Precipitation strengthening of Cu-3Ti-1Cd alloy has been studied using hardness and tensile tests, electrical resistivity measurements, and transmission electron microscopy. The alloy exhibited a hardness of 117 Hv in solution-treated (ST) condition and attained a peak hardness of 288 Hv after aging at 450 °C for 72 h. Electrical conductivity increased from 7%IACS (International Annealed Copper Standard) in ST condition to 13%IACS on aging at 450 °C for 16 h. The alloy exhibited yield strength (YS) of 643 MPa and ultimate tensile strength (UTS) of 785 MPa in peak-aged (PA) condition. Strengthening in Cu-3Ti-1Cd alloy in PA condition is attributed to solid solution strengthening effect of cadmium (Cd) as well as fine scale precipitation of metastable and coherent β′-Cu4Ti phase. On overaging at 450 or 500 °C, the alloy showed a decrease in hardness as a result of formation of equilibrium precipitate β-Cu3Ti as continuous precipitation within the matrix and as discontinuous precipitation at the grain boundaries. While the tensile properties are better, the electrical conductivity of Cu-3Ti-1Cd alloy is less than that of binary Cu-2.7Ti alloy. The strengthening mechanism is the same in both binary and ternary alloys of Cu-Ti, i.e., precipitation of metastable and coherent β′-Cu4Ti phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper and Cu-base alloys are widely used because of their excellent electrical and thermal conductivities, outstanding resistance to corrosion, ease of fabrication as well as good mechanical properties (Ref 1). Age hardenable Cu-Be alloys possess good combination of high strength and medium electrical conductivity (Ref 2), but with the limitations of high cost and toxicity associated with their preparation and processing. Worldwide efforts have, therefore, been concentrated on developing a substitute for Cu-Be alloys. Literature survey carried out indicated that Cu-Ti alloys have a good potential in this respect. Earlier studies have shown that binary Cu-Ti alloys are precipitation strengthened on aging by spinodal decomposition mechanism involving clustering and ordering in the initial stages and that maximum strength is associated with the formation of a coherent transition phase β′ (Cu4Ti) (Ref 3-6). Prolonged aging (overaging) characterizes cellular or discontinuous precipitation along grain boundaries with the formation of equilibrium precipitate β (Cu3Ti) (Ref 7, 8). Nagarjuna et al. (Ref 9) reported that the modulated structure with fine scale precipitation would be formed even during water quenching in Cu-Ti alloys with Ti content greater than 4 wt.%.
Numerous attempts have been made to enhance the tensile properties and electrical conductivity of binary Cu-Ti alloys with ternary additions like V, Al, Sn, B, and Co (Ref 10-14). Cu-Cd alloys were strengthened by solid solution strengthening with slight reduction in electrical conductivity (Ref 15). It was thought that addition of Cd might improve the properties of binary Cu-Ti alloys. Our work on Cu-4Ti-1Cd alloy (Ref 16) confirmed the earlier reports of formation of modulated structure during water quenching (Ref 9) and solid solution strengthening (Ref 15). This alloy exhibited better tensile properties in the peak-aged (PA) condition than those of Cu-4.5Ti alloy (Ref 9) and even comparable to those of Cu-2Be-0.5Co alloy (Ref 2). However, the electrical conductivity of Cu-4Ti-1Cd alloy was inferior to both Cu-4.5Ti and Cu-2Be-0.5Co alloys in solution-treated (ST) as well as PA conditions. In order to increase the electrical conductivity, an alloy with lower solute content in Cu matrix is necessary. Therefore, investigation on a ternary alloy having lower Ti content but keeping Cd content the same, i.e., Cu-3Ti-1Cd was undertaken. Little work has been reported on precipitation hardening in this new ternary alloy. This paper presents hardness, tensile properties, electrical conductivity, and microstructure of precipitation-hardened Cu-3Ti-1Cd alloy.
Experimental Procedure
Cu-3.0 wt.%Ti-1.0 wt.%Cd was prepared by melting in a Stokes Vacuum Induction Melting (VIM) furnace and cast as a 30 kg ingot. Oxygen-free high-conductivity (OFHC) copper and pure Ti and Cd metals were used as raw materials. After homogenization at 800 °C for 24 h, the ingot was analyzed for Ti and Cd concentrations. The analyzed composition matched with the aimed concentration of Ti and Cd. The ingot was initially hot forged and then rolled at 850 °C to 10 mm thick plates and 14 mm diameter rods. Specimens were cut from rods and solution treated at 860 °C for 2 h and quenched rapidly in water. These samples were aged at 400, 450, and 500 °C for different times. Vickers hardness (Hv) of ST samples was measured at 10 kg load for different times of aging. Five readings were taken for each measurement and their average is reported here.
Threaded tensile samples, as per ASTM E8M-97 standard, with a gauge diameter of 6 mm and length of 40 mm were prepared in ST as well as PA (at 450 °C) conditions and tested for tensile properties at ambient temperature in an INSTRON Universal Testing machine at the strain rate of 10−3 s−1.
The ST alloy rods were cold drawn to 2 mm diameter wire with intermittent annealings. Electrical resistance of 300 mm long wire in ST and aged conditions was measured using Micro-ohmmeter (model no. OM-15) and electrical conductivity was evaluated as per ASTM B193-95 specification.
Optical microscopy of the alloy in ST, PA, and overaged conditions was carried out by mechanical polishing followed by etching in a solution of 10 g K2Cr2O7, 5 ml H2SO4, 80 ml distilled water, and 2 drops of HCl. Slices of 0.5 mm thickness cut from the alloys using a Buehler’s Isomet low-speed saw, were mechanically polished to 50 μm thickness. Discs of 3 mm diameter were punched out from the thin slices and electro-polished in a twin jet electro-polisher using an electrolyte of 30 vol.% HNO3 and 70 vol.% methanol at −35 °C and 30 V. The thin foils were examined at 160 kV in a JEOL 200CX Transmission Electron Microscope. The fractured surfaces of tensile-tested samples were examined in a JEOL 840A Scanning Electron Microscope.
Results
Hardness
Figure 1 shows the influence of aging time on hardness of the ST Cu-3Ti-1Cd alloy aged at 400, 450, and 500 °C. A hardness of 117 Hv in ST condition has reached a maximum (235 Hv) after aging for 80 h at 400 °C but not overaged even up to 128 h. A peak hardness of 288 Hv was observed on aging for 72 h at 450 °C, beyond which the overaging was fast. Rapid overaging, however, was noticed in 4 h of aging at 500 °C when it had a hardness of 242 Hv.
Age hardening curves of Cu-3Ti-1Cd alloy aged at 400, 450, and 500 °C are shown respectively, in Fig. 2(a) to (c) in which, the data on binary Cu-2.7Ti alloy as reported by Nagarjuna et al. (Ref 9), are included for comparison. The hardness of Cu-3Ti-1Cd alloy in the ST condition is nearly same as that of Cu-2.7Ti alloy. The maximum hardness of ternary Cu-3Ti-1Cd alloy was observed to be similar to binary Cu-2.7Ti alloy in PA condition but the peak aging time was very long (72 h) compared to binary alloy (16 h) at 450 °C. However, overaging was very rapid in the present ternary alloy after attaining peak hardness at 500 °C compared to binary Cu-2.7Ti alloy.
Influence of Cd on age hardening of Cu-3Ti alloy compared with Cu-2.7Ti (Ref 9). (a) 400 °C (b) 450 °C, and (c) 500 °C
Tensile Properties
The tensile properties along with hardness and electrical conductivity of the present Cu-3Ti-1Cd alloy are compared with those of binary Cu-2.7Ti (Ref 9), Cu-4Ti-1Cd (Ref 16), and Cu-0.5Be-2.0Co (Ref 17) alloys in Table 1. The YS and UTS are higher than that of binary alloy in ST as well as PA conditions. The YS of the present alloy increased from 268 MPa in ST condition to 643 MPa on peak aging while the UTS increased from 484 to 785 MPa on aging at 450 °C for 72 h. These results show that the YS in Cu-3Ti-1Cd alloy has increased by 140%, while the corresponding increment in UTS was 62% on peak aging at 450 °C.
The Cu-3Ti-1Cd alloy exhibited higher ductility than Cu-2.7Ti alloy in the ST condition (46% elongation) and maintained similar relative property ratios on PA at 450 °C (24%). The fractographs in Fig. 3 showed micro-void coalescence indicating that ductile fracture occurred in this alloy in ST as well as PA conditions.
Electrical Conductivity
The effect of aging on electrical conductivity of Cu-3Ti-1Cd alloy at 400, 450 and 500 °C is shown in Fig. 4(a) to (c), respectively, in which the electrical conductivity curves of binary Cu-2.7Ti alloy reported by Nagarjuna et al. (Ref 9) are also included for comparison. The EC of the ternary alloy was much lower than that of binary Cu-2.7Ti alloy in ST as well as aged conditions. The ST alloy exhibited an electrical conductivity of 7.0%IACS, while that reported for Cu-2.7Ti alloy was 10%IACS. On aging at 450 °C for 16 h, alloy exhibited an EC of 13%IACS, while aging at 500 °C for 2 h resulted in a considerable increase in electrical conductivity, to 15%IACS. The addition of 1.0% Cd to Cu-3Ti alloy has, thus, resulted in decreased electrical conductivity in ST as well as aged conditions.
Electrical conductivity of Cu-3Ti-1Cd alloy compared with Cu-2.7Ti (Ref 9) aged at (a) 400 °C (b) 450 °C, and (c) 500 °C
Optical Microscopy
The optical micrographs of Cu-3Ti-1Cd alloy in ST, PA, and overaged conditions are shown in Fig. 5. Single phase with equiaxed grain structure was observed in ST (Fig. 5a) and PA (450 °C) conditions (Fig. 5b), while discontinuous precipitation was seen along grain boundaries in overaged (450 °C/112 h) condition (Fig. 5c). Annealing twins were observed in all three conditions studied. Discontinuous precipitation was also observed in the alloy overaged at 500 °C (Fig. 5d).
Transmission Electron Microscopy
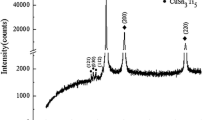
Figure 6 is the bright field (BF) image of ST Cu-3Ti-1Cd alloy, which reveals a single-phase structure with dislocations. TEMs (Transmission electron micrographs) of the present alloy peak aged at 450 °C is shown in Fig. 7. Tweed structure with copious amount of precipitation was observed in the BF and dark field (DF) images in Fig. 7(a) and (b). The SAD and its schematic shown respectively in Fig. 7(c) and (d) identify the fine precipitate to be β′-Cu4Ti.
When the alloy was overaged at 450 °C for 112 h, the matrix exhibited continuous precipitation, while discontinuous precipitation was observed along the grain boundaries as shown in Fig. 8. Figure 8(a) and (b) are the BF and DF images of a grain boundary region exhibiting the discontinuous precipitation. The SAD and its schematic in Fig. 8(c) and (d) confirm the precipitate to be the equilibrium β phase having an orthorhombic crystal structure with the lattice parameters a = 5.162 Å, b = 4.347 Å, and c = 4.53 Å (Ref 7). TEM of the alloy aged at 500 °C for 16 h (overaged) reveals discontinuous precipitation in Fig. 9. Figure 9(a) and (b) are respectively, the BF and DF images of discontinuous precipitation.
Discussion
Solution Treatment
The present investigation shows that the addition of 1% Cd to Cu-3Ti alloy has resulted in a substantial increase in YS and UTS in the ST condition (Table 1). The YS of the Cu-3Ti-1Cd alloy was higher by 40% as compared to the Cu-2.7Ti alloy in ST condition (Ref 9) while the corresponding increase in UTS was 12%. The alloy exhibited a single-phase structure in this condition in contrast to the tweed structure shown in Cu-4Ti-1Cd alloy (Ref 16). For this reason, the YS and UTS of the present alloy were nearly half of those of the Cu-4Ti-1Cd alloy (Ref 16).
The atomic misfit between Cu and Cd is 16.4%, which was the primary reason for large solid solution strengthening effect in the ternary alloy. The YS and UTS are therefore, higher in Cu-3Ti-1Cd alloy than in binary Cu-2.7Ti alloy (Table 1) in this condition. This explains the solid solution strengthening effect of Cd in Cu-3Ti alloy. The TEM (Fig. 6) showed single phase without any modulated structure in Cu-3Ti-1Cd alloy and our observation that modulated structure was absent in Cu-3Ti-1Cd alloy is in agreement with the reported phenomenon (Ref 3-6, 9). The alloy has shown good ductility (∼46% elongation) in ST condition, which is due to the single-phase structure. The electrical conductivity, however, decreased in the ST condition considerably due to the addition of Cd because of the strains developed due to the large misfit in the solid solution.
Peak Aging
The YS and UTS of the Cu-3Ti-1Cd alloy increased considerably on PA at 450 °C (∼140% in YS and 60% in UTS). Similar increases were reported for binary Cu-2.7Ti alloy (Ref 9). The increments in YS and UTS for Cu-4Ti-1Cd alloy were 42% and 18%, respectively (Ref 16). However, time for PA is considerably large; 16 h for binary Cu-2.7Ti and 72 h for Cu-3Ti-1Cd alloy. Like in binary Cu-Ti alloy (Ref 9, 18), increase in YS and UTS of the ternary alloy on PA is attributed to the precipitation of coherent and metastable β′-Cu4Ti phase. The fine scale precipitation of β′-Cu4Ti formed in the present alloy is similar to that in binary Cu-Ti (Ref 9, 18) as well as Cu-4Ti-1Cd alloys (Ref 16). A considerable fraction of the Cd was retained in solid solution on PA causing large solid solution strengthening as well.
The electrical conductivity of Cu-3Ti-1Cd alloy was comparable to that of binary Cu-2.7Ti alloy in aged condition. Addition of 1.0 wt.% Cd to pure Cu decreases the electrical conductivity to 90%IACS (Ref 15), whereas the present Cu-3Ti-1Cd alloy had shown 13%IACS on peak aging compared to 17%IACS for Cu-2.7Ti alloy (Ref 9, 18) while the Cu-4Ti-1Cd alloy exhibited 10%IACS (Ref 16). Therefore, the addition of Cd to Cu-3Ti alloy has improved the strength properties, but lowered the electrical conductivity. The electrical conductivity increases during aging, as the Ti comes out of the matrix (solid solution) to precipitate as metastable phase (β′) without losing coherency. Hence, the electrical conductivity of the ST alloy increases with aging time at any given aging temperature.
Over Aging
Discontinuous precipitation is seen at grain boundaries in the optical micrographs of Cu-3Ti-1Cd alloy in the overaged condition (Fig. 5c and d). Precipitation of β-Cu3Ti within the matrix and as discontinuous precipitation at the grain boundaries is noticed in the overaged alloy (Fig. 8 and 9) and the associated loss of coherency strains is responsible for overaging (Table 1 and Fig. 1). The discontinuous precipitation was reported in overaged binary Cu-2.7Ti (Ref 9) alloys as well as Cu-4Ti-1Cd alloy (Ref 16).
It was reported by Piotrowski and Gawronski (Ref 10) that V retards the continuous precipitation of metastable phase β′ in Cu-Ti alloys and increases the rate of kinetics of discontinuous precipitation but the addition of Al to Cu-2.1Ti alloy did not promote discontinuous precipitation (Ref 11). It was shown that the addition of Sn to Cu-1.5Ti alloy improved the tensile properties significantly and electrical conductivity moderately, on PA at 450 °C/2 h. However, further aging at 450 °C/8 h improved only electrical conductivity but reduced the strength (Ref 12). Addition of B to Cu-Ti alloys resulted in a good combination of strength and electrical conductivity by dispersion strengthening mechanism (Ref 13). In the present study, addition of 1% Cd to Cu-3Ti alloy prolonged peak aging, i.e., longer times are required for attaining peak hardness. Further, overaging characterized formation of discontinuous precipitation. Hence, our observations on the precipitation hardening of Cu-3Ti-1Cd alloy are similar to the behavior reported for various ternary-alloying additions to Cu-Ti alloys (Ref 10-13).
The preceding discussions lead to the conclusion that the ternary Cu-3Ti-1Cd alloy shows single-phase structure in ST condition like in binary Cu-2.7Ti alloy (Ref 9) but not similar to that in Cu-4Ti-1Cd alloy (Ref 16). However, formation of coherent and metastable β′-Cu4Ti precipitate in Cu-3Ti-1Cd alloy on peak aging is similar to binary Cu-Ti alloys and Cu-4Ti-1Cd alloy, which caused strengthening. Overaging of this alloy, again similar to both binary Cu-Ti alloys and Cu-4Ti-1Cd alloy, transpired with the formation of equilibrium precipitate, β Cu3Ti as discontinuous precipitation.
Conclusions
-
1.
Single-phase structure was observed in the ST condition of Cu-3Ti-1Cd alloy. The higher YS and UTS obtained in this condition as compared to binary Cu-2.7Ti alloy are attributed to substitutional solid solution strengthening of Cd.
-
2.
Peak aging is delayed and YS and UTS were increased by 140% and 60% in the ternary Cu-3Ti-1Cd alloy.
-
3.
The electrical conductivity of Cu-3Ti-1Cd alloy is lower than the binary Cu-2.7Ti alloy in both the ST as well as aged conditions.
-
4.
Strengthening in Cu-3.0Ti-1Cd alloy is due to the fine scale precipitation of coherent β′-Cu4Ti phase, which is similar to that reported in binary Cu-2.7Ti and Cu-4Ti-1Cd alloys.
-
5.
Discontinuous precipitation is observed in the ternary alloy overaged at 450 and 500 °C by the formation of equilibrium β-phase.
References
Meta ls Hand Book, Desk Edition, ASM, Ohio, USA, 1984, p 7.1
“Properties & Selection: Non-Ferrous Alloys and Special Purpose Materials,” Metals Hand Book, 10th ed., Vol. 2, ASM International, Ohio, USA, 1990, p 284
Cornnie J.A., Dutta A., Soffa W.A. 1973 An Electron Microscopy Study of Precipitation in Cu-Ti Sideband Alloys. Metall. Trans. A 4:727–733
Laughlin D.E., Cahn J.W. 1975 Spinodal Decomposition in Age Hardening Copper-Titanium Alloys. Acta Metall. 23:329–339
Datta A., Soffa W.A. 1976 The Structure and Properties of Age Hardened Cu-Ti Alloys. Acta Metall. 24:987–1001
Porter A., Thompson A.W. 1984 On the Mechanism of Precipitation Strengthening in Cu-Ti Alloys. Scripta Metall. 18:1185–1188
Karlson N. 1951 An X-ray Study of the Phases in the Copper-Titanium System. J. Inst. Metals 78:391–405
Michels H.T., Cadoff I.B., Levine E. 1972 Precipitation Hardening in Cu-3.6 wt% Ti. Metall. Trans. 3:667–674
Nagarjuna S., Balasubramanian K., Sarma D.S. 1999 Effect of Prior Cold Work on Mechanical Properties, Electrical Conductivity and Microstructure of Aged Cu-Ti Alloys. J. Mater. Sci. 34:2929–2942
Piotrowski W., Gawrouski Z. 1980 Influence of Vanadium on Structure and Kinetic Transformations in Cu-Ti Alloys. Metals Technol. 12:502–508
Vaidyanathan T.K., Mukharjee K. 1976 Precipitation in Cu-Ti and Cu-Ti-Al Alloys; Discontinuous and Localised Precipitation. Mater. Sci. Eng. 24:143–152
Saarivirta M.J. (1961) Development of Copper Base High Strength-Medium Conductivity Alloys Cu-Ti-Sn and Cu-Ti-Sn-Cr. Trans. Met. Soc. AIME 221:596–606
Bozic D., Mitkov M. 1992 Strengthening of Cu-Ti Alloys by Addition of Boron. Mater. Sci. Technol. 8:1108–1115
Nagarjuna S., Sarma D.S. 2002 Effect of Cobalt Additions on the Age Hardening of Cu-4.5Ti Alloy. J. Mater. Sci. 37:1929–1940
D.S. Clark and W.R. Varney, Physical Metallurgy for Engineers, 2nd ed., Litton Educational Publishing Inc, 1962, 392 p
Markandeya R., Nagarjuna S., Sarma D.S. 2004 Precipitation Hardening of Cu-4Ti-1Cd Alloy. J. Mater. Sci. 39:1579
“Properties and Selection: Non-Ferrous Alloys and Pure Metals”, Metals Hand Book, 10th ed., vol. 2, ASM International, Ohio, USA, 1990, p 288
Nagarjuna S., Balasubramanian K., Sarma D.S. 1997 Effect of Prior Cold Work on Mechanical Properties and Structure of an Age-Hardened Cu-1.5 wt%Ti Alloy. J. Mater. Sci. 32:3375–3385
Acknowledgments
The authors gratefully acknowledge Head, Department of Metallurgical Engineering, ITBHU, Varanasi, for providing laboratory facilities. The authors are thankful to Defence Research and Development Organization, New Delhi for financial support. The authors thank Director, Defence Metallurgical Research Laboratory, Hyderabad for permission to publish this work. One of the authors (RM) is thankful to Jawaharlal Nehru Technological University, Hyderabad for granting leave under QIP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markandeya, R., Nagarjuna, S. & Sarma, D. Precipitation Hardening of Cu-3Ti-1Cd Alloy. J. of Materi Eng and Perform 16, 640–646 (2007). https://doi.org/10.1007/s11665-007-9082-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-007-9082-7