Abstract
Pb(Zr0.49Ti0.51)0.7(Zn1/3Nb2/3)0.3O3 (PZN–PZT) piezoceramics with Sr2+ ion substitution were sintered as low as 900°C by the solid-state reaction route. The specimens with various Sr2+ ion concentrations were compared according to their microstructure, phase structure, piezoelectricity, ferroelectricity, and dielectric relaxation. The substitution of the smaller Sr2+ ions for the A-site Pb2+ ions in the PZN–PZT system improves the electrical properties. The samples with 4 mol% Sr2+ substitutions have optimized piezoelectric properties (piezoelectric constant d33 = 496 pC/N, Curie temperature Tc = 244°C, electromechanical coupling coefficient kp = 0.58). Rietveld refinement from the XRD data reveals that the tetragonal lattice constants along with the a and c axes (a, c) for the PZN–PZT decrease with an increased Sr2+ substitution content. The c decreases faster than the a, which leads to decreased tetragonality (c/a), thus leading to shrinkage of the oxygen octahedrons in the ABO3 perovskite lattice. This lattice distortion facilitates mass transport, thereby increasing the bulk density and grain size of the perovskite PZN–PZT system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pb(Zr,Ti)O3 (PZT)-based perovskite piezoceramics have been widely utilized as piezoelectric transformers, sensors, actuators, and other applications in light of their excellent electrical properties and low cost.1,2 However, the conventional sintering temperature for PZT-based piezoelectric piezoceramics is approximately 1200°C. At this temperature, a large amount of PbO volatilizes, which not only causes environmental pollution but also causes a non-stoichiometric ratio. In addition, multilayer piezoelectric ceramics need to co-fire the internal metal layers with the ceramic layers to improve the integration of the piezoelectric components.3 At about 1200°C sintering temperature, the internal electrodes have to use expensive platinum or palladium. If the sintering temperature is lower than 900°C, the internal electrodes can use pure silver, thus greatly reducing the production cost.4,5 To meet the requirements of commercialization and miniaturization for piezoelectric devices, the piezoceramic layers in a multilayer sheet need to be co-fired with the silver inner electrode layers below 900°C. The Curie temperature (Tc) for the piezoceramics needs to be as high as possible (> 230°C) to use the lead-free Zn-Sn-based solder to weld the conducting wire and components.
As a typical ferroelectric relaxor, Pb(Zn1/3Nb2/3)O3 (PZN) piezoceramics with good electrical properties and a Tc of 140°C have been broadly utilized as electromechanical devices.5,6,7,8 The PZT has a high Tc (232−492°C), but relatively low piezoelectric performance. Excellent electrical performance and stable perovskite structure can be obtained by forming solid solutions with a certain proportion of the PZN and the PZT.9,10,11,12
The modification of PZN–PZT piezoceramics has attracted much attention.13,14 Deng et al.15 synthesized La-substituted 0.3PZN–0.7PZT(51/49) piezoceramics by a hot-pressing method and obtained outstanding piezoelectric properties (kp = 0.70 and d33 = 845 pC/N). Vittayakorn et al.16 prepared 0.3PZN–0.7PZT(50/50) piezoceramics using the columbite method at a sintering temperature of 1225°C and achieved outstanding electrical properties (kp = 0.70, d33 = 690 pC/N, and Tc = 299°C). Chandarak et al.17 concluded that the MPB composition for PZN–PZT(52/48) system is clearly at 0.3PZN–0.7PZT.
Low-temperature sintering of PZN–PZT piezoceramics has also been reported in recent years. Fan et al.18 synthesized 0.3PZN–0.7PZT(49/51)+Li2CO3+Sm2O3 piezoceramics at a sintering temperature of 900°C and achieved optimal electrical properties (d33 = 483 pC/N, Tc = 394°C, and kp = 0.65). Ngamjarurojana et al.19 reported that 900°C sintering 0.16Pb(Zn1/3Nb2/3)O3–0.04Pb(Ni1/3Nb2/3)O3–0.8Pb(Zr0.48Ti0.52)O3 piezoceramics have outstanding electrical properties (kp = 0.56, d33 = 350 pC/N, and Qm = 1042). Hou et al.20 prepared (Pb0.95Sr0.05)(Zr0.50Ti0.50)0.80(Zn1/3Nb2/3)0.20O3 +MnO2 piezoceramics at a sintering temperature of 1000°C and obtained good piezoelectric properties (Qm = 1360, kp = 0.62, tanδ = 0.002, εr = 1240, Tc = 320°C, and d33 = 325 pC/N). Lee et al.21 prepared 0.5 wt% MnO2 doped 80% PZT(50/50)–20%PZN piezoceramics at a sintering temperature of 930°C and obtained outstanding electrical properties (kp = 0.62, d33= 330 pC/N, and Qm = 1000).
In this work, we prepared Pb1−xSrx(Zr0.49Ti0.51)0.7(Zn1/3Nb2/3)0.3O3 (PZN–PZT) piezoceramics at a low sintering temperature (900°C) by the traditional solid-reaction method. The substitution of various content Sr2+ ions for the A-site Pb2+ ions enhances the piezoelectric properties.
Experimental Process
The PZN–PZT piezoceramics were fabricated via the solid-state reaction route. The starting powders were reagent grade ZrO2 (99%), PbO (99%), TiO2 (98%), SrCO3 (99%), ZnO (99%), Nb2O5 (99.5%), and Li2CO3 (99%). According to the stoichiometric ratios of the formula Pb1−xSrx(Zr0.49Ti0.51)0.7(Zn1/3Nb2/3)0.3O3 (x = 0, 2, 4, 6, and 8 mol%), the powders were weighed and 0.30 wt% Li2CO3 sintering aids were added. The Li2CO3 with a low melting point (780°C) can form a liquid phase during the sintering process and reduce the sintering temperature.22 The powder was put into a pot with ethanol for ball-milling for 12 h. The dried powder was calcined at 850°C for 3 h and ball-milled for 20 h. The powder was added with an 8% polyvinyl-acetate binder after drying, and pressed into discs with a diameter of 11 mm under 10 MPa. To remove the PVA binder, the disks were burned off at 600°C for 3 h. Then, the disks were sintered as low as 900°C for 3 h to obtain good properties. The sintered piezoceramics were coated with Ag pastes and fired at 720°C for 15 min. The ceramics were poled under a DC voltage of 30 kV/cm in high-temperature silicone oil at 120°C. All the samples were aged for 48 h before the electrical properties were measured.
The phase structure was determined using x-ray diffraction (XRD) (X’Pert Pro MPD instrument; PANalytical, Netherlands). The microstructure was observed using FE–SEM (JSM–7500F; JEOL, Japan). The dielectric properties were analyzed by impedance meters (HP 4294A and HP 4980A; Agilent, USA) according to IEEE standards. A ferroelectric test system (TF Analyzer 2000 E; aixACCT, Germany) was used to measure the polarization versus electric field (P–E) loops. The piezoelectric constant (d33) was measured via a quasi-static piezoelectric constant (d33) meter (ZJ-3A; Institute of Acoustics, Chinese Academy of Sciences, China).
Results and Discussion
X-ray Diffraction Analysis
Figure 1 displays the bulk density for the PZN–PZT piezoceramics with different SrCO3 contents. As the SrCO3 content increases, the bulk density increases from 7.85 g/cm3 with x = 0 to 7.94 g/cm3 with x = 4, and then decreases to 7.84 g/cm3 with x = 8. Figure 1 indicates that the Sr2+ ion substitution can effectively improve the bulk density of the PZN–PZT piezoceramics. The sample with 4 mol% Sr2+ ion content has a bulk density up to a maximum of 7.94 g/cm3. As the Sr2+ ion content further increases, the bulk density decreases. The substitution of Sr2+ ions (1.44 Å radius) for Pb2+ ions (1.49 Å radius) can improve the bulk density of the PZN–PZT piezoceramics. This is because the substitution leads to shrinkage of the oxygen octahedron and facilitates mass transport.23,24,25,26 In addition, the excessive substitution of Sr2+ ions for Pb2+ ions leads to the decreased bulk density of the PZN–PZT piezoceramics, because the decreased Pb2+ ion content leads to weakening of the liquid phase sintering.
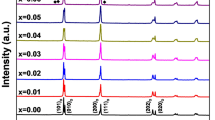
Figure 2 shows the room temperature XRD patterns in the 2θ range from 20° to 70° for the piezoceramics sintered at 900°C with different SrCO3 contents. As shown in Fig. 2, all the piezoceramics show a perovskite structure. Meantime, the typical splitting of the diffraction peaks (002) and (200) indicates that the tetragonal phase exists in the piezoceramics. The splitting of the (002) and (200) diffraction peaks gradually shifts to a higher angle as the Sr2+ ion content increases, which means that the lattice constants decrease.27 The Pb2+-ion radius is larger than the Sr2+-ion radius, and Sr2+ ions enter the lattice in the perovskite structure to replace the Pb2+ ions at the A-site, thus causing the lattice distortion.
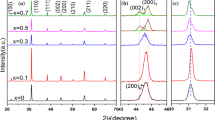
Rietveld refinement of the XRD data for the PZN–PZT piezoceramics was performed at room temperature using the Maud program to study the influence of SrCO3 amount on the crystal structure in the piezoceramics. Figure 3 illustrates the final refined profiles for the PZN–PZT piezoceramics with various SrCO3 contents. The Rietveld fitting requires Sig less than 2 and Rw less than 8%. As shown in Fig. 3, the values of Sig (1.92–1.98) and Rw (6.74–7.12%) demonstrate that the refined profile fits well with the experimental data.
According to the results of the Rietveld refinement, the tetragonal phase content (CTP) increases with the SrCO3 content, as shown in Fig. 4a. The CTP increases from 70.4% with x = 0 to 81.6% with x = 8. The lattice parameters (a, c) for the PZN–PZT piezoceramics were calculated from the Rietveld refinement. Figure 4b displays the change of the lattice parameters with the SrCO3 substitution content. It can be observed that both c and a decrease as the SrCO3 content increases, and c decreases faster than a, which leads to a decreased tetragonality (c/a). A partially enlarged view of the (002) and (200) diffraction peaks in Fig. 2 shows that the spacing of the two diffraction peaks decreases with the increased Sr2+ content. This result also follows the conclusion in Fig. 4b, that the tetragonality (c/a) decreases as the Sr2+ content increases. Figure 4c illustrates the influence of Sr2+ substitution on the crystal structure. The substitution of smaller size Sr2+ ions for Pb2+ ions results in shrinkage of the oxygen-octahedral and volume reduction of the unit cell with shortened a- and c-axes in the crystal structure.23,28
Morphology Analysis
Figure 5a–e show SEM images for the PZN–PZT piezoceramics with different SrCO3 contents. As can be seen from the images, all samples show a uniform grain shape. Figure 5f shows the average grain size for the PZN–PZT piezoceramics with various SrCO3 contents. The substitution of Pb2+ ions with smaller Sr2+ ions makes Sr2+ more easily enter the lattice, leading to shrinkage of the oxygen octahedron, lattice distortion, and mass transport, thus promoting grain growth.23,24,25,26 The larger average grain size means a smaller grain boundary, which is beneficial for mass transport and domain rotation, thus enhancing the electrical properties.29,30 When x increases from 4% to 8%, the decrease of Pb2+ ion content weakens the liquid phase sintering, which hinders mass transport for the PZN–PZT piezoceramic. Excessive Sr2+ ions aggregate at the grain boundary, which hinders the grain growth and deteriorates the electrical properties.
Piezoelectric Properties
Figure 6a displays the piezoelectric properties for the PZN–PZT piezoceramics with the different SrCO3 content. As shown in Fig. 6a, both kp and d33 exhibit similar changing trends with the increased SrCO3 amount. The sample with 4 mol%SrCO3 content has both kp and d33 up to the maximum of 0.59 pC/N and 496 pC/N, respectively. However, the mechanical quality factor (Qm) has an opposite changing trend to that of the d33 or kp. These results may be attributed to the fact that the substitution of Sr2+ ions for Pb2+ ions at the A-site leads to lattice distortion and the promotion of domain wall movement, which is beneficial to the improvement of the piezoelectric response.31 In addition, the kp and d33 have the same changing trends as the average grain size increases. The clamping effect of the grain boundary on the domain wall motion greatly reduces due to the increased grain size with x from 0% to 4%, which enhances the piezoelectric properties.32 With x increasing from 4% to 8%, the average grain size gradually decreases, and more grain boundaries between the small grains inhibit the domain rotation and the domain wall movement, resulting in the deterioration of the electrical properties.33,34,35
Dielectric Properties
Figure 6b exhibits the dielectric permittivity (εr) and dielectric loss (tanδ) for the PZN–PZT piezoceramics with different SrCO3 substituting amounts measured at 1 kHz and 20°C. As seen in Fig. 6b, the dielectric permittivity almost linearly increases as the SrCO3 content increases, while the dielectric loss changes little. The enhancement of the dielectric permittivity may be due to the A-site substitution of the Sr2+ for Pb2+ ions, which causes lattice distortion and promotes domain wall movement.31
Figure 7 illustrates the temperature-dependent dielectric permittivity (εr) for the PZN–PZT piezoceramics with different SrCO3 substitution amounts measured at various frequencies (1, 10, and 100 kHz). As shown in Fig. 7, due to the coexistence of the R-T phases from room temperature to the Curie temperature (Tc), there is no dielectric abnormal.36 Only one dielectric peak is observed for all the samples, which is closely related to the paraelectric‒ferroelectric phase transition. The Tc linearly decreases as the SrCO3 content increases, which can be attributed to the substitution of Sr2+ ions and the decreased tetragonality (c/a).37,38 The dielectric peaks broaden, decrease, and shift towards higher temperatures with the increased frequency, as indicated by the arrows in the insets of Fig. 7, which exhibit typical relaxor behaviors in the piezoceramics.39,40 The relaxor behaviors originate from the temperature evolution and dynamic behaviors of nanoregions with the compositionally disordered crystals.31
Figure 8 shows the Curie temperature (Tc) as a function of the SrCO3 content for the piezoceramics. As observed in Fig. 8, the experimental data match well with the red dotted line of the linear fitting. It can be concluded from the fitting line that the Curie temperature decreases by 11.2°C when the SrCO3 content increases by 1%.41,42
Figure 9a shows the curves of ln(1/ε−1/εmax) versus ln(T−Tmax) for the PZN–PZT piezoceramics with different SrCO3 substitution amounts measured at 1 kHz. The closer the γ value is to 2, the stronger the relaxation characteristics of the piezoceramics.43 As can be seen from Fig. 9a, all the samples show the characteristics of ferroelectric relaxors. Figure 9b displays the variations of the γ and the Curie temperature (Tc) with the increased SrCO3 content. The γ fluctuates between 1.79 and 1.86, and the Tc linearly decreases with the increased SrCO3 substitution amount. The values of ΔTm = Tm (at 100 kHz)−Tm (at 1 kHz) with different x are listed in Table I. With increasing Sr2+ ion content, the ΔTm gradually increases, which means enhanced relaxation in the ceramics.
Ferroelectric Properties
Figure 10a depicts the ferroelectric hysteresis loops for the PZN–PZT piezoceramics with different SrCO3 amounts. All the P–E loops are saturated. Figure 10b shows the Ec and Pr with the increased SrCO3 content. The remanent polarization gradually increases to a maximum of 32.8 μC/cm2 with 4 mol% SrCO3 content, then gradually decreases. The remanent polarization has an opposite changing trend to that of the coercive field. The Ec reduces to a minimum of 0.98 kV/mm with 4 mol% SrCO3 content. The results may be attributed to the lattice distortion and the increased grain size induced by the Sr2+-ion substitution for the A-site Pb2+ ions, which promotes the domain wall movement. High piezoelectric activities are because the domains rotate more easily.44,45
Conclusions
PZN–PZT piezoceramics were sintered as low as 900°C. Optimized piezoelectric properties (kp = 0.58, Tc = 244°C, d33 = 496 pC/N, Qm = 40, εr = 3000, Pr = 32.80 μC/cm2, and Ec = 0.98 kV/mm) were obtained in 4 mol% Sr2+ substituted piezoceramics. As the SrCO3 content increases, the grain size first increases to a maximum and then gradually decreases. Most electrical properties such as d33, kp, and Pr show similar trends.
The lattice parameters (a, c) demonstrate that c and a decrease with the increased SrCO3 content, while c decreases faster than a, which leads to a decreased tetragonality (c/a). The substitution of Pb2+ ions at the A-site with the Sr2+ ions leads to shrinkage of the oxygen octahedron. The lattice distortion promotes mass transport, causes the grains to grow, and increases the bulk density.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the [data-origin_files-OPJ.rar] repository, [https://share.weiyun.com/7REYAvbL]
References
F. Li, S. Zhang, T. Yang, Z. Xu, N. Zhang, G. Liu, J. Wang, J. Wang, Z. Cheng, Z.G. Ye, J. Luo, T.R. Shrout, and L.Q. Chen, The origin of ultrahigh piezoelectricity in relaxor-ferroelectric solid solution crystals. Nat. Commun. 7, 13807 (2016).
R. Nie, Q. Zhang, Y. Yue, H. Liu, Y. Chen, Q. Chen, J. Zhu, P. Yu, and D. Xiao, Phase structure–electrical property relationships in Pb(Ni1/3Nb2/3)O3–Pb(Zr, Ti)O3-based ceramics. J. Appl. Phys. 119, 124111 (2016).
J.H. Yoo, C.B. Lee, Y.H. Jeong, K.H. Chung, D.C. Lee, and D.S. Paik, Microstructural and piezoelectric properties of low temperature sintering PMN-PZT ceramics with the amount of Li2CO3 addition. Mater. Chem. Phys. 90, 386 (2005).
W. Zhang, and R.E. Eitel, Sintering behavior, properties, and applications of co-fired piezoelectric/low temperature co-fired ceramic (PZT-SKN/LTCC) multilayer ceramics. Int. J. Appl. Ceram. Technol. 2, 354 (2013).
J.J. Choi, J.H. Lee, B.D. Hahn, W.H. Yoon, and D.S. Park, Co-firing of PZN-PZT/Ag multilayer actuator prepared by tape-casting method. Mater. Res. Bull. 43, 483 (2008).
Y. Yue, Y. Hou, M. Zheng, X. Yan, and M. Zhu, Submicron crystalline buildup and size-dependent energy harvesting characteristic in PZN–PZT ternary ferroelectrics. J. Am. Ceram. Soc. 100, 5211 (2017).
J. Lou, M. Liu, D. Reed, Y. Ren, and N.X. Sun, Giant electric field tuning of magnetism in novel multiferroic FeGaB/lead zinc niobate-lead titanate (PZN–PT) heterostructures. Adv. Mater. 21, 4711 (2009).
N. Vittayakorn, G. Rujijanagul, X. Tan, H. He, M.A. Marquardt, D.P Cann, Dielectric properties and morphotropic phase boundaries in the xPb(Zn1/3Nb2/3)O3–(1–x)Pb(Zr0.5Ti0.5)O3 pseudo-binary system. J. Electroceram. 16, 141 (2006)
H.L. Li, Y. Zhang, J.J. Zhou, X.W Zhang, H. Liu, J.Z. Fang, Phase structure and electrical properties of xPZN–(1–x)PZT piezoceramics near the tetragonal/rhombohedral phase boundary. Ceram. Int. 41: 4822 (2015)
A. Halliyal, and A. Safari, Synthesis and properties of lead zinc niobate: Pb(Zn1/3Nb2/3)O3 based relaxor ferroelectrics, Ferroelectrics. Ferroelectrics 158, 295 (1994).
H. Fan, and H.E. Kim, Perovskite stabilization and electromechanical properties of polycrystalline lead zinc niobate-lead zirconate titanate. J. Appl. Phys. 91, 317 (2002).
M. Zheng, Y. Hou, M. Zhu, M. Zhang, and H. Yan, Shift of morphotropic phase boundary in high-performance fine-grained PZN–PZT ceramics. J. Eur. Ceram. Soc. 34, 2275 (2014).
Z. Yang, H. Li, X. Zong, and Y. Chang, Structure and electrical properties of PZT–PMS–PZN piezoelectric ceramics. J. Eur. Ceram. Soc. 26, 3197 (2006).
X. Zeng, A.L. Ding, T. Liu, G.C. Deng, X.S. Zheng, and W.X. Cheng, Excess ZnO addition in pure and La-substituted PZN–PZT ceramics. J. Am. Ceram. Soc. 89, 728 (2006).
G.C. Deng, Q.R. Yin, A.L. Ding, X.S. Zheng, W.X. Cheng, and P.S. Qiu, High piezoelectric and dielectric properties of La-Doped 03Pb(Zn1/3Nb2/3)O3–07Pb(ZrxTi1− x)O3 ceramics near morphotropic phase boundary. J. Am. Ceram. Soc. 88, 2310 (2005).
N. Vittayakorn, G. Rujijanagul, X. Tan, H. He, M.A. Marquardt, and D.P. Cann, Dielectric properties and morphotropic phase boundaries in the xPb(Zn1/3Nb2/3)O3–(1–x)Pb(Zr05Ti05)O3 pseudo-binary system. J. Electroceram. 16, 141 (2006).
S. Chandarak, M. Unruan, A. Prasatkhetragarn, and R. Yimnirun, Structural investigation of PZT–PNN and PZT–PZN probed by synchrotron X-ray absorption spectroscopy. Ferroelectrics 455, 117 (2013).
G.F. Fan, M.B. Shi, W.Z. Lu, Y.Q. Wang, and F. Liang, Effects of Li2CO3 and Sm2O3 additives on low-temperature sintering and piezoelectric properties of PZN–PZT ceramics. J. Eur. Ceram. Soc. 34, 23 (2014).
A. Ngamjarurojana, S. Ural, S.H. Park, S. Ananta, R. Yimnirun, and K. Uchino, Piezoelectric properties of low temperature sintering in Pb(Zr, Ti)O3–Pb(Zn, Ni)1/3Nb2/3O3 ceramics for piezoelectric transformer applications. Ceram. Int. 34, 705 (2008).
Y.D. Hou, M.K. Zhu, H. Wang, B. Wang, H. Yan, and C.S. Tian, Piezoelectric properties of new MnO2-added 0.2 PZN–0.8 PZT ceramic. Mater. Lett. 58, 1508 (2004).
S.M. Lee, S.H. Lee, C.B. Yoon, H.E. Kim, and K.W. Lee, Low-temperature sintering of MnO2-doped PZT–PZN Piezoelectric ceramics. J. Electroceram. 18, 311 (2007).
Q. Zhang, Y. Yue, R. Nie, H. Liu, Q. Chen, P. Yu, J. Zhu, and D. Xiao, Achieving both high d33 and high TC in low-temperature sintering Pb(Ni1/3Nb2/3)O3–Pb(Mg1/2W1/2)O3–Pb(Zr0 5Ti0 5) O3 ceramics using Li2CO3. Mater. Res. Bull. 85, 96 (2017).
T. Zaman, M.K. Islam, M.A. Rahman, A. Hussain, M.A. Matin, and M.S. Rahman, Mono and co-substitution of Sr2+ and Ca2+ on the structural, electrical and optical properties of barium titanate ceramics. Ceram. Int. 45, 10154 (2019).
M.P. Zheng, Y.D. Hou, F.Y. Xie, J. Chen, M.K. Zhu, and H. Yan, Effect of valence state and incorporation site of cobalt dopants on the microstructure and electrical properties of 0.2PZN–0.8PZT ceramics. Acta Mater. 61, 1489 (2013).
D. Wei, and H. Wang, Low-temperature sintering and enhanced piezoelectric properties of random and textured PIN–PMN–PT ceramics with Li2CO3. J. Am. Ceram. Soc. 100, 1073 (2017).
C.C. Tsai, S.Y. Chu, C.S. Hong, and S.F. Chen, Effects of ZnO on the dielectric, conductive and piezoelectric properties of low-temperature-sintered PMnN-PZT based hard piezoelectric ceramics. J. Eur. Ceram. Soc. 31, 2013 (2011).
H.W. Zhu, D.Y. Zheng, X.J. Wang, L. Yang, C. Fang, and Z.H. Peng, Effects of Ta2O5 addition on relaxation behavior and electric properties of PMS–PNN–PZT ceramics. J. Mater. Sci. Mater. Electron. 29, 16864 (2018).
N. Vittayakorn, S. Uttiya, G. Rujijanagul, and D.P. Cann, Dielectric and ferroelectric characteristics of 0.7PZT–0.3PZN ceramics substituted with Sr. J. Phys. D 38, 2942 (2005).
W. Jo, R. Dittmer, M. Acosta, J. Zang, C. Groh, E. Sapper, K. Wang, and J. Rödel, Giant electric-field-induced strains in lead-free ceramics for actuator applications–status and perspective. J. Electroceram. 29, 71 (2012).
C.A. Randall, N. Kim, J.P. Kucera, W. Cao, and T.R. Shrout, Intrinsic and extrinsic size effects in fine-grained morphotropic-phase-boundary lead zirconate titanate ceramics. J. Am. Ceram. Soc. 81, 677 (1998).
C.H. Wang, The piezoelectric and dielectric properties of PZT–PMN–PZN. Ceram. Int. 30, 605 (2004).
Y.D. Hou, L.M. Chang, M.K. Zhu, X.M. Song, and H. Yan, Effect of Li2CO3 addition on the dielectric and piezoelectric responses in the low-temperature sintered 05PZN–05PZT systems. J. Appl. Phys. 102, 084507 (2007).
K. Okazaki, and K. Sakata, Space charge polarization and aging of barium titanate ceramics. Electrotechnol. J. Jpn. 7, 13 (1962).
J. Schultheiß, S. Checchia, H. Uršič, T. Frömling, J. Daniels, B. Malič, T. Rojac, and J. Koruza, Domain wall-grain boundary interactions in polycrystalline Pb(Zr07Ti03)O3 piezoceramics. J. Eur. Ceram. Soc. 40, 3965 (2020).
X. Li, Y. Cheng, F. Wang, Q. Xu, Y. Chen, L. Xie, Z. Tan, J. Xing, and J. Zhu, Enhancement of energy storage and hardness of (Na0.5Bi0.5)0.7Sr0.3TiO3-based relaxor ferroelectrics via introducing Ba(Mg1/3Nb2/3)O3. Chem. Eng. J. (2021). https://doi.org/10.1016/j.cej.2021.133441.
C. Yuan, J. Xing, C. Wu, T. Wang, L. Xie, Y. Liu, X. Xu, K. Wang, D. Xiao, and J. Zhu, Investigation of high piezoelectric properties of KNNSb-SrxBNZ ceramics. J. Alloys Compd. 815, 152252 (2020).
M.R. Suchomel, and P.K. Davies, Enhanced tetragonality in xPbTiO3–(1–x)Bi(Zn1∕2Ti1∕2)O3 and related solid solution systems. Appl. Phys. Lett. 86, 262905 (2005).
J. Kelly, M. Leonard, C. Tantigate, and A. Safari, Effect of composition on the electromechanical properties of (1–x)Pb(Mg1/3Nb2/3)O3−xPbTiO3 Ceramics. J. Am. Ceram. Soc. 80, 957 (1997).
Y. Yue, Q. Zhang, R. Nie, H. Liu, Q. Chen, P. Yu, J. Zhu, and D. Xiao, Influence of sintering temperature on phase structure and electrical properties of 0.55Pb(Ni1/3Nb2/3)O3–0.45Pb(Zr0.3Ti0.7)O3 ceramics. Mater. Res. Bull. 92, 123 (2017).
J. Su, X. Lu, Y. Liu, J. Zhang, G. Li, X. Ruan, F. Huang, J. Du, and J. Zhu, Multiferroicity in 07Pb(Zr0.52Ti048)O3–0.3Pb(Ni1/3Nb2/3)O3 ceramics. Appl. Phys. Lett. 100, 102905 (2012).
A. Kumar, I. Rivera, and R.S. Katiyar, Investigation of local structure of lead-free relaxor Ba(Ti0.70Sn0.30)O3 by Raman spectroscopy. J. Raman Spectrosc. 40, 459 (2009).
N. Vittayakorn, Effects of strontium on the characteristics of Pb(Zr1/2Ti1/2)O3−Pb(Zn1/3Nb2/3)O3 ceramics. Phase Transit. 80, 813 (2007).
H. Liu, R. Nie, Y. Yue, Q. Zhang, Q. Chen, J. Zhu, P. Yu, D. Xiao, C. Wang, and X. Wang, Effect of MnO2 doping on piezoelectric, dielectric and ferroelectric properties of PNN–PZT ceramics. Ceram. Int. 41, 11359 (2015).
H. Liu, J. Chen, L. Fan, Y. Ren, Z. Pan, K.V. Lalitha, J. Rodel, and X. Xing, Critical role of monoclinic polarization rotation in high-performance perovskite piezoelectric materials. Phys. Rev. Lett. 119, 017601 (2017).
H. Chen, J. Xing, J. Xi, T. Pu, H. Liu, and J. Zhu, Phase, domain, and microstructures in Sr2+ substituted low-temperature sintering PZT-based relaxor ferroelectrics. J. Am. Ceram. Soc. 104, 6266 (2021).
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (grant number: 20826041E4280), the Key Research and Development Program of Sichuan Province (grant number: 2021YFG0234), the National Natural Science Foundation of China (grant number: 52032007), the research fund of National Major Science and Technology Projects of China (grant number: 2019zx06002021), and the Applied Basic Research of Sichuan Province (grant number: 2020YJ0317).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Pu, T., Luo, Y. et al. Enhancement of Piezoelectric Properties in Low-Temperature Sintering PZN–PZT Ceramics by Sr2+ Substitution. J. Electron. Mater. 51, 1261–1271 (2022). https://doi.org/10.1007/s11664-021-09393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09393-7