Abstract
Nanocomposites of bismuth ferrite-tungsten trioxide (BiFeO3-WO3) were synthesized from sol–gel reaction followed by processing at two different calcination temperatures, 550°C and 650°C. The calcined composite products were investigated using powder XRD, FE-SEM, EDX, UV–Vis and FTIR to assess the influence of calcination temperature on their structural, morphological, and optical absorption properties and their photocatalytic characteristics. A photodegradation efficiency test was conducted for the synthesized BiFeO3-WO3 nanocomposite powders, with methylene blue (MB) used as the dye source. The optimal experimental conditions required for the effective and maximized degradation of the MB pollutant were also determined, and the data authenticity was validated through an artificial intelligence (AI)-based support vector machine (SVM) tool. SVM predictions agreed reasonably well with the experimental results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water contamination through industrial processes is a growing concern, as many organic dyes and medical wastes are discharged into water bodies. More specifically, dye processing and textile industries release several organic dye pollutants, including methylene blue, azo-dye, and phenols, into water resources.1,2,3 Among the industrial effluents, methylene blue dye is of specific interest as it has adverse effects on the reproductive and neural functions in both human beings and animals. It also poses a serious threat to human life and aquatic habitats. Hence, various pollution control boards have framed rules for the maximum allowable concentration of dye pollutants to be discharged into the water drainage upon treating industrial wastes.4,5 To reduce the level of these kinds of contaminants from water resources, commercial water purification techniques have been used, but they are still not up to the mark due to their high input costs, high energy consumption and some environmental effects created by the chemicals used in them.6,7 In addition, researchers are also facing great challenges in designing the photocatalysts with superior adsorption properties in degrading pharmaceutical effluents and dye molecules. To overcome the above disadvantages, various alternative approaches have been proposed, including advanced oxidation processes (AOP), Fe (VI) treatment technology, and adsorption and biological methods such as membrane processes, including microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO).8 Among these methods, the advanced oxidation process has gained significant interest due to the use of non-toxic, lower-cost and easily prepared photocatalytic materials. Materials commonly chosen for AOP should be capable of facilitating the generation of hydroxyl radicals required for oxidizing the dye molecules in wastewater.9,10
Among the photocatalytic materials, metal oxide-based semiconductors with a wide band gap such as TiO2 and ZnO have been used for dye degradation using UV light. Dye degradation of TiO2 material was first reported by Fujishima and Honda in 1972.11 The prime disadvantages of wide band gap photocatalysts (Eg = 3–3.4 eV) is that they cannot trap the visible portion of light and are responsive only to the UV regime.12 In such instances, designing a visible light active photocatalyst having excellent stability with less recombination rate has become one of the challenges in photochemistry. Recently, perovskite oxide-based semiconductors have shown good performance in photocatalysis due to their excellent nature of light capturability and narrow band gap.13 Bismuth ferrite (BiFeO3), in particular, is a material with optimum band gap (~ 2.0 eV) and good chemical stability.14,15,16 This material is quite an attractive candidate for photocatalytic activity. However, its photocatalytic degradation efficiency seems to be limited due to the quick recoupling of photogenerated charge carriers. Therefore, heterogeneous photocatalysts are preferable, as they have a high separation rate for photogenerated electron–hole charge carriers and exhibit magnificent photocatalytic efficiency.
It may not be possible for any single semiconductor-based photocatalyst to meet all essential criteria such as visible light activeness, high stability and a lower rate of recombination. As an alternate option, new types of photocatalysts deduced by the way of coupling two or more semiconductors have recently been introduced and they were synthesized with the intention of improving their photocatalytic properties.17,18 More specifically, tungsten oxide (WO3) is a unique metal oxide and it possesses characteristic electrical conductance and photocatalytic activity.19,20 It also possess a narrow band gap of 2.4 eV. In addition, its non-toxicity, good stability, low density and lower cost have attracted researchers for its potential use in gas sensing elements, solar cells, and photocatalytic applications.21 However, WO3 exhibits lower photocatalytic characteristics because there are fewer active sites on its agglomerated surface. For instance, Mioduska et al.22 reported on the synthesis of WO3/TiO2, wherein it was found that combining TiO2 helped to improve the crystallinity of WO3, reduced the optical band gap and most importantly, decreased the agglomeration in the nanocomposite form. Therefore, WO3 can be finely distributed with the low band gap semiconductor material. As such, combining with BiFeO3 with WO3 at different calcination temperatures of 550°C and 650°C is a viable option to extend the range of the visible light capture and to enhance the separation of the photogenerated electron–hole carriers for the betterment of methylene blue dye degradation. Based on the literature survey, the adoption of the support vector machine (SVM) method to estimate MB dye removal using BiFeO3-WO3 nanocomposite and optimization of process parameters at different temperatures has not been investigated elsewhere. Therefore, in the present work, we choose two different calcination temperatures, 550°C and 650°C, for the preparation of BiFeO3-WO3 nanocomposites. We also proposed to determine its impact on their photocatalytic activity.
Further, photocatalytic degradation investigations carried out earlier on BiFeO3-WO3 did not focus on the optimization of process parameters such as the pH of the reaction medium, the concentration of the pollutant and irradiation exposure time. These factors mainly determine the MB degradation efficiency. In our earlier work, BiFeO3-Bi2S3 photocatalysts were identified with promising characteristics for the degradation of endocrine-disrupting compounds.23 Therein, a complex non-linear relationship among the process parameters could be established and was also reflected in the outputs. Hence, it is strongly believed that the design of experiments and optimizing the process parameters would greatly reduce time and ambiguity. The modelling experiments use a novel artificial intelligence tool to help minimize the wastage of chemicals and also the experimental time. In order to predict the optimum process parameters for improving the degradation of dye molecules, an artificial intelligence-based support vector machine tool is preferred because the SVM tool consumes less time and is also cost-effective. It has also been extensively used in all fields of engineering and sciences.24,25 In fact, support vector machine and artificial neural network (ANN) tools have already been adopted for predicting the degradation efficiency of Acid Red 73 using TiO2 nanoparticles as the photocatalyst.26 But none of them was found to have utilized the AI principles to validate the experimental dye degradation data and, therefore, it will be generally more beneficial to use AI to obtain reliable and authentic results. With this main objective, synthesis of BiFeO3-WO3 at 550°C and 650°C has been proposed to remove the methylene blue dye pollutants and to evaluate the influence of calcination temperature on its structural, topographical and optical absorption properties. In addition, the interactive effects of the process parameters involved in the MB dye degradation were also analysed to determine the optimal conditions for achieving higher efficiency to degrade methylene blue dye using the SVM tool.
Experimental Procedure
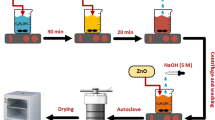
The starting materials used were of analytical grade (Merck, 99.9% Purity). Bismuth nitrate pentahydrate (Bi(NO3)3.5H2O), iron nitrate nonahydrate (Fe(NO3)3.9H2O), tungsten(VI) oxide (WO3), conc. nitric acid (HNO3), tartaric acid (C4H6O6) and ethanol (C2H5OH) were the precursor chemicals. The synthesis procedure of BiFeO3-WO3 nanocomposites, as appended in our reported work27 at 500°C, has now been repeated at different calcination temperature, 550°C and 650°C.
Material Characterization
Powder XRD patterns of the synthesized nanocomposites were recorded using a PANalytical X’Pert PRO x-ray diffractometer with CuKα radiation of wavelength λ = 1.54056 Å. An FE-SEM (Carl Zeiss) attached to an EDX analyser was utilized to determine the quantitative presence of elements and the topographical features of the synthesized composite samples. Optical absorption measurements were done in the wavelength range 200 to 800 nm using a UV-2450 SHIMADZU spectrophotometer. In order to study the vibrational structure of the composite sample, FT-IR spectra have been recorded at room temperature in the frequency range 400–4000 cm−1 using a Shimadzu IR Affinity-1 spectrophotometer.
Photodegradation Test
Optical absorption of the photocatalyst has been recorded using a LAB INDIA double beam UV–Vis spectrophotometer. Ten milligrams of synthesized composite (as a photocatalyst) and 10 mL of methylene blue dye (10 mg/L) were dispersed in the aqueous solution containing the photocatalyst. The solution was then stirred under dark conditions for 120 min to ensure equilibrium between adsorption and desorption. Upon attaining equilibrium, the MB composite solution mixture was kept under natural sunlight. At every 3-h interval, 4-mL aliquots were pipetted out of the glass tube and centrifuged to prepare the solution for degradation analysis.
Powder XRD Analysis on BiFeO3-WO3 Nanocomposites
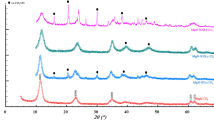
Diffraction patterns recorded in the 2θ range 20°–60° at a scan rate of 2°/min for the synthesized BFO-WO3 composites calcined at 550°C and 650°C are shown in Fig. 1. The diffracted peak positions for the obtained composite samples have been compared with the standard JCPDS files of BiFeO3 and WO3.27 All peaks with moderate intensities were correctly indexed to their corresponding (hkl) planes, confirming the presence of the rhombohedral phase of BiFeO3 as well as the monoclinic nature of WO3. The mixed crystalline phases as evidenced from XRD would authenticate the formation of BFO-WO3 nanocomposites. With increasing calcination temperature from 550°C to 650°C, the diffraction peaks sharpened, indicating improvement in the degree of crystallinity and structural coarsening. In addition, the particle sizes increased, which could be perceived from peak broadening, as expected.
In order to assess the crystalline strain and size, Williamson-Hall (W–H) analysis28 was done for the calcined samples of BiFeO3-WO3 samples. Using the computed trigonometric values of 4sinθ and βcosθ, linear fits were obtained for the composite powders calcined at 550°C and 650°C and are shown in Fig. 2. Crystallite size and induced strain were calculated for the BiFeO3-WO3 composites calcined at 550°C and 650°C as per the following equation.
where D is the crystallite size, K is the shape factor (0.94), λ is the wavelength of Cukα radiation, θ is the Bragg angle, βhkl is the full width at half maximum, and ε is the crystalline strain.
Table I indicates an increased crystallite size and decreased induced strain for the composite calcined at 650°C when compared to the composite calcined at 550°C. This may be due to the decrease in crystalline defects. Consequently, its crystallinity appears to have improved.
FE-SEM Analysis of BiFeO3-WO3 Nanocomposites
Particle growth and surface morphology are considered to be key factors affecting photocatalytic activity. Therefore, topographical features of BiFeO3-WO3 nanocomposites calcined at 550°C and 650°C were analysed through FE-SEM images, and they are shown in Fig. 3a and b. In the case of BiFeO3-WO3 nanocomposites calcined at 550°C, the sample surface was found to be distributed with BiFeO3 spherical-shaped particles and WO3 appeared to be plate-like particles, as indicated by arrows in FE-SEM images. From Fig. 3b, the 2D structure of the BiFeO3-WO3 calcined at 650°C can be found with increased grain growth and surface is also more agglomerated when compared to the sample calcined at 550°C. The increase in temperature makes the composite powders coarser and more agglomerated and in turn may decrease the surface area. This is likely to influence its physical properties. Cai et al.29 observed that the higher calcination temperature of 600°C for Mn-C-codoped TiO2 had strong effects on the photocatalytic properties. Hence, upon increasing the calcination temperature, the surface morphology of the BiFeO3-WO3 nanocomposites show a shrinking in surface area, growth of crystallite size and also enhanced agglomeration, resulting in a decrease in the photocatalytic activity. These changes in the morphological features of BiFeO3-WO3 would drastically modify their photocatalytic behaviour. Energy-dispersive x-ray (EDX) patterns, as shown in Fig. 3c and d, depict the percentage composition of Bi, W, Fe, and O present in the synthesized composites calcined at 550°C and 650°C. The chemical compositions of the individual elements in the as-synthesized nanocomposites did not show much variation with the increase in calcination temperature. However, the measurement clearly substantiates that both bismuth ferrite and tungsten oxide molecules are non-uniformly distributed throughout the composite system.
UV–Vis-NIR Studies of BFO-WO3 Nanocomposites
Photoabsorption properties of BiFeO3-WO3 composite samples calcined at 550°C and 650°C were analysed using the UV–Vis-NIR spectra and are shown in Fig. 4a and b. By observing the graphs, it is noticed that compared with the BiFeO3-WO3 composite sample calcined at 550°C, the composite sample calcined at 650°C shows a significant change in the optical absorption, and λmax appears to be shifting towards a longer wavelength side, indicating a redshift due to the increased crystallite size upon the increase in calcination temperature. The band gap energies (Eg) have been computed using the Kubelka–Munk (KM) function30 and Tauc plots have been drawn and are given in Fig. 4b. The obtained Eg value of the BiFeO3-WO3 nanocomposite calcined at 650°C (1.98 eV) was found to be relatively smaller than that of BiFeO3-WO3 calcined 550°C (2.0 eV). The decrease in Eg from 2.0 eV to 1.98 eV increased the sample's optical absorption in the visible region. Therefore, it can be concluded that temperature variation likely aids in tuning the band gap energy and in turn may influence the photocatalytic activity.29 In addition, the band gap value of bare BiFeO3 was reported in an earlier work as ~ 1.8 eV,27 and the bare WO3, which was purchased commercially, has a band gap value of 2.4 eV.
Vibrational Analysis of BiFeO3-WO3 Nanocomposites
FT-IR spectra recorded in the frequency range 400-1000 cm−1 on BiFeO3-WO3 composites calcined at 550°C and 650°C are represented in Fig. 5. A broad IR absorption noticed in the region of 400–700 cm−1 for both calcined composite samples corresponds to the interaction binding between Bi-O, Fe–O, and W–O. This specific absorption perhaps indicates the formation bismuth ferrite with tungsten oxide as a nanocomposite.31,32 Further, this broad IR band is also slightly shifted towards the low-frequency side for the nanocomposite samples calcined at 650°C.
Based on the above observation, it is clear that the calcination temperature has once again played a key role in the optical absorption properties even in the IR regime. In the frequency interval 700–4000 cm−1 (shown as an inset in Fig. 5), no additional functional groups are seen other than BiFeO3-WO3, so it can be concluded that the synthesized composites contained only the expected crystalline phases without any additional impurities.
Photocatalytic Dye Degradation Activity of BiFeO3-WO3 Composites
In order to study the photocatalytic behaviour, synthesized composites have been utilized as photocatalysts, and methylene blue (MB) was used as an industrial effluent. Degradation experiments were performed under natural sunlight illumination on the synthesized BiFeO3-WO3 composite in the presence of known concentrations of MB dye solution. A few milliliters of degraded dye solution was taken every 3 h and the absorption spectra were also recorded at regular intervals of time. The obtained spectra are shown in Fig. 6a and b. The rate of dye degradation with respect to exposure time and degradation efficiencies for various illumination periods have been computed and are presented in Fig. 6c and d.
While observing the photodegradation activity of MB dye/BiFeO3-WO3 photocatalyst solution, the maximum absorbance peak appearing initially at 652 nm was found to gradually decrease with increasing time of irradiance. Dye degradation efficiency was thus estimated from the decrease in the absorbance rate through UV–visible spectra, and efficiencies are given in Table II.
From the above table, it is inferred that an increase in calcination temperature likely causes a decrease in the photodegradation efficiency, and this implies the influence of surface morphological features and crystallite sizes on the photocatalytic behaviour of the composite used for study. As a consequence, visible light seems to be much more active for a good surface area with smaller particles. They possess good contact with dye molecules and also produce low chances of recombination of charge carriers. Low MB dye degradation for the BiFeO3-WO3 sample calcined at 650°C was observed due to its increased particle sizes and because of its more agglomerated characteristics. Accordingly, the surface contact of the photocatalyst with dye molecules decreases and leads to the incomplete decomposition of methylene blue. The methylene blue degradation efficiencies have been calculated for the synthesized composite samples calcined at 550°C and 650°C using an efficiency formula adopted elsewhere33 and confirm that the sample calcined at 550°C exhibited better photocatalytic properties over that calcined at 650°C.
The possible mechanism for the photocatalytic degradation of methylene blue dye using the nanocomposite of BiFeO3-WO3 calcined at 550°C is shown in Fig. 7. To hypothesize the process of photocatalytic degradation of methylene blue dye using BiFeO3-WO3 photocatalysts, the locations of the valence band (VB) and conduction band (CB) edges of BiFeO3 and WO3 semiconductors must be determined using the Mullikan electronegativity approach34,35 by following steps 1 and 2 as follows.
Step 1: Compute the conduction band positions (ECB) of BiFeO3 and WO3 semiconductors using the relation
where X is the electronegativity value of the BiFeO3 and WO3 semiconductors; Ee is the energy of a free electron on a hydrogen scale (4.5 eV); and Eg is the band gap energy value of the BiFeO3 (~ 1.8 eV) and WO3 (~ 2.4 eV) semiconductors.
Step 2: Using ECB and Eg values, compute the value of valence band positions (EVB; with respect to the standard hydrogen electrode) of BiFeO3 and WO3 using the formula
Based on this, the conduction band potentials of BiFeO3 and WO3 were computed as 0.4 eV and 0.8 eV, and their valence band potentials were found to be 2.2 eV and 3.2 eV, respectively. These band gap edges are incredibly beneficial for developing a unique heterojunction system between the p-type BiFeO3 and n-type WO3 during interface. Prior to contact, the Fermi energy level of p-type BiFeO3 and n-type WO3 are close to their valence band. After making contact, the Fermi energy levels move towards each other, and the movement of photogenerated charge carriers occurs at the p-type BiFeO3 and n-type WO3 hetero-interface. This resulted in the formation of an internal electric field, which might provide a potent driving force for easing the process of electron transfer and segregation.36,37,38 Upon illumination with natural sunlight, the photogenerated charge carriers, such as electron (e−) and holes (h+) pairs, are produced inside the BiFeO3-WO3 nanocomposite. Initially, the photogenerated electrons in the valence band of BiFeO3 are excited to its conduction band and then transferred to the conduction band of WO3 because of the CB offset. At the same time, the holes on the valence band of the WO3 moved to the valence band of BiFeO3 due to the VB offset. Then these charge carriers migrate onto the photocatalyst surface to take part in subsequent photocatalytic reactions. The electrons in the CB of WO3 generate superoxide radicals (·O2−) by reacting with molecular oxygen in the reaction chamber. Then ·O2− reacts with water molecules to create hydroxyl (·OH) radicals. Similarly, photoinduced holes in the VB of BiFeO3 oxidize water molecules to produce ·OH radicals. These hydroxyl and superoxide radicals react with the methylene blue dye adsorbed on the photocatalyst surface to degrade into carbon dioxide and water molecules upon sunlight illumination. In addition, higher MB degradation efficiency of BiFeO3-WO3 calcined at 550°C was also strongly dependent on parameters such as calcination temperature, surface morphological features and crystallite sizes. They also play a vital role in generating ·O2− and ·OH species, which supports enhancement of the photocatalytic characteristics of the BiFeO3-WO3 nanocomposites. In summary, the higher MB degradation behaviour of the BiFeO3-WO3 calcined at 550°C was observed with the combined influence of wider surface area, narrower band gap, effective charge transfer kinetics, and formation of built-in electric field at the BiFeO3 and WO3 interface.
SVM Computation of Dye Degradation Rate and Efficiencies
The support vector machine is a traditional machine learning approach that can be utilized for classification and regression applications. SVM augments fundamental machine learning algorithms with features that make them more effective in a variety of applications.39,40 The support vector machine tool was first introduced by Oyehan et al.41 and it is a robust machine learning technique that can be used to model and validate the complex non-linear relationship between process variables and their outputs.42 The SVM tool has been applied here to validate the experimental data used for the removal of MB dye in aqueous solution using four operational variables, which are termed as the estimators. The input parameters such as pH, dye concentration, photocatalyst dose and irradiation exposure time were provided to run the SVM algorithm and are tabulated in Table III.
The inputs of MB degradation models are randomly divided into two subsets used: 85% for training and 15% for testing. Dye degradation efficiency [DDE (%)] and rate constant performance (R2) for the synthesized samples were predicted and compared with the experimental results. The predicted result is useful to analyse the influence of MB dye degradation process parameters and to find the optimal conditions for obtaining the higher rate of methylene blue degradation. In order to evaluate through SVM, two formulae are used for the measuring the rate constant performance (R2) and DDE (%) are shown as the Eqs. 8 and 9.
Figure 8a shows the SVM-generated values of MB dye degradation efficiencies for the photocatalyst samples calcined at 550°C and 650°C. According to the SVM prediction, the optimal condition for maximum methylene blue degradation efficiency of 67% was obtained from BiFeO3-WO3 composite synthesized at the calcination temperature of 550°C with an irradiation time of 9 h, whereas for the same exposure time, the degradation efficiency of BiFeO3-WO3 composite synthesized at 650°C was predicted as 49.5%. This shows the excellent agreement between predicted and experimental degradation values, confirming that SVM can be used as a tool for validating the MB dye degradation experimental results. The results of R2 obtained experimental data fit have also been compared with SVM prediction and are tabulated in Table IV.
From Fig. 8b, it has been found that the R2 values obtained through SVM for the BiFeO3-WO3 composite calcined at 550°C are reasonably appropriate with the experimental fit, indicating that the present tool is a good kinetic model. The SVM tool can also produce excellent fits with the experimental results in a shorter time, and obtained R2 values are nearly unity. Hence, data validation from SVM converges with the experimental observations that BiFeO3-WO3 composites calcined at 550°C are capable of producing good degradation efficiency for MB dye with a higher rate constant when compared to that of BiFeO3-WO3 calcined at 650°C. The relative significance of input variables on the methylene blue dye removal efficiency is shown as a percentile score in Table V.
It has been identified that all input variables played a role in MB dye removal. However, the greatest influence was shown by dye concentration, which primarily determined the effluent removal rate. The analysis of the SVM model has shown that the methylene blue dye can be degraded using BiFeO3-WO3 nanocomposites calcined at 550°C and 650°C. Their outputs clearly demonstrated that under enriched optimum conditions such as an initial dye concentration of 10 mg/L, constant pH of 5, photocatalyst dosage (1 g/L), and contact time (9 h), BiFeO3-WO3 calcined at 550°C is capable of degrading MB dye effectively. It has been found that these results are qualitatively similar to the experimental result obtained through a laboratory photocatalytic setup. If more analysis is performed through the SVM model, there exists an ample chance of improving this model in degradation experiments.
Conclusion
In summary, BiFeO3-WO3 nanocomposites have been successfully synthesized through sol–gel process at two different calcination temperatures, 550°C and 650°C. The synthesized nanocomposite powders of BiFeO3-WO3 were used for XRD structural analysis, revealing that the diffraction peaks of the composite indicate both rhombohedral BiFeO3 and monoclinic WO3 material. It has also been found that a sample calcined at 650°C appears to exhibit higher crystallinity through the observation of high-intensity x-ray diffraction peaks and higher grain growth was observed from FE-SEM images when compared to composite powders prepared at 550°C. SEM images also suggest that the synthesized composites have different morphologies, i.e. spherical-shaped nanoparticles, indicating that the BFO particles belong to WO3. The surface morphology of BiFeO3-WO3 calcined 550°C was found to have a unique surface area with less agglomeration and porosity when compared with BiFeO3-WO3 prepared at 650°C. Upon increasing the calcination temperature from 550°C to 650°C, a reduction in the band gap energy was noticed for the title composites. FT-IR measurement confirmed the vibrational structure of composite and evidenced IR absorptions due to Bi-O, Fe–O, and W–O at 400–700 cm−1 which substantiates the formation of BiFeO3-WO3 nanocomposite. Photocatalytic dye degradation measurements revealed that the BiFeO3-WO3 nanocomposites calcined at 550°C exhibited better degradation ability for degrading methylene blue dye than that of the sample calcined at 650°C. These results confirmed that MB degradation efficiency depends not only on the optical absorption and band gap energy but is also influenced by the calcination temperature, surface morphology and crystallite size. Since the surface morphology and crystallite sizes of photocatalysts played a crucial role in generating ·O2− and ·OH species, effective removal of MB dye for the BiFeO3-WO3 sample calcined at 550°C is more pronounced. The intelligent SVM model has been utilized to validate the experimental degradation data of methylene blue and its predictions were found to be in good agreement with experimental data. The convergence of experimental and theoretical data on photocatalytic MB dye degradation activity of BiFeO3-WO3 indicated the reliability of the obtained results.
References
S.K. Marttinen, R.H. Kettunen, and J.A. Rintala, Occurrence and removal of organic pollutants in sewages and landfill leachates. Sci. Total Environ. 301, 1–12 (2003).
K. Pazdzior, L. Bilinska, and S. Ledakowicz, A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 376, 120597 (2019).
Q. Ke, Y. Zhang, X. Wu, X. Su, Y. Wang, H. Lin, R. Mei, M.Z. Yu Zhang, C. Hashmi, and J.C. Chen, Sustainable biodegradation of phenol by immobilized Bacillus sp SAS19 with porous carbonaceous gels as carriers. J. Environ. Manage 222, 185 (2018).
O.S. Abu-Rizaiza, Modification of the standards of wastewater reuse in Saudi Arabia. Water Res. 33, 2601 (1999).
J.A. Faby, F. Brissaud, and J. Bontoux, Wastewater reuse in France: water quality standards and wastewater treatment technologies. Water Sci Technol. 40, 37 (1999).
G. Crini, and E. Lichtfouse, Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 17, 145 (2019).
F.C. Moreira, R.A.R. Boaventura, E. Brillas, and V.J.P. Vilar, Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 202, 217 (2017).
E. Manoli, and C. Samara, The removal of polycyclic aromatic hydrocarbons in the wastewater treatment process: experimental calculations and model predictions. Environ. Pollut. 151, 477 (2008).
M. Mehrjouei, S. Müller, and D. Möller, A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 263, 209 (2015).
L.G.C. Villegas, N. Mashhadi, M. Chen, D. Mukherjee, K.E. Taylor, and N. Biswas, A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2, 157 (2016).
A. Fujishima, and K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
N. Daneshvar, M.H. Rasoulifard, A.R. Khataee, and F. Hosseinzadeh, Removal of C.I. acid orange 7 from aqueous solution by UV irradiation in the presence of ZnO nanopowder. J. Hazard. Mater. 143, 95–101 (2007).
A. Dandia, P. Saini, R. Sharma, and V. Parewa, Visible light driven perovskite-based photocatalysts: a new candidate for green organic synthesis by photochemical protocol. Curr. Opin. Green Sustain. Chem. 3, 100031 (2020).
S. Irfan, Z. Zhuanghao, F. Li, Y.X. Chen, G.X. Liang, J.T. Luo, and F. Ping, Critical review: Bismuth ferrite as an emerging visible light active nanostructured photocatalyst. J. Mater Res. Technol. 8, 6375 (2019).
Y. Huo, M. Miao, Y. Zhang, J. Zhu, and H. Li, Aerosol-spraying preparation of a mesoporous hollow spherical BiFeO3 visible photocatalyst with enhanced activity and durability. Chem. Commun. 47, 2089 (2011).
J. Jiang, J. Zou, M.N. Anjum, J. Yan, L. Huang, Y. Zhang, and J. Chen, Synthesis and characterization of wafer-like BiFeO3 with efficient catalytic activity. Solid State Sci. 13, 1779 (2011).
Y. Subramanian, B. Mishra, S. Mandal, R. Gubendiran, and Y.S. Chaudhary, Design of heterostructured perovskites for enhanced photocatalytic activity: insight into their charge carrier dynamics. Mater. Today: Proc. 35, 179 (2021).
Y. Ma, P. Lv, F. Duan, J. Sheng, S. Lu, H. Zhu, M. Du, and M. Chen, Direct Z-scheme Bi2S3/BiFeO3 heterojunction nanofibers with enhanced photocatalytic activity. J. Alloys Comp. 834, 155158 (2020).
D.P. DePuccio, P. Botella, B. O’Rourke, and C.C. Landry, Degradation of methylene blue using porous WO3, SiO2–WO3, and their Au-loaded analogs: adsorption and photocatalytic studies. ACS Appl. Mater. Interf. 7, 1987 (2015).
X. Liu, H. Zhai, P. Wang, Q. Zhang, Z. Wang, Y. Liu, Y. Dai, B. Huang, X. Qin, and X. Zhang, Synthesis of a WO3 photocatalyst with high photocatalytic activity and stability using synergetic internal Fe3+ doping and superficial Pt loading for ethylene degradation under visible-light irradiation. Catal. Sci. Technol. 9, 652–658 (2019).
M.B. Tahir, G. Nabi, N.R. Khalid, and N.R. Khalid, Synthesis of nanostructured based WO3 materials for photocatalytic applications. J. Inorg. Organomet. Polym. 28, 777 (2018).
J. Mioduska, A. Zielińska-Jurek, M. Janczarek, J. Hupka, The effect of calcination temperature on structure and photocatalytic properties of WO3/TiO2 nanocomposites. J. Nanomaterials. 3145912 (2016).
Y. Subramanian, B. Mishra, P.M. Rajashree, N. Kumar et al., Efficient degradation of endocrine-disrupting compounds by heterostructured perovskite photocatalysts and its correlation with their ferroelectricity. New J. Chem. 46, 11851 (2022).
N.M. Mahmoodi, J. Abdi, M. Taghizadeh, A. Taghizadeh, B. Hayati, A.A. Shekarchi, and M. Vossoughi, Activated carbon/metal-organic framework nanocomposite: preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J. Environ. Manag. 233, 660 (2019).
M. Ateia, M.G. Alalm, D. Awfa, M.S. Johnson, and C. Yoshimura, Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: a critical review. Sci. Total Environ. 698, 134197 (2019).
M.A.S. VaezZarringhalam MoghaddamAlijani, Optimization and modeling of photocatalytic degradation of Azo dye using a response surface methodology (RSM) Based on the central composite design with immobilized titania nanoparticles. Indus. Eng. Chem. Res. 51, 4199 (2012).
Y. Subramanian, V. Ramasamy, R.K. Gubendiran, G.R. Srinivasan, and D. Arulmozhi, Structural, optical, thermal and photocatalytic dye degradation properties of BiFeO3-WO3 Nanocomposites. J. Electron. Mater. 47, 7212 (2018).
H. Heryanto, B. Abdullah, and D. Tahir, Analysis of structural properties of X-ray diffraction for composite copper-activated carbon by modified Williamson-Hall and size-strain plotting methods. J. Phys: Confer. Series. 1080, 012007 (2018).
J. Cai, W. Xin, G. Liu, D. Lin, and D. Zhu, Effect of calcination temperature on structural properties and photocatalytic activity of Mn-C-codoped TiO2. Mater. Res. 19, 401 (2016).
R. Ghosh, K.S. Asha, SMd. Pratik, A. Datta, R. Nath, and S. Mandal, Synthesis, structure, photocatalytic and magnetic properties of an oxo-bridged copper dimer. RSC Adv. 4, 21195 (2014).
U. OparaKrasˇovec, A.S. ˇurcaVuk, and B. Orel, IR Spectroscopic studies of charged–discharged crystalline WO3 films. Electrochim. Acta 13, 1921 (2001).
T. Gao, Z. Chen, Y. Zhu, F. Niu, Q. Huang, L. Qin, X. Sun, and Y. Huang, Synthesis of BiFeO3 nanoparticles for the visible-light induced photocatalytic property. Mater. Res. Bull. 59, 6 (2014).
S. Alkaykh, A. Mbarek, and E.E. Ali-Shattle, Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon. 6, e03663 (2020).
Y. Subramanian, V. Ramasamy, R. Karthikeyan, G. Srinivasan, D. Arulmozhi, R. Gubendiran, and M. Sriramalu, Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3-GdFeO3 nanocomposite photocatalyst. Heliyon 5, e01831 (2019).
R. Cheng, L. Shen, J. Yu, S. Xiang, and X. Zheng, Photocatalytic inactivation of bacteriophage f2 with Ag3PO4/g-C3N4 composite under visible light irradiation: performance and mechanism. Catalysts 8, 406 (2018).
H. Yang, A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 142, 111406 (2021).
T. Cheng, H. Gao, G. Liu, Z. Pu, S. Wang, Z. Yi, X. Wu, and H. Yang, Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloids Surf., A 633, 127918 (2022).
Y. Gu, B. Guo, Z. Yi, X. Wu, J. Zhang, and H. Yang, Morphology modulation of hollow-shell ZnSn(OH)6 for enhanced photodegradation of methylene blue. Colloids Surf., A 653, 129908 (2022).
M.S. Mazloom, F. Rezaei, A. Hemmati-Sarapardeh, M.M. Husein, S. Zendehboudi, and A. Bemani, Artificial intelligence based methods for asphaltenes adsorption by nanocomposites: application of group method of data handling, least squares support vector machine, and artificial neural networks. Nanomaterials 10, 890 (2020).
Y. Subramanian, R. Veena, S. Muhammed Ali, A. Kumar, R. Gubediran, A. Dhanasekaran, D. Gurusamy, and K. Muniandi, Artificial intelligence technique based performance estimation of solid oxide fuel cells. Mater. Today: Proc. (2021). https://doi.org/10.1016/j.matpr.2021.06.412.
T.A. Oyehan, M.A. Liadi, and I.O. Alade, Modeling the efficiency of TiO2 photocatalytic degradation of MTBE in contaminated water: a support vector regression approach. SN Appl. Sci. 1, 386 (2019).
T. Zhang, C. Wu, Z. Xing, J. Zhang, S. Wang, X. Feng, J. Zhu, X. Lu, and L. Mu, machine learning prediction of photocatalytic lignin cleavage of C-C bonds based on density functional theory. Mater. Today Sustain. 20, 100256 (2022).
Acknowledgments
One of the authors, Dr. G.Ramesh Kumar, wishes to acknowledge all his collaborators for their kind research and moral support to bring this work to the level of publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subramanian, Y., Gajendiran, J., Veena, R. et al. Structural, Photoabsorption and Photocatalytic Characteristics of BiFeO3-WO3 Nanocomposites: An Attempt to Validate the Experimental Data Through SVM-Based Artificial Intelligence (AI). J. Electron. Mater. 52, 2421–2431 (2023). https://doi.org/10.1007/s11664-022-10188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-10188-7