Abstract
In this study, 6060 aluminum alloy was coated by plasma electrolytic oxidation (PEO) process. The effect of sodium silicate concentration (A solution-7.5 g/L—B solution-15 g/L) on various morphological properties and corrosion resistance of the surface was investigated. The correlation between the microwave sintering of 6060 aluminum alloy coated by PEO and non-microwave sintering of 6060 aluminum alloy properties are discussed. Detailed estimation of the quality of the coated metal surface was performed by additional testing of chemical compositions by EDS, crystalline structure of the films was examined using x-ray diffraction (XRD) and scanning electron microscope (SEM). The results showed that the oxidation layer was of typical morphology for the PEO process. The porosity amount of 6060 aluminum sample coated with 15 g/L was obtained higher than that of 7.5 g/L. In addition to, the porosity of all coated samples was decreased with increasing microwave sintering time. The corrosion resistance of coated samples with microwave sintering process was better than non-microwave sintering of 6060 aluminum alloy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum alloys have become increasingly important for the automobile, aerospace, industrial fields, etc., owing to their high specific strength, low density, relatively well physical and chemical performance, and very abundance reserves in the earth’s crust. However, the extensive usage of aluminum alloys is limited in their relatively lower wear and corrosion resistance than that of steel. That’s why, plasma electrolytic oxidation (PEO) can be used to produce ceramic coatings on aluminum with potentially advantageous tribological and corrosion performance compared with other surface treatment technologies (Ref 1-5).
The PEO technique is an electrochemical formation of anodic films on non-ferrous metals by spark/arc microdischarges, which are initiated at potentials above the breakdown voltage of the growing oxide film and move rapidly across the anode surface (Ref 5-7). The properties of PEO coatings are affected by the processing parameters, such as composition of electrolyte, voltage, current density, time, etc. (Ref 8, 9).
Recently, many studies (Ref 10-13) mainly focused on optimization of the electrolyte composition to obtain coatings. However, the systematic effects of the sodium silicate concentration and the correlation between the microwave sintering on the structural and tribological properties of such coatings are not clear yet.
In this study, 6060 aluminum alloy was modified by PEO. The effect of different solution constant sodium silicate (A solution-7.5 g/L—B solution-15 g/L) on various morphological properties and corrosion resistance of the surface was investigated. And the correlation between the microwave sintering of 6060 aluminum alloy coated by PEO and non-microwave sintering of 6060 aluminum alloy properties are discussed. The results are hoped to give some useful information to the additive studies.
Materials and Experimental Details
Samples and Plasma Electrolytic Oxidation Processing
Cylindrical coupons (diameter-6 mm) of aluminum alloy were used as substrates for aluminum oxide coating deposition.
The aluminum oxide coating was fabricated using a 5 kW PEO unit, which consists of an insulated electrolyte bath, a thermometer, a stirrer, and a high-voltage DC power supply with approximate 1000 V of the maximum voltage amplitude. One output of the power supply was connected to the electrolyte bath and another output to a specimen immersing into the electrolyte. A constant current density (21 A/dm2) at the coating surface was maintained by controlling the voltage amplitude for 150 min. Stirring and cooling systems maintained the temperature of electrolyte at 20-30 °C during the process. The electrolyte was prepared from a solution of 7.5 g/L Na2SiO3-2.5 g/L KOH-2 g/L KF (A solution) and 15 g/L Na2SiO3-2.5 g/L KOH-2 g/L KF (B solution) in distilled water (Table 1).
Microwave Sintering
The aluminum alloys was microwave sintered with 2.5 GHz. Three different sintering times were chosen (5, 10, and 20 min).
Corrosion Test
Immersion test was carried out in 10 wt.% NaOH solution for 15 h in open system, the corrosion products were cleaned in distilled water with an ultrasonic cleaner, all the samples were weighted by JA5003N electronic balance (accuracy: 1 mg) before and after the immersion test, and the corrosion rate calculated from weight loss data.
Testing Methods
The surface roughness (Ra) of PEO coatings was detected by Mahr, Perthometer M1 surface roughometer. The surface and cross-sectional morphologies and the elemental compositions of PEO coatings were examined by EDS and SEM. The crystalline structure of the films was examined using x-ray diffraction (XRD) with a Cu K α source. The porosity was calculated by Archimedes method. The uncoated and coated Al 6060 alloys were measured using a FUTURE TECH-CORP.FM-700 microhardness tester at a load of 10 g and for a loading duration of 10 s.
Results and Discussion
Figure 1 shows the surface morphologies of the different concentration solution coatings prepared by PEO on Al 6060 alloy at 20 min microwave sintering treatment time. Figure 1(a) and (b) shows the surface morphologies at 20 min sintered Al 6060 with A solution. Figure 1(c) and (d) also shows the surface morphologies of the coating prepared by B solution on Al 6060 alloy at 20 min microwave sintered. It could be seen that the typical PEO porous structure is observed at different microwave sintering times. In addition, with increasing the microwave sintering time, the amount of porosity on the coating surface was reduced. What is more (Fig. 2), the results indicate that the coatings become more compact with raising the microwave sintering time. From Fig. 2, it can be seen that the surface porosity of the PEO coatings increase as the Na2SiO3 concentrations increased.
During the PEO process, when the applied voltage exceeds the critical voltage for barrier layer breakdown, a large number of visible sparks occur. When a spark extinguishes, melted substances are erupted into the discharge channel, leaving a craterlike hole on the coating surface (shown in Fig. 3), so a porous and coarse structure is formed on the surface of the sample. These results demonstrate that the surface morphology of the coatings formed by PEO mostly rely on the evolution of the sparks during the process of PEO. In Fig. 3, the surface image of the PEO coating on the Al 6060 substrate exhibited traces for microdischarge channels. After the microwave sintering process, the microdischarge channels is closed, was as seen in Fig. 3(b).
Table 2 shows the Ra and surface hardness of the PEO coatings formed in electrolytes with various concentrations of Na2SiO3.
Many studies showed that the solution conductivity varied in direct proportion to the solution concentration within a certain range (Ref 8, 9). Based on literature (Ref 10, 14, 15), PEO process depended mainly on whether the instantaneous plus voltage peak (V ps) is higher than the breakdown voltage of PEO coating or not. The higher the V ps, the stronger the instantaneous energy, then the faster the growth rate of PEO coating. In accordance with literature, we observed that the solution conductivity and the V ps were increased with raising the concentration of Na2SiO3 and the growth rate of PEO coatings was improved. Furthermore, the Ra of the coatings increased. In addition to, the roughness of all coated samples was decreased with increasing microwave sintering time.
The microhardness of coatings was determined from the average of measurements made on polished cross-sections in the inner part of the coatings. The results are listed in Table 2, which includes the maximum and minimum values of the hardness. Measurements of the cross-sectional microhardness of coatings indicated that the highest coating hardness was 1814 HV0.01 for coated with A solution and sintered 5 min. It is noted that the average microhardness exhibited by bare 6060 Al alloy substrate is only 90 HV0.01.
In terms of the microhardness analysis, we can see that all samples show a high microhardness through the PEO technique, which can satisfy the requirements for the mechanical application.
The chemical composition of the coating was analyzed by EDS. As shown in Fig. 4, the element of Al comes from the substrate alloy, additionally the elements of Si, K come from the solution. EDS analyses (Fig. 4), it can be indicated that only aluminum element was oxidized to form alumina (Al2O3) during the PEO process.
In Fig. 5, the element of Al come from the substrate alloy, additionally the element of Si, K, Na, and O come from the solution.
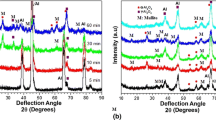
Figure 6 shows the XRD patterns of Al 6060 alloy and the PEO coatings formed in electrolytes containing various concentrations of Na2SiO3. The Al 6060 alloy is composed of Al, but XRD patterns indicate that the Al 6060 alloy is composed of Al, γ-Al2O3, and mullite phase. It can be seen that the sintering process did not change the phase composition. The strongest peak of mullite is from coating obtained in A solution.
Al 6060 alloys with and without PEO coatings, after microwave sintered coatings have been immersed in 10 wt.% NaOH solution for 15 h, weight loss method was used to calculate the corrosion rate and the results were shown in Fig. 7. The different solution constant and microwave sintering time has influenced the surface morphology, phase and elemental composition of the coatings, and therefore, it is bound to influence the corrosion resistance of the coatings.
Figure 7 shows that the corrosion rates of all the samples with PEO coatings are lower than that of Al 6060 alloy. When the Na2SiO3 concentration is increased from 7.5 to 15 g/L, the anticorrosion capacity of the samples with PEO coatings not influence the corrosion rate. However, when all the samples sintered, the anticorrosion capacity of the coatings decreased. It also can be seen that the best corrosion resistance coating is formed in electrolyte containing 15 g/L Na2SiO3, whose anticorrosion capacity is nearly three times higher than that of Al 6060 alloy.
Conclusions
-
Different coatings were formed on Al 6060 alloy in electrolytes containing various concentration of Na2SiO3. As a special additive, Na2SiO3 could effectively increase the surface porosity of the coatings. After the microwave sintering treatment, the amount of porosity on the coating surface was decreased.
-
The introduction of Na2SiO3 into the electrolyte leads to significant change in the discharge characteristics of the sparks at the surface. High Na2SiO3 leads to increased sparking discharge intensity and contributes the accumulation of the coating products and forms a high Ra. The low Na2SiO3 tends to form fine-grain structure and causes lower Ra contrarily to high sodium silicate. After the microwave sintering treatment, the roughness of the coatings was decreased.
-
The coatings produced in each of two different electrolytic solutions provide a high surface hardness.
-
Corrosion tests show that the corrosion resistance of Al 6060 alloy could be significantly improved by PEO treatment, moreover, the corrosion resistance of the PEO coatings also could be increased by adding proper concentration of Na2SiO3. The best corrosion resistance coating was obtained in electrolyte containing 15 g/L Na2SiO3 and also microwave sintering.
References
E. Arslan, Y. Totik, E.E. Demirci, Y. Vangol, A. Alsaran, and I. Efeoglu, High Temperature Wear Behavior of Aluminum Oxide Layers Produced by AC Micro Arc Oxidation, Surf. Coat. Technol., 2009, 204, p 829–833
H. Ding, Z. Dai, S. Skuiry, and D. Hui, Corrosion Wear Behaviors of Micro-Arc Oxidation Coating of Al2O3 on 2024Al in Different Aqueous Environments at Fretting Contact, Tribol. Int., 2010, 43, p 868–875
X. Shi-Gang, S. Li-Xin, Z. Rong-Gen, and H. Xing-Fang, Properties of Aluminium Oxide Coating on Aluminium Alloy Produced by Micro-Arc Oxidation, Surf. Coat. Technol., 2005, 199, p 184–188
E. Matykina, R. Arrabal, A. Mohamed, P. Skeldon, and G.E. Thompson, Plasma Electrolytic Oxidation of Pre-Anodized Aluminium, Corros. Sci., 2009, 51, p 2897–2905
M.H. Zhu, Z.B. Cai, X.Z. Lin, P.D. Ren, J. Tan, and Z.R. Zhou, Fretting Wear Behaviour of Ceramic Coating Prepared by Micro-Arc Oxidation on Al-Si Alloy, Wear, 2007, 263, p 472–480
Y. Oh, J. Mun, and J. Kim, Effects of Alloying Elements on Microstructure and Protective Properties of Al2O3 Coatings Formed on Aluminum Alloy Substrates by Plasma Electrolysis, Surf. Coat. Technol., 2009, 204, p 141–148
L.R. Krishna, A.S. Purnima, and G. Sundararajan, A Comparative Study of Tribological Behavior of Microarc Oxidation and Hard-Anodized Coatings, Wear, 2006, 261, p 1095–1101
A. Polat, M. Makaraci, and M. Usta, Influence of Sodium Silicate Concentration on Structural and Tribological Properties of Microarc Oxidation Coatings on 2017A Aluminum Alloy Substrate, J. Alloys Compd., 2010, 504, p 519–526
H.Y. Zheng, Y.K. Wang, B.S. Li, and G.R. Han, The Effects of Na2WO4 Concentration on the Properties of Microarc Oxidation Coatings on Aluminum Alloy, Mater. Lett., 2005, 59, p 139–142
L. Yerokhin, X. Nie, A. Leyland, and A. Matthews, Characterisation of Oxide Films Produced by Plasma Electrolytic Oxidation of a Ti-6Al-4V Alloy, Surf. Coat. Technol., 2000, 130, p 195–206
W. Xue, C. Wang, R. Chen, and Z. Deng, Structure and Properties Characterization of Ceramic Coatings Produced on Ti-6Al-4V Alloy by Microarc Oxidation in Aluminate Solution, Mater. Lett., 2002, 52, p 435–441
J. Tian, Z. Luo, S. Qi, and X. Sun, Structure and Antiwear Behavior of Micro-Arc Oxidized Coatings on Aluminum Alloy, Surf. Coat. Technol., 2002, 154, p 1–7
Y. Han, S.H. Hong, and K.W. Xu, Porous Nanocrystalline Titania Films by Plasma Electrolytic Oxidation, Surf. Coat. Technol., 2002, 154, p 314–318
M.H. Zhu, Z.B. Cai, X.Z. Lin, J.F. Zheng, J. Luo, and Z.R. Zhou, Fretting Wear Behaviors of Micro-Arc Oxidation Coating Sealed by Grease, Wear, 2009, 267, p 299–307
W. Xiaohong, J. Zhaohua, L. Huiling, X. Shigang, and H. Xinguo, Photo-Catalytic Activity of Titanium Dioxide Thin Films Prepared by Micro-Plasma Oxidation Method, Thin Solid Films, 2003, 441, p 130–134
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becerik, D.A., Ayday, A., Kumruoğlu, L.C. et al. The Effects of Na2SiO3 Concentration on the Properties of Plasma Electrolytic Oxidation Coatings on 6060 Aluminum Alloy. J. of Materi Eng and Perform 21, 1426–1430 (2012). https://doi.org/10.1007/s11665-011-0022-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-011-0022-1