Abstract
SiO2 plays an important role in the formation of calcium ferrite as a main binding phase in iron ore sinter. But the mechanism of SiO2 influence on the crystallization of calcium ferrite has been still unclear. The crystallization behavior of Fe2O3–CaO–SiO2–Al2O3 melt in a non-equilibrium state was investigated using X-ray diffraction, optical microscopy, scanning electron microscopy, and energy-dispersive spectroscopy. The results show that SiO2 was an essential impact on the amount formation and morphology of calcium ferrite crystallization during the sintering process. With the increase of SiO2 content, the crystal morphology changed in the order of blocky, columnar, acicular to grains. The crystallization size increased first and then decreased. The crystallization sequence of samples with 4.0 mass pct SiO2 was observed as (Fe2O3, C4F14, SFCA-I) → CF→ SFCA → β-C2S. Increase of the cooling rate promoted the formation of Fe2O3, C4F14, and glass phase and the transformation of SFCA-I to SFCA. However, it also inhibited the crystallization of CF and SFCA, simultaneously the transformation of β-C2S to γ-C2S. The thermodynamic calculation shows that the crystallization temperatures and the primary crystal phase varied with the increase of SiO2 content, which agreed with the experimental results. In addition, the increase of SiO2 significantly reduced the liquid phase amount, simultaneously increased its viscosity at high-temperature range, which was contributing to decrease of the crystallization size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sinter is the primary iron-bearing material for ironmaking in blast furnaces.[1] The quality of the sinter depends on its strength and reducibility,[2,3,4,5,6,7] which are mainly affected by the nature, abundance, and morphology of minerals.[8,9,10] SiO2 is a main gangue component in the sinter, and its content is closely related to the bonding phase quality of the sinter.[11] Previous studies[12,13,14,15] show that calcium ferrite was the most critical bonding phase in high basicity sinter, with a relatively low melting point, high strength, and superior reducibility. Therefore, understanding the influence of SiO2 on the formation of calcium ferrite during the sintering process is the key to improving the quality of the sinter.
Webster and Pownceby et al.[16,17] found that the ternary phase (CFA) was generated before SFCA formed. It was confirmed that Al2O3 developed a solid solution with calcium ferrite and then reacted with SiO2 to generate SFCA.
Research on the crystal structure of silico-ferrite of calcium (SFC) provides a deeper understanding of the structure of SFCA. The SFC phase was identified by Hamilton et al.[18] They recognized that SFC was an alumina-free equivalent of SFCA within a compositional range in the CaO·3FeO (CF3) and 4CaO·3SiO2 (C4S3) pseudo-binary phase diagram in the Fe2O3–CaO–SiO2 system. The compositions of SFC are distributed along a line connecting between 7.0 and 11.7 mass pct C4S3.[19,20,21,22] Ding and Guo[23] examined the effect of SiO2 on the formation of SFC, which is the precursor phase of SFCA, suggesting that the solid solution process of SiO2 is related to the Ca channel composed of Ca octahedron in the SFC structure. Si mainly occupies the center position of the upper and lower tetrahedron near the Ca channel.
Many studies[24,25] have shown that there are mainly two kinds of complex calcium ferrite in iron ore sinter: SFCA (high Si and high Al) and SFCA-I (low Si and low Al). The cross-sectional morphology of SFCA-I is usually needle-shaped, and SFCA is usually prismatic, columnar, or lath-shaped. Compared with SFCA, SFCA-I has better mechanical behavior.[26] The performance of calcium ferrite is related to its composition, simultaneously affected by its morphology.[27,28] Park and Choi et al.[29] investigated the non-equilibrium crystallization behavior of Fe2O3–CaO–SiO2 systems with different SiO2 content and cooling rate by confocal laser scanning. They found that the primary phase’s formation temperature and equilibrium temperature were different. Wang and Yang et al.[30] found that the platy and needle-like complex calcium ferrite is the longest in one direction in its 3D form. The solid solution of Si4+ into the calcium ferrite crystal change the habitus of crystal growth from no obvious orientation to preferential orientation along the (040) or (320) surface, determined by the first-principles calculation and the XRD detection.
The formation of calcium ferrite during the sintering process determines the initial liquid phase, but the crystallization morphology is produced during the solidification process. However, the previous studies for crystallization of calcium ferrite mainly focused on the binary system (Fe2O3–CaO),[28] and the ternary system (Fe2O3–CaO–SiO2),[29,30] It is insufficient to reflect the quaternary system (Fe2O3–CaO–SiO2–Al2O3) in crystallization process.
In this work, the crystallization behavior and morphology of calcium ferrite in the Fe2O3–CaO–SiO2–Al2O3 melt with change of SiO2 contents were investigated in a non-equilibrium state. The crystallization device was designed so that the cooling rate can be accurately measured. This method can ensure more truly the crystal morphology and its composition. Results from this work are expected to helpfully understand the phase transformation in the crystallization process of the Fe2O3–CaO–SiO2–Al2O3 system, which will provide a theoretical guide to optimize the quality of iron ore sinter.
Experimental Procedures
Preparation of Samples
Samples were prepared using analytical grade reagents of Fe2O3, CaCO3, SiO2, and Al2O3. The chemical composition of the starting samples is listed in Table I. In the actual sintering process, the molar ratio of Fe2O3 and CaO is about 2.5:1. In iron ore fines, the adhesive powder with the smaller size is kept at 40 to 50 mass pct while the nuclear granules with the large size about 50 to 60 mass pct.[31] During the sintering process, normally the adhesive powder is completely transformed into a liquid phase due to contacting with the basic flux very well. It is assimilated to form the calcium ferrite with lower melting point. Furthermore, the nuclear granules also resolves partially into the liquid phase. Therefore, the molar ratio of Fe2O3 to CaO was selected to 1.3:1 in this work. As the gangue in iron ore fines, the Al2O3 is selected at 1.6 mass pct, while the SiO2 content at 1.0 to 6.0 mass pct as shown in Table I.
Sinter Process
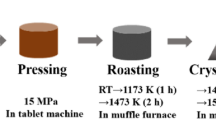
The pre-sintered hematite fines were blended with CaO, SiO2, Al2O3 according to the compositions as shown in Table I. CaO was replaced by equimolar CaCO3 for accurately weighing. 20g of each sample were homogenized by grinding in a mortar grinder (Fritsch Pulverisette 2, Idar-Ober-sttein, Germany) for 30 minutes and wetted with alcohol to mix again in an agate mortar for 10 minutes. It was then dried at 100 °C for 2 hours. The sample was pressed into a tablet with a dimension of 20 mm in diameter and approximately 20 mm in height using an electro-hydraulic briquetting machine at a pressure of 5 MPa for 3 minutes. Each sample was placed in a platinum crucible, and the experiment was carried out in an electric resistance furnace. According to previous studies,[32,33] the equilibrium state of the molten slag can be achieved by maintaining the temperature above the liquidus temperature for 2 hours. Therefore, the sample was first heated to 900 °C and held for 2 hours to completely convert CaCO3 into CaO, then the samples were heated to 1350 °C at 5 °C/min. The samples were held for 4 hours to from a homogenized liquid phase. After that, the samples were cooled down at different cooling rates for investigation of the crystallization behavior for calcium ferrite.
Quenching Method
To investigate the crystallization morphology and sequence of calcium ferrite in the CaO–Fe2O3–SiO2–Al2O3 melt at different cooling rates, an experimental device is as shown in Figure 1.
A B-type thermocouple was enclosed in a platinum protective tube for temperature acquisition. It was then inserted into a platinum crucible containing 20 g of sample. The cooling rate was selected to investigate the effect of SiO2 content on crystallization morphology as following.
-
(1)
The cooling rate was controlled by a temperature-controller of the resistance furnace from 1350 °C to room temperature.
-
(2)
The sample with the platinum crucible was quickly taken out of the furnace cooling to room temperature from 1350 °C
-
(3)
The sample with the platinum crucible was dropped into a liquid nitrogen tank for quenching from 1350 °C.
-
(4)
The sample was dropped into a water tank for quenching from 1350 °C.
The temperature values were collected by the B-type thermocouple transmitting to computer through a scanning thermometer. The corresponding four cooling rates are 0.02, 5, 15 and 65 °C/s respectively as shown in Figure 2.
To investigate the crystallization sequence of calcium ferrite, samples with the platinum crucible at 1350 °C for 4 hours were cooled down to 1300 °C, 1290 °C, 1280 °C, 1250 °C, 1200 °C, 1150 °C and 1100 °C, respectively, at the cooling rate of 0.02 °C/s, and then vertically dropped into the water tank in the bottom of resistance furnace
Phase Determination
The samples were ground into fine powders to less than 100μm in an agate mortar, X-ray diffractometer (Rigaku Corporation, Tokyo) was used to determine different phases in Cu Κα as the radiation source (40 KV, 400 mA) with a graphite monochromator in the diffraction beam path. The XRD data were collected using continuous scanning mode, at a scanning speed of 10 deg/min. The scanning angle range (2θ) is 10 to 90 deg. The XRD data were analyzed by the Crystallographic Search-Match software package (Oxford Cryosystems Ltd., Oxford, UK).
Three pieces were randomly selected from each sample to embed into the phenolic mounding powders by a metallographic inlaying machine, which was polished and observed using optical microscope and scanning electron microscope (SEM). A Zeiss-Evo18 scanning electron microscope was used with an accelerating voltage of 30kV. The chemical composition of different phases was qualitatively determined using a Zess-5010 Energy-dispersive spectroscopy (EDS).
Results and Discussion
The Effect of SiO2
The prepared samples with different SiO2 content were melted at 1350 °C for 4 hours and then cooled to room temperature at a cooling rate of 0.02 °C/s. They are used as the reference sample comparing the crystallization sample of the Fe2O3–CaO–SiO2–Al2O3 melt at the faster cooling rate. Figure 3 shows the XRD patterns of samples at different SiO2 content. The SEM and EDS results are shown in Figure 4 and Table II. As shown in Figure 3, CF (CaFe2O4), γ-C2S (γ-Ca2SiO4), CFA (Ca3.18Fe15.48Al1.34O36), SFCA (Ca5Si2(Fe, Al)18O36) and Fe2O3 were observed in samples at a cooling rate of 0.02 °C/s. Simultaneously with the increase of SiO2 content, the CF intensity decreased, while CFA intensity increased. As shown in Figure 4 and Table II, the Si element dissolved into CFA to form a solid solution, which indicates that SiO2 promoted the formation of CFA, simultaneously transforming SFCA. According to above results, it is considered as follows.
(1) When SiO2 was not added, CaO reacted with Fe2O3 to form CF, and then CF and Al2O3 reacted to form CFA. The final phases were CF and CFA.
(2) When SiO2 was added in the range of 1.0 to 3.0 mass pct, CaO reacted with Fe2O3 to form calcium ferrite (CF, CFA), and then a part of SiO2 entered into the CFA lattice forming SFCA-I, while another part of SiO2 reacted with CaO forming γ-C2S due to Fe2O3 much more than SiO2. The final phases were CF, SFCA-I, and γ-C2S.
(3) When the SiO2 content reached and exceeded 4.0 mass pct, the samples were obvious degraded, simultaneously a part of SFCA-I was transformed into SFCA (high Si). The cause of the degradation is due to increasing of phase transformation from β-C2S (metastable state) to γ-C2S, along with a volume expansion of 12 pct.[34,35] The final phases were CF, SFCA-I, SFCA, Fe2O3 and γ-C2S.
The reactions of C2S and CF formation would be as shown as following in Eqs. [1] and [2] respectively. Thermodynamically, the Gibbs standard free energy of C2S formation is significantly more negative than that of CF,[30] which indicates that C2S is more stable to generate easier than calcium ferrite. However, the C2S content was very low when w(SiO2) is less than 3.0 mass pct, due to the mass fraction of SiO2 much smaller than Fe2O3, simultaneously SiO2 also forming SFCA-I or SFCA.
In this experiment, the sample was cooled at a slow cooling rate (0.02 °C/min) to make the sample crystallization very well, which is beneficial to discuss the crystalline phase and it’s changing with the cooling rate.
The Sequence of Mineral Crystallization
To investigate the crystallization sequence of minerals at different temperatures, the samples with 4.0 mass pct (No. 5) were kept at 1350 °C for 4 hours in an air atmosphere, cooled to different target temperatures at a cooling rate of 0.02 °C/s, and then quenched in water tank.
The XRD patterns of samples obtained are shown in Figure 5. When the sample was cooled down directly from 1350 °C, the crystalline phases were Fe2O3, SFCA-I, and Ca4Fe14O25 (C4F14). When the samples were cooled, respectively, to 1300 °C, 1290 °C, 1280 °C, 1250 °C, 1200 °C, 1150 °C and 1100 °C at 0.02 °C /s, and then quenched, the results show that C4F14 disappeared, while Fe2O3 and CFA increased at 1300 °C, indicating that all C4F14 participated in the reaction to form SFCA-I; CF appeared while Fe2O3 and SFCA-I decreased at 1290 °C; SFCA-I further reduced, while CF and Fe2O3 increased at 1280 °C; SFCA-I phase transformed into a new SFCA phase, simultaneously Fe2O3 decreased, while CF increased at 1250 °C, indicating that Fe2O3 was consumed in the generation of CF, while CF and SFCA-I participated in the reaction of formation of SFCA; another new phase of β-C2S appeared, simultaneously SFCA increased, while CF decreased at 1200 °C. Furthermore, when the target temperature cooled at 0.02 °C /s was lowered respectively to 1150 °C and 1100 °C, the crystallization phase had not changed, but the Fe2O3 decreased, while the SFCA increased. In addition, to verify the change of minerals below 1200 °C, another sample was taken to cool down to 1200°C at 0.02°C/s, held for 10 minutes at 1200 °C, and then quenched in water tank. It was no significant change comparing with the above sample by quenching directly at 1200°C as shown in Figure 5.
Figure 6 and Table III show the SEM images of the cross-sectional structure of the sample at different quenching temperatures. The crystalline products were Fe2O3, C4F14, Slag phase, SFCA-I, SFCA, CF, and β-C2S. With the decrease of the target temperature, Fe2O3 had grown in a massive shape, calcium ferrite (CF, C4F14, SFCA-I, and SFCA) developed from a needle shape to a columnar shape, and β-C2S had formed in a striped shape. Especially at 1250 °C, the complex calcium ferrite changed obvious from needle-shaped SFCA-I to columnar SFCA. It can be considered obvious differences in the microstructure of the samples at different temperatures.
According to the above experimental results, we know that the crystallization sequence was (Fe2O3, Ca4Fe14O25, SFCA-I) → CF → SFCA → β-C2S as shown in Figure 5(c).
Effect of SiO2 Content and Cooling Rate on Crystallization Process
The XRD patterns of samples with different SiO2 content cooled at 5, 15 and 65 °C/s, respectively, from 1350 °C to room temperature are shown in Figures 7 to 9. The corresponding optical micrographs of samples are shown in Figure 10. It shows the SiO2 content significantly affects the type, quantity and morphology of the phases formed in the sintered samples. Table IV shows that seven different phases were observed as CF, CFA, C4F14, SFCA-I, SFCA, Fe2O3, and β-C2S.
As shown in Figure 10, CF and CFA were in a block shape, C4F14 and SFCA-I were in a columnar and needle shape, and SFCA was completely columnar. But β-C2S was not observed. As the SiO2 content increased to 4.0 mass pct, CF and C4F14 gradually decreased, while the SFCA-I content increased. When more than 4.0 mass pct of SiO2, SFCA-I transformed completely into SFCA in sample cooled at 5 °C/s, while still existed partly in samples cooled at 15 °C and 65 °C, respectively. Because the Fe content in SFCA-I was higher than that of SFCA, Fe2O3 also increased in transformation process from SFCA-I to SFCA. The morphology of calcium ferrite crystallization changed from columnar to needle-like with increase of cooling rate.
SEM image of the samples with different SiO2 content cooled at 5°C/s is shown in Figure 11. The corresponding EDS result is listed in Table V. It shows that with the increase of SiO2 content, the SFCA-I structure developed from the needle to the column, simultaneously the content of Si and Al elements in SFCA-I phase increased. When the SiO2 content reached 4.0 mass pct, SFCA-I was transformed completely into SFCA. As the SiO2 content increased, the sizes of CF and C4F14 gradually shrank, simultaneously the Si content in complex calcium ferrite gradually increased. Comparing with a cooling rate of 0.02 °C/s, the C4F14 and β-C2S were promoted, while the γ-C2S was inhibited. Since the crystallization time was relatively shortened by increasing the cooling rate, the size of each crystalline phase also became smaller.
Table V shows that when SiO2 was not added, only C4F14, CF and CFA phases were formed, while after adding SiO2, seven phases appeared as C4F14, CF, SFCA-I, SFCA, Fe2O3, β-C2S, and the slag phase.
In the above samples, the sample with 3.0 mass pct SiO2 cooled at 5 °C/s to room temperature was a relatively representative, the SEM-EDS mapping, morphology and element distribution is shown in Figure 12. It can be seen that a part of Si element entered the CFA lattice to form SFCA-I, while the other part existed in β-C2S and slag. Fe and Ca elements were mainly present in CF and SFCA-I, and Al element was mainly in the CFA phase.
When the cooling rate increased from 5 to 15 °C/s, the crystallization of all phases was suppressed, and the size was reduced significantly due to decrease of crystalline growth time. Thus, the complex calcium ferrite developed from a columnar shape to a needle shape. At the same time, it promoted the early appearing of Fe2O3, C4F14, and glass phase. It was found that β-C2S disappeared, indicating that increasing the cooling rate had an inhibitory effect on β-C2S crystallization. Therefore, when the cooling rate increased from 15 to 65 °C/s, the dendrite arms of SFCA-I and SFCA became short, the crystal morphology of other minerals also shrank, and the slag phase was filled in calcium ferrite vacancies around.
In order to explore the influence of SiO2 on the morphology of complex calcium ferrite (SFCA-I and SFCA). A particle size analysis software (Nano Measurer 1.2, published by Fudan University)[36] was used to calculate the particle size of the complex calcium ferrite in Figure 10, measuring 20 data for each image to take an average value. Figure 13 shows the particle size distribution of complex calcium ferrite in samples with different SiO2 content at different cooling rate. The result shows that with the increase of SiO2 content, the particle size first increased and then decreased. The maximum size was changed with the cooling rate and the SiO2 content, as 29.68, 16.21 and 11.10 μm, respectively corresponding to 5 °C/s and 4.0 mass pct, 15 °C/s and 2.0 mass pct, and 65 °C/s and 2.0 mass pct. It was found that the decrease of the crystallization size was accompanied by the precipitation of Fe2O3. It can be considered that increase of the cooling rate promoted the crystallization of Fe2O3 due to Fe2O3 having the higher melting point than other crystallization phases in the Fe2O3–CaO–SiO2–Al2O3 melt. The precipitation of Fe2O3 was promoted, resulting in decrease of Fe2O3 in the slag, which would inhibit the formation of calcium ferrite.
Thermodynamic Analysis of Crystallization Mechanism
In order to explain the experimental phenomenon, FactSage software package was used to calculate equilibrium state crystallization and the phase diagram. It was found that the formation of the primary crystal phase not only depended on chemical composition but also was greatly affected by the cooling rate. Due to no thermodynamic database of C4F14, SFCA-I and SFCA crystallization phases, here the existence of thermodynamic database was only used to explain the crystallization in the Fe2O3–CaO–SiO2–Al2O3 melt.
Figure 14 shows the quantitative relationship between the temperature and the amount of crystallization phase in samples with different SiO2 content simulated by FactSage software.[37] It shows that when the SiO2 content was 0 mass pct, M2O3 (≥ 99.50 mass pct Fe2O3 and ≤ 0.50 mass pct Al2O3), Ca(Al, Fe)2O4 (≥ 99.60 mass pct CaFe2O4 and ≤ 0.40 mass pct CaAl2O4), CF2 (CaFe4O7), CFA-I (Ca2(Al, Fe)2O5), and CFA-II (Ca(Al, Fe)6O10) would be crystallized during the cooling process, while when the SiO2 content increased from 1 to 6 mass pct, CFA-I disappeared, and the initial crystallization temperatures of M2O3 and α′-C2S would increase respectively from 1173 °C to 1288 °C, and 1195 °C to 1249 °C, while Ca(Al, Fe)2O4 and CaFe4O7 decreased respectively from 1196 °C to 1175 °C, and 1201 °C to 1189 °C. At the same time, the corresponding crystallization amounts of M2O3 and α′-C2S increased respectively from 17.78 to 43.44 mass pct, and 2.87 to 17.20 mass pct, while Ca(Al, Fe)2O4 and CaFe4O7 decreased respectively from 66.82 to 27.16 mass pct, and 12.54 to 12.20 mass pct. This is consistent with our experimental results.
Table VI listed the primary crystallization phase and temperature in thermodynamic equilibrium state, it also shows the crystallization is closely related to the SiO2 content.
Viscosity is also an important effect factor for crystallization of complex calcium ferrite kinetically. The viscosity of the Fe2O3–CaO–SiO2–Al2O3 melt at 1350 to1270 °C was calculated by the FactSage software[37] as shown in Figure 15. It shows that the viscosity increased with increases of SiO2 content, while decreased with increase of temperature. When the content of SiO2 was at 4.0 to 6.0 mass pct, the primary crystalline product was Fe2O3 which precipitated at 1251 and 1288 °C, respectively as shown in Table VI. When the solid-liquid phases existed simultaneously, the viscosity were calculated by the Einstein–Roscoe formula as shown in Eq. [3],[38] it shows that the viscosity value increased significantly after precipitation of Fe2O3, resulting in deviation from the linear relationship. Due to hindering the mass transferring of crystallization, the size is reduced, and the morphology of the complex calcium ferrite developed from columnar to needle-like or granular.
\(\eta \) is a viscosity of liquid-solid mixture; \(\eta \)0 is a viscosity of liquid phase in the mixture; c is a mass fraction of solids in the mixture.
Isothermal cross-section of Fe2O3–CaO–SiO2–Al2O3 system at different SiO2 content and different temperature simulated by FactSage are shown in Figure 16. It shows that the liquid phase area of the Fe2O3–CaO–SiO2–Al2O3 system gradually shrank as the SiO2 content increased, which indicates that the amount of the liquid phase was significantly reduced due to precipitation of M2O3. For example, the red point is corresponding to chemical composition of samples at 1270 °C, it shows that the phase region of the red point changed from Lα corresponding to sample with 1.0 mass pct to M2O3 + Lα + Lβ corresponding to sample with 6.0 mass pct. The precipitation of M2O3 resulted in increase of the viscosity and decrease of the Fe3+ content in the melt, which would be against the crystallization of complex calcium ferrite. But an appropriate amount of SiO2 existed in Fe2O3–CaO–SiO2–Al2O3 melt, is conducive to crystallization of complex calcium ferrite due to demanding SiO2 thermodynamically. For examples, the sample with 3.0 mass pct at 1270 °C as shown in Figure 16, it has still retained a single liquid phase, simultaneously the Fe3+ content has not also decreased.
Conclusion
To investigate the crystallization behaviors and morphology of the Fe2O3–CaO–SiO2–Al2O3 melt at different cooling rate and different SiO2 content, the cooling experiments were carried out from 1350 °C to room temperature. XRD, optical microscopy, and SEM-EDS were used to determine the crystal phase compositions and morphology. The following conclusions were summarized.
-
1.
In crystallization of calcium ferrite, SiO2 plays an essential role. As the content of SiO2 increased, the crystal morphology of complex calcium ferrite changed in the order of blocky, columnar to acicular, while the crystallization size increased first and then decreased.
-
2.
At the cooling rate of 0.02 °C/s, CF decreased while β-C2S increases with increase of the SiO2 content. When SiO2 content reached 4.0 mass pct, SFCA-I had transformed to SFCA, simultaneously the samples appeared a degradation due to the volume expansion of β-C2S to γ-C2S. The crystallization phases were CF, γ-C2S, CFA, SFCA, and Fe2O3, while the sequence of crystallization was (Fe2O3, C4F14, SFCA-I), CF, SFCA to β-C2S.
-
3.
With increase of the cooling rate, SFCA-I, Fe2O3, C4F14 and glass phases increased while SFCA decreased. At the same time, it can inhibit the crystallization of CF and SFCA, and the transformation of β-C2S to γ-C2S improving the degradation of samples.
-
4.
As increase of the SiO2 content, the temperature and sequence of the primary crystal phase had changed. When the SiO2 content increased from 4.0 to 6.0 mass pct, the primary crystal temperature of Fe2O3 increased from 1251 °C to 1288 °C in the Fe2O3–CaO–SiO2–Al2O3 melt. The precipitation of Fe2O3 would increase the viscosity, simultaneously decrease the Fe3+ content in the melt, which suppressed the crystallization of calcium ferrite.
References
C.E. Loo and W. Leung: ISIJ Int., 2003, vol. 43(9), pp. 1393–1402.
L.X. Yang and C.E. Loo: ISIJ Int., 1997, vol. 37(5), pp. 449–57.
S.L. Wu, J. Zhu, J.X. Fan, G.L. Zhang, and S.G. Chen: ISIJ Int., 2013, vol. 53(9), pp. 1561–70.
B.G. Ellis, C.E. Loo, and D. Witchard: Ironmak. Steelmak., 2007, vol. 34(2), pp. 99–108.
H. Guo and X.M. Guo: Metall. Mater. Trans. B., 2018, vol. 49B(4), pp. 1974–84.
Y.L. Liu, J.S. Wang, H.J. Zhang, J.Z. Liu, X.F. She, and Q.G. Xue: Ironmak. Steelmak., 2015, vol. 42(5), pp. 358–65.
S.L. Wu, G.L. Zhang, S.G. Chen, and B. Su: ISIJ Int., 2014, vol. 54(3), pp. 582–88.
A. Cores, A. Babich, M. Muñiz, S. Ferreira, and J. Mochon: ISIJ Int., 2010, vol. 50(8), pp. 1089–98.
L.H. Hsieh and J.A. Whiteman: ISIJ Int., 1989, vol. 29(8), pp. 625–34.
T.J. Park, J.S. Choi, and D.J. Min: Met. Mater. Int., 2020, vol. 26, pp. 532–40.
N.A.S. Webster, D.P. O’Dea, B.G. Ellis, and M.I. Pownceby: ISIJ Int., 2017, vol. 57(1), pp. 41–47.
A.M. Nyembwe and A.M. Garbers-Craig: Ironmak. Steelmak., 2014, vol. 41(3), pp. 173–81.
Z. Wang, D. Pinson, S. Chew, B.J. Monaghan, H. Rogers, and G.Q. Zhang: ISIJ Int., 2016, vol. 56(4), pp. 505–12.
L. Lu, R.J. Holmes, and J.R. Manuel: ISIJ Int., 2007, vol. 47(3), pp. 349–58.
S.Y. Cheng, M. Shevchenko, P.C. Hayes, and E. Jak: Metall. Master. Trans. B., 2021, vol. 52B, pp. 1891–1914.
N.A.S. Webster, M.I. Powceby, and I.C. Madsen: ISIJ Int., 2013, vol. 53(8), pp. 1334–40.
N.A.S. Webster, M.I. Powceby, I.C. Madsen, A.J. Studer, J.R. Manuel, and J.A. Kimpton: Metall. Mater. Trans. B., 2014, vol. 45B(6), pp. 2097–2105.
J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, and M.A. Montague: Neues Jahrb Min. Abh., 1989, vol. 161(1), pp. 1–26.
M.I. Pownceby, J.M.F. Clout, and M.J. Fisher-White: Trans. Inst. Min. Metall., 1998, vol. 107, pp. C1-10.
M.I. Pownceby and J.M.F. Clout: Trans Inst. Min. Metall., 2003, vol. 112, pp. 44–51.
M.I. Pownceby and T.R.C. Patrick: Eur. J. Mineral., 2000, vol. 12, pp. 455–68.
M.I. Pownceby and T.R.C. Patrick: Metall Mater. Trans. B., 2002, vol. 33B(1), pp. 79–89.
X. Ding and X.M. Guo: Metall. Mater. Trans. B., 2014, vol. 45B, pp. 1221–31.
N.A.S. Webster, M.I. Pownceby, I.C. Madsen, and J.A. Kimpton: Metall Mater. Trans. B., 2012, vol. 43B(6), pp. 1344–57.
K.H. Yajima and S.M. Jung: ISIJ Int., 2012, vol. 52(3), pp. 535–37.
L.H. Hsieh and J.A. Whiteman: ISIJ Int., 1993, vol. 33(4), pp. 462–73.
B. Cai, T. Watanabe, C. Kamijo, and M. Susa: ISIJ Int., 2018, vol. 58(4), pp. 642–51.
C.Y. Ding, X.W. Lv, S.W. Xue, J. Qiu, Y. Chen, and C.G. Gang: ISIJ Int., 2017, vol. 57(4), pp. 634–42.
T.J. Park, J.S. Choi, and D.J. Min: Metall Mater. Trans. B., 2019, vol. 50B(2), pp. 790–98.
W. Wang, D.W. Yang, Z.L. Ou-Yang, R.S. Xu, and M.M. Song: Metall Mater. Trans. B., 2019, vol. 50B(2), pp. 678–87.
X.H. Fan: Principle and Technology of Optimum Blending of Iron Ore Sintering, Metallurgical Industry Press, Beijing, 2013, pp. 62–65.
Z.J. Wang, Y. Sun, S. Sridhar, M. Zhang, and Z. Zhang: Metall Mater. Trans. B., 2015, vol. 46B(5), pp. 2246–54.
Z.J. Wang, Y. Sun, S. Sridrar, Z. Mei, and Z. Zhang: Metall Mater. Trans. B., 2017, vol. 48B(1), pp. 527–37.
R.T.D. Cruz and S.R. Bragança: Ceramic. Int., 2015, vol. 41, pp. 4775–81.
I. Nettleship, K.G. Slavick, Y.J. Kim, and W.M. Kriven: J. Am. Ceramic. Soc., 1992, vol. 75(9), pp. 2400–06.
R.G. Chang, Y.T. Yan, J.Y. Zhang, Z.L. Zhu, and J.H. Gu: Thin Solid Films., 2020, vol. 712, p. 138279.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melancon, A.D. Pelton, and S. Petersen: Calphad., 2002, vol. 26(2), pp. 189–228.
R. Roscoe: Br. J. Appl. Phys., 1952, vol. 3, pp. 267–69.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (Nos. 51774029 and U1460201) for financial support of this research. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted December 14, 2021; accepted March 14, 2022.
Rights and permissions
About this article
Cite this article
Xin, RF., Guo, XM. Effect of SiO2 on Crystallization of Calcium Ferrites in Fe2O3–CaO–SiO2–Al2O3 System in Cooling Process. Metall Mater Trans B 53, 1904–1919 (2022). https://doi.org/10.1007/s11663-022-02501-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02501-w