms, which increases the as-quenched hardness of both heats. Although retained austenite is present in both heats after quenching from austenitizing temperatures of 1075 °C or lower, retained austenite is not present in high enough quantities to reduce the as-quenched hardness significantly. Higher austenitizing temperatures raise the amount of carbon and alloying elements in solution in the austenite, and depress the martensite transformation range to lower temperatures. At temperatures higher than approximately 1075 °C, increased carbide dissolution results in higher retained austenite contents (as shown in Fig. 18), particularly in HEAT 1, and a considerable reduction in hardness. The higher retained austenite content and lower as-quenched hardness of HEAT 1 after austenitizing at higher temperatures can probably be attributed to the higher alloying content of this steel. More molybdenum in solid solution causes a considerable reduction in the martensite transformation range, resulting in higher levels of retained austenite.
The dissolution of carbides during austenitizing also affects the austenite grain size, as shown in Fig. 19 at austenitizing temperatures between 1000 and 1100 °C. The average ASTM grain size number remains stable at well above 9 for austenitizing temperatures below about 1075 °C. At austenitizing temperatures between 1075 and 1200 °C, the grain size increases rapidly from an average ASTM grain size number of 9 to around 3. This increase in grain size is associated with the increase in temperature (providing a higher driving force for grain growth during heat treatment), compounded by the dissolution of grain-pinning carbides. Grain growth in HEAT 1 is suppressed at temperatures below approximately 1120 °C because of the higher stability of carbides in this steel. Once all the alloying elements are in solid solution due to the dissolution of carbides at higher temperatures, the austenite grain sizes of the two heats are similar.
The average carbide diameters of the two heats are shown in Fig. 20, as a function of austenitizing temperature. The average carbide diameter measured in HEAT 1 decreases from approximately 1.3 μm at 1000 °C to 0.8 μm at 1100 °C. This reduction in average carbide diameter is less evident in HEAT 2, with the diameter decreasing from approximately 0.75 μm at 1000 °C to below 0.6 μm diameter at 1100 °C.
Conclusions
Medium-carbon AISI 420 martensitic stainless steel is recommended for use in applications requiring moderate corrosion resistance, high hardness, excellent wear resistance, and good edge retention in cutting surfaces. The microstructure and properties of this steel depend strongly on the hardening heat treatment, and in particular the austenitizing treatment that the steel receives. The austenitizing temperature controls the partitioning of alloying elements between the austenite and carbides at elevated temperature, and affects the martensite transformation range, grain size, hardness, and the retained austenite content of the steel in the as-quenched condition. This project aimed at studying the effect of the austenitizing heat treatment on the microstructure and properties of two heats of as-quenched AISI 420 martensitic stainless steel. The following conclusions are drawn:
-
Higher austenitizing temperatures lead to increased carbide dissolution. The carbide densities in both steels decrease with an increase in austenitizing temperature. The higher molybdenum content of HEAT 1 retards carbide dissolution at higher austenitizing temperatures because of the increased stability of the carbides (as confirmed by phase diagrams constructed using the CALPHAD model). Complete carbide dissolution takes place at temperatures higher than 1175 °C in both heats.
-
The gradual dissolution of carbides at austenitizing temperatures up to 1175 °C (where complete carbide dissolution is observed in both heats) affects the measured retained austenite content, as-quenched hardness, and grain size of the steels. The measured hardness values of both heats increase slightly with an increase in austenitizing temperature up to 1075 °C. This can be attributed to the partial dissolution of M23C6 carbides, which raises the carbon content of the austenite phase, as a result of which a higher carbon martensite forms on quenching.
-
Higher austenitizing temperatures raise the amount of carbon and alloying elements in the solution in the austenite, and depress the martensite transformation range to lower temperatures. At temperatures higher than approximately 1075 °C, increased carbide dissolution results in higher retained austenite contents, particularly in HEAT 1, and a considerable reduction in hardness.
-
The dissolution of carbides during austenitizing affects the austenite grain size. The average ASTM grain size number remains stable at well above 9 for austenitizing temperatures below about 1075 °C. At austenitizing temperatures between 1075 and 1200 °C, the grain size increases rapidly. This increase in grain size is associated with the increase in temperature (thereby providing a higher driving force for grain growth during heat treatment), compounded by the dissolution of grain-pinning carbides.
References
W.F. Smith, Principles of Materials Science and Engineering, 3rd ed., McGraw-Hill, New York, 1996, p 517–551
F.B. Pickering, The Metallurgical Evolution of Stainless Steels, a Discriminative Selection of Outstanding Articles and Papers from the Scientific Literature, American Society for Metals, Metals Park, OH, 1979, p 2–44
D.S. Clark and W.R. Varney, Metallurgy for Engineers, D. van Nostrand Company, New York, 1965, p 206–333
Interalloy Pty Ltd, Data Sheet for 420 Martensitic Stainless Steel Bar, available at http://www.interlloy.com.au/data_sheets/stainless_steel/stainless_pdf/interlloy_420_Martensitic_Stainless_Steel_Bar.pdf
A. Rajasekhar, G.M. Reddy, T. Mohandas, and V.S.R. Murti, Influence of Austenitizing Temperature on Microstructure and Mechanical Properties of AISI, 431 Martensitic Stainless Steel Electron Beam Welds, Mater. Des., 2009, 30(5), p 1612–1624
I. Calliari, M. Zanesco, M. Dabala, K. Brunelli, and E. Ramous, Investigation of Microstructure and Properties of a Ni-Mo Martensitic Stainless Steel, Mater. Des., 2008, 29(1), p 246–250
S.S.M. Tavares, D. Fruchart, S. Miraglia, and D. Laborie, Magnetic Properties of an AISI, 420 Martensitic Stainless Steel, J. Alloy. Compd., 2000, 312(1–2), p 307–314
Latrobe Steel, Datasheet for LSS 420 HC stainless steel, 2008, available at http://www.matweb.com/search/datasheet.aspx?matguid=ed94b14f5a6b463e8ffee7b89def0cec&ckck=1
C. Garcia de Andrés, L.F. Alvarez, and V. Lopez, Effects of Carbide Forming Elements on the Response to Thermal Treatment of the X45Cr13 Martensitic Stainless Steel, J. Mater. Sci., 1998, 33, p 4095–4100
G.F. vander Voort, Committee E-4 and Grain Size Measurements, ASTM Standardization News, May 1991
C. Garcia de Andrés, G. Caruna, and L.F. Alvarez, Control of M23C6 Carbides in 0.45C-13Cr Martensitic Stainless Steel by Means of Three Representative Heat Treatment Parameters, Mater. Sci. Eng. A, 1998, A241, p 211–215
A. Bjarbo and M. Hatterstrand, Complex Carbides Growth, Dissolution, and Coarsening in a Modified 12 Pct Chromium Steel—An Experimental and Theoretical Study, Metall. Mater. Trans. A, 2001, 32A, p 19–27
R.A. Higgins, Engineering Metallurgy, Part 1—Applied Physical Metallurgy, 5th ed., Hodder and Stoughton, New York, 1983, p 295–319
S.H. Avner, Introduction to Physical Metallurgy, International Student Edition, McGraw-Hill, New York, 1974, p 349–367
S. Salem, Alloyed Steel Intended for Hot Rolling Mill Rolls, Met. Sci. Heat Treat., 1993, 35(11), p 657–659
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
A summary of the results obtained for HEAT 1 and HEAT 2 after various austenitizing heat treatments
Austenitizing temperature | Hardness (HV) | Microstructure | % Retained austenite | Carbide density per mm2 |
|---|---|---|---|---|
HEAT 1 | ||||
1000 °C | 664 | Martensite, retained austenite and carbides | 4 | 181 |
1050 °C | 678 | Martensite, retained austenite and carbides | 5 | 131 |
1075 °C | 684 | Martensite, retained austenite and carbides | 15 | 87 |
1100 °C | 653 | Martensite, retained austenite and carbides | 23 | 84 |
1130 °C | 474 | Martensite, retained austenite and carbides | 25 | 81 |
1150 °C | 308 | Martensite and retained austenite | 27 | 43 |
1175 °C | 279 | Martensite and retained austenite | 29 | 0 |
1200 °C | 270 | Martensite and retained austenite | 33 | 0 |
HEAT 2 | ||||
1000 °C | 639 | Martensite, retained austenite and carbides | 4 | 227 |
1050 °C | 665 | Martensite, retained austenite and carbides | 6 | 184 |
1075 °C | 674 | Martensite, retained austenite and carbides | 10 | 117 |
1100 °C | 639 | Martensite, retained austenite and carbides | 12 | 63 |
1130 °C | 620 | Martensite, retained austenite and carbides | 15 | 32 |
1150 °C | 609 | Martensite and retained austenite | 17 | 14 |
1175 °C | 488 | Martensite and retained austenite | 21 | 0 |
1200 °C | 459 | Martensite and retained austenite | 24 | 0 |
Rights and permissions
About this article
Cite this article
Barlow, L.D., Du Toit, M. Effect of Austenitizing Heat Treatment on the Microstructure and Hardness of Martensitic Stainless Steel AISI 420. J. of Materi Eng and Perform 21, 1327–1336 (2012). https://doi.org/10.1007/s11665-011-0043-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-011-0043-9