Abstract
It is the objective of this article to investigate the influence of surface preparation on the cold roll bonding (CRB) process. In this context, the effects of surface preparation parameters consisting of surface preparation method, surface roughness, scratch-brushing parameters, and the delay time between surface preparation and rolling are investigated on the bond strength of aluminum strips. The bond strength of two adjacent aluminum strips produced by the CRB process is evaluated by the peeling test. Furthermore, the interface region is investigated by metallographic observations. Our findings indicate that higher surface roughness values and shorter delay times improve the bond strength. It is also found that degreasing followed by scratch-brushing yield the best bonding properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold roll bonding (CRB) is a solid-phase welding process whereby the bonding is established by the joint plastic deformation of the metals to be bonded. Bonding is obtained when the surface expansion causes the surfaces of the virgin metal to be exposed or when the pressure reaches a value large enough to extrude the virgin material through the cracks of the fractured layer, which results in the establishment of contact and bonding between opposing virgin surfaces (Ref 1-3).
The solid-state joining technique in the CRB can be applied to a large number of either same materials possessing identical attributes, or different materials possessing widely varying mechanical and metallurgical properties (Ref 1-3). In comparison with other methods, CRB is simple and can be easily automated.
Metal surfaces are typically rough, and when two absolutely clean surfaces are pressed together, contact is expected. In practice, metal surfaces are covered with oxide films and other surface contaminants (Ref 4, 5), such as grease, chemical compounds remaining after pickling, and adsorbed moisture. These inhibit bonding, at least at room temperature. Consequently, the surface condition before CRB is a very important factor influencing the bond strength.
Of utmost importance in the CRB process is the removal of contaminant layers from the surface by chemical and mechanical treatments (surface preparation). This typically involves the proper cleaning and preparing of surfaces in order to remove any contaminants (bonding barriers) on the surfaces of the two metals to be joined (Ref 6-8).
Many research studies have been conducted to investigate the parameters involved in the bonding process in an attempt to gain an understanding of the complex nature of bonding mechanisms and to obtain an empirically based definition of the process conditions. It has been reported that the roll bonding of metals is affected by such various factors as reduction of thickness during rolling (Ref 1-3, 9-11), bonding temperature (Ref 11, 12), annealing treatment before and/or after the CRB process (Ref 10, 13, 14), rolling speed (Ref 10, 11, 15, 16), rolling direction (Ref 10), and initial thickness (Ref 10, 11). However, no conclusive research has been reported on the effects of surface preparation parameters on the bond strength of the strip when CRB is used.
Recent applications of CRB to a range of materials have prompted this study on the influence of process parameters on bonding quality. The surface preparation parameters of CRBed aluminum strips consist of surface roughness, scratch-brushing parameters, delay time between surface preparation and rolling, and the surface preparation method employed. The parameters involved in scratch-brushing consist of peripheral speed, brushing load, and brush stiffness (Ref 17). Also, the present authors have found two other important parameters of scratch-brushing, namely, wire diameter and wire length. However, no research is reported in the literature on the effects of wire diameter and length on bond strength. For this reason, the peripheral speed and brushing load were assumed constant in this study and the effects of brush stiffness on bond strength were investigated as assessed by wire diameter and length. The different methods of surface preparation consisted of degreasing followed by scratch-brushing, scratch-brushing followed by degreasing, electrochemical Ni plating (Matt Ni and Bright Ni), chemical Ni plating, machining, anodizing, and electropolishing (Ref 5, 17). It was found that scratch-brushing gave the best bonding properties (Ref 17). This is because scratch-brushing produces rough and brittle surfaces which provide a greater amount of surface asperities and promote localized shear deformation to break unavoidable surface oxide films during rolling. For this reason, the first and second methods were further investigated for evaluation.
The objective of this study is to investigate the effects of surface preparation parameters such as surface roughness, scratch-brushing parameters, delay time between surface preparation and rolling, and the surface preparation method used in CRBed aluminum strips. To determine the influence of surface preparation, a series of peeling tests were performed.
Experimental Procedure
Materials
As-received commercial purity aluminum strips with the specifications given in Table 1 (achieved by a quant meter apparatus) were used in this study. Strips of 150 × 30 × 1.5 mm3 were cut from a cold-rolled sheet, parallel to the original rolling direction.
Surface Preparation
To produce a satisfactory metallurgical bond by the CRB process, it is essential to remove the contamination layers on the surfaces of the two metals to be joined. The identical preparation process used for all the samples included degreasing in an acetone bath followed by scratch-brushing the surfaces with a rotating stainless steel brush machine (maintaining a constant pressure on the strips). Also, to investigate the influence of the surface preparation method on bond strength, some of the specimens were first scratch-brushed and then degreased. To investigate the effect of surface roughness on bond strength, brushes were used with wire diameters of 0.24, 0.25, and 0.26 mm and wire lengths of 25, 29, 32, and 35 mm. Surface roughness was measured by the SM7 roughness profile meter apparatus and according to ASTM-D7127 standard. Roughness was measured randomly at 10 different points in the longitudinal and transverse rolling directions for each sample. Maximum and minimum measurement values were disregarded, and the mean roughness value was calculated using the remaining eight values. The surfaces were subsequently placed on each other and riveted in order to ensure alignment during rolling. It is important that the clean surface thus prepared should not be touched because grease or oil on the faying surfaces might impair the formation of a strong joint. Welding should take place immediately after degreasing and scratch-brushing to avoid any interference with bonding from oxidation.
Cold Roll Bonding Process
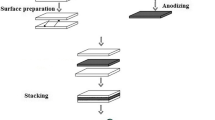
After surface preparation, the handling of strips was performed carefully to avoid renewed contamination. Furthermore, to avoid the strip sliding on each other, the stack was fixed by copper wires at four corner points. The time between surface preparation and rolling was kept to less than 1 min in an attempt to avoid the formation of a thick and continuous oxide layer on the bond strip surfaces. Delay times of 5, 10, 15, 30, 60, 90, and 120 min were used to investigate effects on bond strength. Finally, the stack was rolled at the ambient temperature to reduce its thickness. The CRB experiments were carried out with no lubricant using a laboratory rolling mill with a loading capacity of 20 tons. The roll diameters were 125 mm, and the rolling speed was 2 m/min. The schematic illustration of CRB for the production of layered alloys is presented in Fig. 1.
Peeling Test
The strips were evaluated for their bond strength using a peeling test according to ASTM-D1876-01. The peel tests were performed using a Hounsfield H50KS tensile testing machine with a 50 kg load cell and a crosshead speed of 20 mm/min. In this test, breaking-off forces were measured as shown in Fig. 2 and the average peel strength was determined using the following equation:
After peeling, the fracture surfaces of the samples were studied by PHILIPS XL30 scanning electron microscopy (SEM) and optical microscopy (OM).
Results
Effect of Surface Roughness
The initial surface roughness (prior to scratch-brushing) of the samples was 0.5 μm along both the rolling and transverse directions. Wire brushes with different specifications were used to create scratches of different surface roughness (Table 2). It can be seen in Table 2 that surface roughness is enhanced by increasing wire diameter and decreasing wire length. Figure 3 shows the effect of surface roughness on bond strength. By increasing surface roughness, the average peel strength and, therefore, the bond strength are enhanced.
Effect of Delay Time Between Surface Preparation and Rolling
Strips of aluminum were rolled after scratch-brushing the surfaces and exposing them to the atmosphere with a temperature of 293 K and a humidity of 20% for periods of 1-120 min. Figure 4 shows the variations in average peel strength versus delay time at a constant reduction (R = 50%). It is seen that bond strength increases by decreasing the delay time between surface preparation and rolling of Al/Al strips. If bonding is carried out within the first 10 min, the bond strength is not affected but longer delay times causes reduced bond strength. The resulting bond strengths were found to decrease markedly with exposure for 15-60 min. Beyond this value, the strength reduction rate takes a slightly falling trend again.
Effect of Surface Preparation Method
Figure 5 shows the effects of two different types of surface preparation on the bonding of aluminum strips:
-
1.
Degreasing in an acetone bath followed by scratch-brushing the surfaces (the method commonly used in most studies),
-
2.
scratch-brushing the surfaces followed by degreasing in acetone (the method used in a few number of studies such as Ref 18).
A series of logarithmic curves were fitted to the experimentally obtained data using a best-fit method. It is evident from Fig. 5 that degreasing followed by scratch-brushing gave a far better bond strength. When the procedure was reversed, a far lower bond strength was obtained. The results also showed a lower threshold deformation (R t) of about 35% for the first method while it was around 50% for the formation of an appropriate bond in the second method. Beyond these values, the bond strength increased rapidly with reducing thickness.
Fractography
Scanning electron microscopy micrographs before and after scratch-brushing of strips are shown in Fig. 6. As shown in Fig. 6(a-d), the surface roughnesses of aluminum strips produced by different wire brushes are 0.5 (for as-received strips), 2.4, 3.2, and 4.2 μm. It can be seen that the brush causes tears in the surface, which are obviously enhanced by increasing surface roughness and the depth and number of tears. Also, the scratch-brushed layer which is relatively hard and brittle consists of striations in the wire brushing direction.
Figure 7 shows the fracture surfaces of the specimens after the peeling test at a constant reduction in thickness for different surface roughnesses. These figures show cracks along with extruded areas of the virgin metal on the peeled surface. The extruded areas of the virgin substrate facilitate the intimate contact and, ultimately, the bonding between the two strips. An increase in surface roughness increases the size and number of these cracks.
The observation of cracks and the extruded virgin metal through them was investigated by several authors (Ref 10, 14, 19-21). They expressed that, since the brittle surface layers (oxidized scratch-brushed layers) on strips have little ductility and also there is a difference between hardness of underlying virgin metal and brittle surface layers, they break up under rolling deformation and cracking occurs.
Scanning electron microscopy micrographs after scratch-brushing and before the CRB are shown in Fig. 8 for the two delay times of 5 and 15 min. It can be seen that the strip surface with a delay time of 5 min is fresher compared to the one with a delay time of 15 min. As mentioned in Section 3.2, 10 min is the crucial delay time since bond strength remains unchanged during this time. It is, therefore, more important to investigate the behavior of the samples at delay times shorter and longer than this.
Optical microscopy micrographs of fractured surfaces for different delay times produced by 50% reduction are shown in Fig. 9. It can be seen that an increase in delay time causes a corresponding decrease in the extruded areas of the virgin metals.
Figure 10 shows the fracture surfaces of the specimens after the peeling test at a constant thickness reduction for different surface preparation methods. Figure 10(a) shows the strip scratch-brushed followed by degreasing in an acetone bath, while Fig. 10(b) shows one sample degreased followed by scratch brushing. It can be seen in Fig. 10(b) that the size and number of cracks and, therefore, the extruded areas of the virgin metals, are greater.
Discussion
Referring to Fig. 3, it is evident that by increasing strip surface roughness, average peel strength and, thereby, the bond strength increases. Increasing surface roughness increases the work hardening of strips and causes a more brittle layer to form on the surface that can be broken more easily which, in turn, causes the virgin metal to be extruded more easily. Figures 6 and 7 reveal that an increase in surface roughness causes the number and size of cracks on the surface layers to increase, providing greater areas of extruded virgin metals to be joined and, thus, enhancing bond strength. Scratch brushing is not only for cleaning, but also for providing rough surfaces, which provide a greater amount of surface asperities and promote a localized shear deformation that breaks unavoidable surface oxide films during roll bonding, contributing to the bonding of two metals. Consequently, surface roughening by scratch brushing in our experiments greatly improved bonding quality, reduced the pressure required to initiate bonding, and gave the highest bond strength in a number of cases. According to Table 2, increased wire diameter and decreased wire length increased surface roughness and, therefore, bond strength. By increasing wire diameter, the depth and number of the tearings are obviously enhanced, which naturally increases surface roughness. Also, by increasing wire length, more wire curves are obtained during scratch-brushing so that pressure decreases because of the increasing contact surface between the wire and the strip. This causes the depth and number of tears to decrease, which in turn decreases surface roughness as a consequence. The scratch brushing of the surfaces to be bonded can improve CRB; thus, it may be reasonable to conclude that roughness is an important factor affecting bonding.
Figure 4 demonstrates bond strength increasing by decreasing the delay time between surface preparation and rolling of Al/Al strips at a constant value of thickness reduction. These results may be attributed to the formation of a thinner layer of oxides, absorbed ions (of sulfur, phosphorus, and oxygen), humidity, and dust particles on the surfaces of the strips. In other words, it seems that the decreased bonding ability was a function of the increasing thickness of the oxide film. The fracture of the work-hardened surface layer or the oxide film and the extrusion of virgin metals through the cracks played very important roles in the real contact between metals. By increasing the thickness of the oxide film, fracturing became more difficult and, therefore, the bond strength decreased. However, the bond strengths slightly decreased after 60 min when the formation of the oxide layer on the surfaces was almost complete. In other words, it is extremely difficult for the oxide layer to grow thicker after 60 min. Figure 9 reveals that only a few surface cracks appear at higher delay times and that the amount of virgin metals in contact with one another is, therefore, very small at the interface. Thus, new metal surfaces cannot bond sufficiently.
It may be concluded from Fig. 5 that degreasing followed by scratch brushing gave far better bonding properties. According to Fig. 10, degreasing followed by scratch-brushing caused the number and size of cracks to grow, giving rise to more extruded areas. Thus, this method enhances bond strength. From these results, it can be concluded that the surface condition in the interface has a significant influence on bonding.
Conclusions
The bond strength between aluminum strips produced by the CRB process at different surface roughnesses, with various delay times between surface preparation and rolling, and by different surface preparation methods were assessed and measured by the peeling test. The conclusions drawn from the experiments can be summarized as follows:
-
1.
Increasing surface roughness of strips increases average peel strength or bond strength. This is because of the increasing rolling force and pressure as well as work hardening by enhanced surface roughness.
-
2.
Surface roughness and, thereby, bond strength increase by increasing wire diameter and decreasing wire length.
-
3.
Bond strength decreases with increasing delay time between surface preparation and rolling at a constant value of thickness reduction. It seems that the decrease in bonding ability is a function of the increase in the oxide film thickness.
-
4.
Degreasing in an acetone bath followed by scratch-brushing of surfaces versus scratch-brushing of the surfaces followed by degreasing in an acetone bath greatly increases bond strength.
References
D. Pan, K. Gao, and J. Yu, Cold Roll Bonding of Bimetallic Sheet and Strips, Mater. Sci. Technol., 1989, 5, p 934–939
N.D. Lukaschkin, A.P. Borissow, and A.I. Elrikh, The System Analysis of Metal Forming Technique in Welding Processes, J. Mater. Proc. Technol., 1997, 66, p 264–269
H.Y. Wu, S. Lee, and J.Y. Wang, Solid State Bonding of Iron-Base Alloy, Steel-Brass and Aluminum Alloy, J. Mater. Proc. Technol., 1998, 75, p 173–179
N. Bay, Cold Welding Part II, Process Variation and Application, Met. Construct., 1986, 18(6), p 486–490
L.R. Vaidyanath and D.R. Milner, Significance of Surface Preparation in Cold Pressure Welding of Metals, Br. Weld. J., 1960, 7, p 1–6
N. Bay and W. Zhang, Influence of Different Surface Preparation on the Bond Formation in Cold Pressure Welding, Proceeding of the Second European Conference on Joining Technology, Italy, 1994, p 379–388
C. Clemensen and O. Jalstrap, Cold Welding-Influence of Surface Preparation on Bond Strength, Met. Construct., 1986, 18(10), p 625–629
K. Thomas, Roll Welding, ASM Handbook, Welding, Brazing, and Soldering, Vol 6, ASM, Materials Park, OH, 1994, p 312–314
M.Z. Quadir, A. Wolz, M. Hoffman, and M. Ferry, Influence of Processing Parameters on the Bond Toughness of Roll-Bonded Aluminum Strip, Scr. Mater., 2008, 58, p 959–962
R. Jamaati and M.R. Toroghinejad, Investigation of the Parameters of the Cold Roll Bonding (CRB) Process, Mater. Sci. Eng., 2010, A527, p 2320–2326
M. Abbasi and M.R. Toroghinejad, Effects of Processing Parameters on the Bond Strength of Cu/Cu Roll-Bonded Strips, J. Mater. Proc. Technol., 2010, 210, p 560–563
M. Eizadjou, H. Danesh Manesh, and K. Janghorban, Investigation of Roll Bonding Between Aluminum Alloy Strips, Mater. Des., 2008, 29, p 909–913
H. Danesh Manesh and A. Karimi Taheri, The Effect of Annealing Treatment on Mechanical Properties of Aluminum Clad Steel Sheet, J. Mater. Des., 2003, 24, p 617–622
M. Movahedi, H.R. Madaah Hosseini, and A.H. Kokabi, The Influence of Roll Bonding Parameters on the Bond Strength of Al-3003/Zn Soldering Sheets, Mater. Sci. Eng., 2008, A487, p 417–423
K.J.B. McEwan and D.R. Miller, Pressure Welding of Dissimilar Metals, Br. Weld. J., 1962, 9, p 406–420
J. Butlin and C.A. Mackay, Experiment on the Roll-Bonding of Tin Coatings to Non-Ferrous Substrate Sheet, Metal. Ind., 1979, p 1063–1072
N. Bay, Cold Welding Part III, Influence of Surface Preparation on Bond Strength, Met. Construct., 1986, 18(6), p 625–629
G. Krallics and J.G. Lenard, An Examination of the Accumulative Roll-Bonding Process, J. Mater. Proc. Technol., 2004, 152, p 154–161
M. Soltan Ali Nezhad and A. Haerian Ardakani, A Study of Joint Quality of Aluminum and Low Carbon Steel Strips by Warm Rolling, Mater. Des., 2009, 30, p 1103–1109
M. Alizadeh and M.H. Paydar, Study on the Effect of Presence of TiH2 Particles on the Roll Bonding Behavior of Aluminum Alloy Strips, Mater. Des., 2009, 30, p 82–86
M. Eizadjou, H. Danesh Manesh, and K. Janghorban, Mechanism of Warm and Cold Roll Bonding of Aluminum Alloy Strips, Mater. Des., 2009, 30, p 4156–4161
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamaati, R., Toroghinejad, M.R. The Role of Surface Preparation Parameters on Cold Roll Bonding of Aluminum Strips. J. of Materi Eng and Perform 20, 191–197 (2011). https://doi.org/10.1007/s11665-010-9664-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-010-9664-7