Abstract
In the present work, the effect of dephosphorization slag basicity on the dephosphorization of hot metal has been studied in the lower temperature range of 1370 °C to 1420 °C and the lower basicity of 1.26 to 2.20 with new double slag converter steelmaking process (NDSP). Based on the ion-molecule coexistence theory (IMCT), the thermodynamic model IMCT-Ni of dephosphorization slag is established. With increasing basicity from 1.26 to 2.20, the phosphorus distribution ratio LP between hot metal and slag increases. The dephosphorization ratio and the decarbonization ratio both increase, while the demanganization ratio decreases. The morphologies of P-rich phase change from long strip shape (B = 1.26-1.37) to dendritic shape (B = 1.50) and to massive shape (B = 1.71-2.20). The area of P-rich phase increases from about 4 μm2 to about 8000 μm2. The content of P2O5 in the P-rich phase increases and the value of the coefficient n in nC2S-C3P of the P-rich phase decreases from 6-20 to 1-2. The phosphorus-enrichment contribution ratio of calcium silicate is in the order of \(R_{{{\text{C2S}}}}\)>\(R_{{{\text{CS}}}}\)>\(R_{{{\text{C3S}}}}\)>\(R_{{{\text{C3S2}}}}\). The phosphorus-enrichment degree in dephosphorization slag is enhanced mainly by C2S-C3P. With increasing basicity, the calculated results of IMCT-(pct C2S-CjP) and \(R_{{{\text{C2S}}}}\) are well consistent with the measurement results of AP-rich phase and (pct P2O5)P-rich phase of industrial experiment, indicating that the IMCT calculated results can correctly express the phosphorus-enrichment degree of dephosphorization slag.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The multi-refining converter (MURC)[1,2,3] developed by Nippon Steel in 2001 is a representative new double slag converter steelmaking process(NDSP). Firstly, desiliconization and dephosphorization are carried out at lower basicity and lower temperature in the converter. After intermediate deslagging of dephosphorization slag, the decarburization are conducted in the same converter, with the decarburization slag left in the furnace for dephosphorization in the next heat,[1,2,3,4,5] so that the lime consumption and slag emission amount can be greatly reduced.
Some scholars have studied the effect of dephosphorization slag basicity on hot metal dephosphorization in NDSP. Fang et al.[6] optimized the process parameters of the dephosphorization stage in NDSP. When the dephosphorization endpoint temperature is 1328 °C, the dephosphorization slag basicity is 1.5, T.Fe content is 12 to 16 pct, and the oxygen supply intensity is 2.0 to 2.7 m3/(t min), the dephosphorization ratio reaches 67.3 pct and the phosphorus content reaches 0.011 pct at the endpoint of dephosphorization. Liu et al.[7] carried out the industrial experiments on NDSP by 80 t converter. With the slag basicity of 1.2 to 2.0 at the dephosphorization stage, the dephosphorization ratio of hot metal is higher, and the optimal deslagging temperature range is 1327 °C to 1427 °C. Using the hot metal with a high phosphorus content in NDSP, Wang et al.[8] showed that with increasing dephosphorization slag basicity from 1.75 to 2.22, the phosphorus content of hot metal decreases linearly at the endpoint of dephosphorization. Based on the 180 ton industrial experiments on NDSP, our previous work[9] studied the behavior of phosphorus enrichment in dephosphorization slag. With increasing basicity, the morphologies of different phases in the dephosphorization slag change greatly, and the area fractions and P2O5 content of the P-rich phase also increase.
In the process of dephosphorization in NDSP, the slag is in the coexistence state of solid phase and liquid phase. Many research results[4,5,6,7,8,9,10,11,12,13,14,15,16,17] showed that the phosphorus oxide combines with calcium silicate in slag to form a solid solution, 2CaO·SiO2-3CaO·P2O5(C2S-C3P), which makes phosphorus exist in slag stably. Xie et al.[10] studied the crystallization kinetics of C2S-C3P solid solution in multiphase slag. The content of C2S-C3P solid solution in multiphase slag increases with increasing P2O5 content and decreasing holding temperature, and decreases with increasing FeOt content. They further studied the mass transfer behavior of phosphorus in multiphase dephosphorization slag at 1350 °C by adding P2O5 powder into CaO-FeOt-SiO2 slag saturated with 2CaO·SiO2(C2S).[11] The added P2O5 powders can form C2S-C3P solid solution rapidly with C2S in the slag, and the rate control step of the process is the mass transfer of phosphorus from C2S-C3P solid solution layer to internal C2S crystal. Kakimoto et al.[12] studied the dissolution behavior of lime in CaO-SiO2-FeO and CaO-SiO2-FeO-5.2 pct P2O5 slag. Adding P2O5 to the slag can promote the dissolution of lime into the slag. The main reason is that part of CaO exists in the slag in the form of C2S and C3P in the form of solid solution. Du et al.[13] obtained that the distribution ratio of P2O5 in solid solution and liquid phase increases with increasing T.Fe content, and has nothing to do with P2O5 content and valence of Fe in CaO-SiO2-FeO-P2O5-Na2O slag.
On the other hand, the ion-molecule coexistence theory (IMCT) is a slag structure theory based on the possible compounds of simple ions, simple molecules and complex molecules in the phase diagram as the structural units, and the mass action law is used to quantitatively calculate the reaction ability of structure units or ion couples in the studied slag.[18,19,20,21,22,23,24,25,26,27,28,29] The coexistence theory model was first proposed by Chuiko[24] and then was further developed by Zhang.[25]
In recent years, some scholars[22,23,25,26] studied the dephosphorization process of steelmaking slag based on IMCT. Yang et al.[22] established the phosphorus distribution ratio prediction model IMCT-logLP of CaO-FeO-Fe2O3-SiO2-MgO-MnO-Al2O3-P2O5, and compared the calculation results with the empirical formula values and the measured values. The IMCT-logLP model can accurately predict the phosphorus distribution ratio of the slag, and the corresponding phosphorus distribution ratio of each component with dephosphorization ability in slag, indicating that C3P is the main contribution component of LP, and its contribution ratio is 96.01 pct. They further established the phosphorus capacity prediction model \( {\text{IMCT}} - \log C_{{{\text{PO}}_{4}^{{3 - }} }} \) and the phosphorus capacity index prediction model \( {\text{IMCT}} - {\text{log}}C_{{{\text{PO}}_{4}^{{3 - }} \;{\text{index}}}} \) under the same slag condition based on IMCT.[23] The results show that the dephosphorization reaction is controlled by the basic compositions of converter slag and iron oxide. CaO + FetO is the main contribution component for phosphorus capacity or phosphorus capacity index of slag, and its contribution ratio reaches 99.996 pct. Li et al.[26] established the phosphorus distribution ratio model of IMCT-logLP which can accurately predict the phosphorus distribution ratio of CaO-FeO-Fe2O3-SiO2-MgO steelmaking slag. With increasing CaO and FetO content and decreasing MgO and SiO2 content, the predicted values of LP, cal is increased which is consistent with the measured values.
Based on IMCT, some scholars studied the phosphorus-enrichment capacity of calcium silicate in slag. Xie et al.[27] discussed the phosphorus-enrichment capacity of calcium silicate in multiphase slag through the phosphorus-enrichment contribution ratio of calcium silicate \(R_{{Ci}}\) (refer to Eq. [16]) defined by IMCT. In CaO-FeO-Fe2O3-SiO2-MgO-MnO-Al2O3-P2O5 slag with the temperature of 1350 °C and the basicity of 0.5 to 4, the phosphorus-enrichment contribution ratio of dicalcium silicate \(R_{{{\text{C2S}}}}\) will be significantly increased by increasing the basicity of slag, and the value of \(R_{{{\text{C2S}}}}\) reaches the maximum value when FeO content is 25 pct. In addition, with increasing m(FeO)/m(Fe2O3) ratio, the value of \(R_{{{\text{C2S}}}}\) increases, while with increasing P2O5 content in slag, the value of \(R_{{{\text{C2S}}}}\) decreases slightly. Li et al.[28] studied the phosphorus-enrichment behavior in CaO-FeO-Fe2O3-SiO2-MgO steelmaking slag at 1450 °C to 1600 °C based on IMCT. C3P formed in slag can combine with C2S easily to form C2S-C3P solid solution at 1500 °C. Under the fixed cooling condition with basicity of 2.5 and the value of (pct FetO)/(pct CaO) of 0.955, the phosphorus-enrichment degree in C2S-C3P solid solution \(R_{{{\text{C2S - C3P}}}}\) (refer to Eq. [15]) defined by IMCT is 0.844. They further studied the phosphorus-enrichment behavior in CaO-FeO-Fe2O3-SiO2-MgO slag at 1500 °C and basicity in the range of 1.0 to 2.0.[29] There is an asymmetric inverted V-shape relationship between the enrichment degree of phosphorus in slag and the binary basicity. When the basicity is 1.3, the maximum content of P2O5 in C2S-C3P solid solution is about 30.0 pct.
From our previous work,[5,9,30,31] the lower temperature range of 1370 °C to 1420 °C and the lower basicity range of 1.26 to 2.20 are the optimal dephosphorization ranges for the NDSP. According to the above literatures, it can be seen that in these temperature and basicity ranges, the researches on the dephosphorization of hot metal and the phosphorus-enrichment capacity of calcium silicate in the dephosphorization slag are quite limited using NDSP.
In the present work, the industrial experiments were carried out by 180 ton top-bottom combined blowing converter for NDSP. When the dephosphorization slag basicity is 1.26 to 2.20 and the dephosphorization endpoint temperature is 1370 °C to 1420 °C, the effect of dephosphorization slag basicity on dephosphorization of hot metal was studied. The effect of dephosphorization slag basicity on the mass action concentration, Ni, of CaO, SiO2, calcium silicate and phosphate in the dephosphorization slag were analyzed by IMCT-Ni thermodynamic model for CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 slag. The effect of dephosphorization slag basicity on the enrichment degree \(R_{{Ci-Pj}}\) of P2O5 containing solid solution and the phosphorus-enrichment contribution ratio of calcium silicate \(R_{{Ci}}\) were studied. In addition, X-ray diffraction (XRD) was used to detect the phase compositions of dephosphorization slag under different basicities. The morphological evolution of P-rich phase in dephosphorization slag was observed and analyzed by SEM, and the P-rich phase compositions were determined by SEM-EDS under different basicities. Combined with the IMCT thermodynamic calculation results and dephosphorization reaction of molten slag, the effect of dephosphorization slag basicity on phosphorus-enrichment behavior was clarified, which was verified by the experimental analysis results.
Industrial Experiments
New Double Slag Converter Steelmaking Process
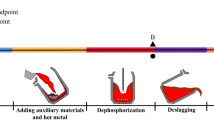
The industrial experiments were carried out with 180 ton top-bottom combined blowing converter in a steelmaking plant of China. Figure 1 shows the NDSP flow chart. The NDSP is mainly divided into eight steps: slag splashing protection → slag solidification confirmation → adding lime and scrap → pouring hot metal → dephosphorization stage → deslagging → decarburization stage → tapping and retaining decarburization slag. According to the silicon content of the hot metal, the quantitatively calculated amounts of auxiliary materials are added in the dephosphorization and decarbonization stages, respectively. Light burned dolomite is used as slag adjusting agent in slag splashing protection.
Industrial Experiment Parameters and Auxiliary Materials
Table I shows the consumptions of auxiliary materials for 180 ton top-bottom combined blowing converter. Lime is put into the bottom of furnace for preheating before dephosphorization stage, so as to improve the utilization rate of lime. The OG(Oxygen converter Gas recovery) slag pellets contain T.Fe of 55 pct, CaO of 12.4 pct and SiO2 of 3.8 pct, mainly including the mud produced by oxygen converter gas recovery.
The experimental converter capacity is 180 ton, the actual amount of hot metal added is 186 to 196 t, and the amount of scrap added is 29 to 33 t. Table II shows oxygen flow rates, blowing times, oxygen consumptions in dephosphorization stage and decarburization stage of 180 ton top-bottom combined blowing converter for the heat numbers of S1 to S6. Low-high-low lance position is used for dephosphorization stage blowing. The initial low lance position (1.7 to 1.8 m) promotes oxygen lance ignition, the subsequent high lance position (1.9 to 2.0 m) promotes rapid slagging in the early stage of dephosphorization, and the low lance position (1.65 to 1.75 m) in the later stage of dephosphorization promotes the agitation in dephosphorization. After the dephosphorization stage, the slag is poured out, and then lime, dolomite and other auxiliary materials are added for decarburization blowing. After decarburization, steel is tapped and decarburization slag is left for the dephosphorization of the next heat.
Compositions of the Initial Hot Metal, the Hot Metal at the Endpoint of Dephosphorization and Dephosphorization Slag
In the smelting process of steel grade Q235B, the hot metal after desulfurization pretreatment is used for dephosphorization. The initial compositions and temperatures of hot metal in 180 ton top-bottom combined blowing converter are shown in Table III.
After the dephosphorization stage, the slag samples were taken from inclined converter by sticking onto a long inserted iron bars, cooling in air and being collected at room temperature. The whole process is very short with about 1 to 2 minutes, so the cooling rate is above 700 °C/ min, especially at the high temperature range. Part of the slag samples were crushed by crusher and passed through a 200-mesh sieve to remove the residual iron particles. The compositions of slag samples were analyzed by M4 TORNADO fluorescence spectrometer of Bruker company in Germany. The temperatures were measured and the hot metal was sampled with a sub-gun. Then, the hot metal samples were analyzed by SPECTROMAXx direct reading spectrometer of German SPECTRO company. The basicities and chemical compositions of dephosphorization slag, chemical compositions and temperatures of hot metal at the endpoint of dephosphorization are shown in Table IV. The dephosphorization slag basicity, B, is calculated by binary basicity, which is expressed as Eq. [1]:
Figure 2 shows the dephosphorization slag compositions with different basicities in CaO-SiO2-FeO-8 pct MgO-8 pct MnO-4 pct P2O5 pseudo ternary phase diagram. The industrial experiments were carried out at the temperature between 1370 °C and 1420 °C. The liquidus projection sections of dephosphorization slag at 1370 °C and 1420 °C were drawn with FactSage8.0 using the database of phase diagram. The compositions of dephosphorization slags selected in the present work are marked in Figure 2. The compositions of six groups of dephosphorization slag all locate outside the liquidus projection area, which indicates that the dephosphorization slags should be in the multiphase state of solid-liquid coexistence.
The phase analysis of dephosphorization slags were carried out by D8 Advance X-ray powder diffractometer (XRD) of Bruker company in Germany. In the range of 2θ = 10 to 90 deg and step size of 0.04°s−1, XRD data were collected by Cu-Kα radiation. A small amount of massive dephosphorization slag was embedded in epoxy resin. Then it was ground and polished by automatic grinding and polishing machine of PRESI company in France. The surface was carbonized by magnetron sputtering MC1000 of HITACHI company in Japan. The morphology of P-rich phase of dephosphorization slags were observed by Zeiss EVO 18 electronic scanning microscope of Zeiss company of Germany, and the chemical compositions of P-rich phase in dephosphorization slags were analyzed by X-MaxN large area energy dispersive spectrometer (SEM-EDS) of Oxford Instruments Company in UK.
Thermodynamic Model IMCT-N i for Calculating Mass Action Concentrations of Structural Units Or Ion Couples In Cao-Sio2-Feo-Mgo-Mno-P2o5-Al2o3 Slags
Hypotheses
According to the classical hypothesis of IMCT summarized by Zhang et al.[25] and Yang et al.,[22] in the thermodynamic model IMCT-Ni for calculating the mass action concentration of structural unit or ion couple in the dephosphorization slag of CaO-SiO2-FeO-MgO-MnO-P2O5-Al2O3 which reacts with hot metal, the main assumptions can be summarized as follows:
-
In the dephosphorization slag in the NDSP, the structural units Ca2 +, Mg2 +, Fe2 +, Mn2 + and O2− exist as simple ions, SiO2, P2O5 and Al2O3 exist as simple molecules; silicate and phosphate are complex molecules. Each structural unit has its own position in the dephosphorization slag.
-
Each kind of cation and anion generated by the same component will participate in the chemical reaction of forming complex molecules in the form of (Me2 + + O2−) ion couple. The chemical reaction of forming complex molecule obeys the mass action law.
-
The structural units in dephosphorization slag which reacts with hot metal have continuity in the studied concentration range.
-
The reaction between simple ions and simple molecules to form complex molecules by bonding ion couples is in chemical dynamic equilibrium.
Selection of Structural Units in CaO-SiO2-FeO-MgO-MnO-P2O5-Al2 O 3 Dephosphorization Slag
At the endpoint of the dephosphorization stage in the NDSP, the dephosphorization slag containing phosphorus reacted with hot metal is chosen as CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5. According to the binary and ternary phase diagrams[25] of CaO-SiO2, MgO-SiO2, FeO-SiO2, MnO-SiO2, MgO-P2O5, CaO-P2O5, FeO-P2O5, MnO-P2O5, CaO-Al2O3, CaO-MgO-SiO2, CaO-Al2O3-MgO, etc., and other binary and ternary phase diagrams related to dephosphorization slag, it is found that there are 32 complex molecules in dephosphorization slag, such as CaO·SiO2, 3CaO·P2O5, 2FeO·SiO2. In Table V, all simple ions, simple molecules and complex molecules in CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 dephosphorization slag at 1370 °C to 1420 °C are summarized and assigned specific numbers.
In 100 g dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 , the initial mole numbers of seven components are expressed as \(a_{{\text{1}}} = n_{{{\text{CaO}}}}^{{\text{0}}}\), \(a_{{\text{2}}} = n_{{{\text{MnO}}}}^{{\text{0}}}\), \(a_{{\text{3}}} = n_{{{\text{MgO}}}}^{{\text{0}}}\), \(a_{{\text{4}}} = n_{{{\text{FeO}}}}^{{\text{0}}}\), \( b_{1} = n_{{{\text{SiO}}_{2} }}^{0} \), \( b_{2} = n_{{{\text{P}}_{2} {\text{O}}_{5} }}^{0} \), \( b_{3} = n_{{{\text{Al}}_{2} {\text{O}}_{3} }}^{0} \), respectively. The total equilibrium mole number ∑ni of all structural units can be expressed as Eq. [2].
According to the definition of mass action concentration Ni[18,19,20,21,22,23,24,25,26,27,28,29] of structural unit or ion couples in slag, Ni is the ratio of equilibrium mole number of structural unit i to the total equilibrium mole numbers ∑Ni of all structural units in slag with a fixed amount. The calculation formula of mass action concentration of structural unit i and ion couples (Me2 + + O2 -) in slag is expressed as Eqs. [3] and [4].[22,23,25]
Table V also lists the ion couples formed by simple ions in the dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5, as well as the equilibrium mole number ni of simple ions, simple and complex molecules and the corresponding mass action concentration Ni.
The chemical reaction for the formation of 32 complex molecules ci in the dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 are listed in Table VI, and the relationship between the variation of standard molar Gibbs free energy \(\Delta _{{\text{r}}} G_{{{\text{m,c}}i}}^{\Theta }\) and temperature T are also given. The expressions of the corresponding standard reaction equilibrium constants \(K_{{{\text{c}}i}}^{\Theta }\) are also listed in Table VI. In addition, the mass action concentrations Nci for all complex molecules expressed using N1 (\( N_{{{\text{CaO}}}} \)), N2 (\(N_ {\text{MnO}} \)), N3 (\(N_ {\text{MgO}} \)), N4 (\(N_{{\text{FeO}}}\)), N5 (\( N_{{{\text{SiO}}_{{\text{2}}} }} \)), N6 (\( N_{{{\text{P}}_{2} {\text{O}}_{5} }} \)), N7 (\(N_{{\text{Al}}2{\text{O}}3}\)) and \(K_{{{\text{c}}i}}^{\Theta }\) are summarized in Table VI.
Establishment of IMCT-Ni Thermodynamic Model for Calculating Mass Action Concentrations of Structural Units or Ion Couples in CaO-FeO-SiO2-MgO-MnO-Al2O3-P2 O 5 Dephosphorization Slag
With the dephosphorization reaction going on, the phosphorus in hot metal gradually enters the dephosphorization slag and partially removed at the end of dephosphorization stage. Combined with IMCT, Ni and \(\sum n_{i}\) in Tables V and VI, the mass conservation equations of seven components in 100 g dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 are established, which are expressed as Eqs. [5] through [11], respectively.
According to the mass conservation law, the sum of mole fractions of all structural units in the dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5 with fixed total mass under equilibrium condition is equal to 1.0, and Eq. [12] can be obtained.
Eqs. Eqs. [5] through [12] are the governing equations for calculating the mass action concentration Ni of structural units or ion couples in the dephosphorization slag of CaO-FeO-SiO2-MgO-MnO-Al2O3-P2O5. According to Eqs. [5] through [12], there are eight unknown parameters of N1, N2, N3, N4, N5, N6, N7 and ∑ni, combined with the eight independent governing equations built by IMCT. The mass action concentration Ni of simple components and the total equilibrium mole number ∑ni can be obtained, thus the mass action concentration Nci of complex components can be calculated, and the reaction ability of complex components can be further studied. The solution of the Eqs. (5-12) can be obtained by Jupyter Notebook in Anaconda, and the accuracy of the solution of nonlinear equations by calling Fsolve Function can reach to be smaller than 10−25. Therefore, it can be considered that the obtained Ni is very accurate.
Definition of Enrichment Possibility \(N_{{Ci-Pj}}\), Enrichment Degree \(R_{{Ci - Pj}}\) of Solid Solution Containing P2O5 and Phosphorus-Enrichment Contribution Ratio of Calcium Silicate \(R_{{Ci}}\)
Senlin Xie et al.[27] and Jingyan Li et al.[28,29] both studied the enrichment behavior of phosphorus in calcium silicate based on IMCT. P2O5 is mainly concentrated in calcium silicate complex molecules, so the solid phase rich in P2O5 can be regarded as the reaction product of calcium silicate and phosphate, independent of other structural units.[27] There are four kinds of calcium silicate molecules and seven kinds of phosphate in the dephosphorization slag in the present work. Therefore, the product of mass action concentration \(N_{{Ci}}\) of calcium silicate and \(N_{{Pj}}\) of containing P2O5 component can be used to describe the enrichment possibility \(N_{{Ci-Pj}}\) of solid solution containing P2O5 in dephosphorization slag,[27,28,29] as shown in Eq. [13]:
Ci includes CS, C2S, C3S, 3CaO·2SiO2(C3S2).
Pj includes 2CaO·P2O5(C2P), 3CaO·P2O5(C3P), 4CaO·P2O5(C4P), 3FeO·P2O5(F3P), 4FeO·P2O5(F4P), 2MgO·P2O5(M2P), 3MgO·P2O5(M3P).
The sum of the enrichment possibility \( N_{{Ci - Pj}} \) of the above-mentioned twenty-eight components containing P2O5 components is expressed by Eq. [14]:
Mi is the relative atomic or molecular mass of i. The ratio of \( N_{{Ci - Pj}} \frac{{M_{{{\text{P}}_{2} {\text{O}}_{5} }} }}{{M_{{Ci - Pj}} }} \) of a certain components to the sum of the enrichment possibility for all the components containing P2O5, \( \sum\limits_{\begin{subarray}{l} i = 1,2,3,4 \\ j = 1,2,3,4,5,6,7 \end{subarray} } {\left( {N_{{Ci - Pj}} \frac{{M_{{{\text{P}}_{{\text{2}}} {\text{O}}_{{\text{5}}} }} }}{{M_{{Ci - Pj}} }}} \right)} \), can be defined as the enrichment degree \(R_{{Ci - Pj}}\) of solid solution containing P2O5.[27,28,29] Taking C2S-C3P as an example, the enrichment degree \(R_{{{\text{C2S - C3P}}}}\) is expressed as Eq. [15]:
In order to describe the phosphorus-enrichment capacity of a certain calcium silicate in the dephosphorization slag, the sum of the enrichment degree \(R_{{Ci - Pj}}\) of all components containing P2O5 in a certain calcium silicate is defined as the phosphorus-enrichment contribution ratio \(R_{{Ci}}\) of calcium silicate.[27] Taking C2S as an example, the phosphorus-enrichment contribution ratio \(R_{{{\text{C2S}}}}\) can be expressed as Eq. [16]:
Analysis and Discussion of Hot Metal Samples and Dephosphorization Slag Samples from Industrial Experiments
Effect of Dephosphorization Slag Basicity on Element Removal Ratio in Hot Metal, Phosphorus Distribution Ratio Between Slag and Hot Metal
In industrial experiment, it is difficult to control all the other parameters to be constants for investigating the effect of dephosphorization slag basicity on hot metal dephosphorization. In the heats of industrial experiments selected in the present work, the dephosphorization endpoint temperatures were 1370 °C to 1420 °C. From the previous industrial experiment results,[5,9,30,31] the slagging effect of dephosphorization slag was favorable in this temperature range, and the thermodynamic conditions were conducive to dephosphorization. The FeO contents changed in a narrow range of 17.04 to 20.48 pct, which ensured a relatively close oxidation capacity. The contents of MgO, MnO and Al2O3 changed little, and these changes did not have a great impact on the dephosphorization results. The changes in the removal ratios of phosphorus, silicon, carbon and manganese in hot metal, phosphorus distribution ratio between dephosphorization slag and hot metal at the different dephosphorization slag basicities from 1.26 to 2.20 were studied.
Figure 3 shows the effect of dephosphorization slag basicity on removal ratios of elements in hot metal. \(\eta _{i}\) is the removal ratio of element i in hot metal, which is calculated by Eq. [17]. [pct i]0 represents the mass fraction of i in the hot metal at the initial point of dephosphorization and [pct i]e represents that at the endpoint of dephosphorization. The chemical reactions of dephosphorization, desilication, demanganization and decarbonization in hot metal are expressed as Eqs. [18] through [21].[32,33,34,35,36]
It can be seen from Figure 3 that with increasing dephosphorization slag basicity, the dephosphorization ratio and the decarbonization ratio both increases, and the demanganization ratio gradually decreases, while the desiliconization ratio does not change significantly and remains at a relatively high level. With increasing dephosphorization slag basicity, the activity of CaO in slag increases, and the calcium silicate is easier to form in the slag, which is conducive to the formation of C2S-C3P solid solution. According to the chemical reaction of Eq. [18], increasing the dephosphorization slag basicity is beneficial to reduce the activity of P2O5 in the slag, so as to improve the phosphorus distribution ratio between slag and hot metal, and further improve the dephosphorization ratio.
With increasing dephosphorization slag basicity, the oxygen potential at the interface between slag and hot metal is increased, and the decarburization reaction is also promoted. However, the decarburization reaction is weak in the dephosphorization stage, and the decarburization ratio is from 28.46 to 33.95 pct. In the dephosphorization stage, the combination ability of silicon and manganese with oxygen is stronger than that of phosphorus, so that silicon and manganese will be oxidized prior to phosphorus. With increasing dephosphorization slag basicity, the activity of SiO2 in the slag decreases, which is conducive to the desilication reaction. The desilication ratio at the end of dephosphorization is always above 94 pct, so that silicon in hot metal is removed to trace at the end of dephosphorization stage. The increase of basicity leads to the existence of a large amount of free CaO in the dephosphorization slag. CaO will replace the MnO in 2MnO·SiO2 to generate the stabler C2S,[36] thus increasing the activity of MnO. This will lead to the occurrence of remanganization reaction, reducing the demanganization ratio. When the dephosphorization slag basicity is 2.20, the dephosphorization ratio can reach 66.20 pct. The desilication ratio, demanganization ratio and the decarburization ratio are 94.22, 35.65 and 33.95 pct, respectively.
Figure 4 shows the effect of dephosphorization slag basicity on P2O5 content in dephosphorization slag, phosphorus content in hot metal and phosphorus distribution ratio LP. Phosphorus distribution ratio LP is calculated by Eq. [22].
With increasing the dephosphorization slag basicity, the phosphorus distribution ratio LP increases, the content of P2O5 in dephosphorization slag increases as a whole and the phosphorus content in hot metal decreases. When the dephosphorization slag basicity is 1.90, the P2O5 content in the slag can reach 5.10 pct. When the dephosphorization slag basicity is further increased to 2.20, the phosphorus distribution ratio increases to 81.3 and the phosphorus content in hot metal decreases to 0.048 pct. The increase of dephosphorization slag basicity improves the apparent diffusion coefficient of phosphorus in solid solution,[37] which makes the phosphorus in hot metal more easily fixed in C2S-C3P solid solution. This increases the content of P2O5 in slag and reduces the phosphorus content in hot metal.
XRD Analysis of Dephosphorization Slag Under Different Basicities
Figure 5 shows the XRD analysis results of dephosphorization slag with basicities of 1.26 to 2.20. At the endpoint of the dephosphorization stage in the NDSP, the dephosphorization slag mainly contains dicalcium silicate Ca2SiO4(C2S), silicate phase Ca3Mg(SiO4)2, calcium ferrite phase Ca2Fe2O5, the Ca(FexMgy)(SiO4)(x+y=1) mixture of Ca3Mg(SiO4)2 and Ca2Fe2O5, metal oxide phase RO and the solid solution of (Ca2(SiO4))6(Ca3(PO4)2)(C2S-6C3P) and Ca15(PO4)2(SiO4)6(6C2S-C3P) containing phosphorus.
By comparing the XRD results of dephosphorization slags with different basicites, it is found that the mixed phase of Ca(FexMgy)(SiO4)(x + y = 1) is widely existed in dephosphorization slag when basicity is 1.26 to 1.37, and there is no oxide phase and the solid solution phase containing phosphorus. Combined with the dephosphorization results in Figure 3 and our previous mineralogical analysis[9] of the dephosphorization slag under low basicity, a reasonable explanation can be obtained. Because there is a large amount of liquid slag matrix phase in the slag at lower basicity, phosphorus in hot metal is difficult to be effectively fixed in dephosphorization slag. Large-area P-rich phase in the dephosphorization slag is hard to form, which leads to the low dephosphorization ratio at lower basicity.
When the basicity range of dephosphorization slag is 1.37 to 1.71, C2S, Ca3Mg(SiO4)2, Ca2Fe2O5 and RO are mainly detected in the dephosphorization slag, which indicates that the dephosphorization slag changes from liquid slag matrix phase to silicate and calcium ferrite. However, the solid solution containing phosphorus has not been detected by XRD in this range. When the basicity range of dephosphorization slag is further increased to 1.90 to 2.20, the diffraction peaks of C2S-6C3P and 6C2S-C3P phases appear, which is consistent with the higher dephosphorization ratio as shown in Figure 3.
Analysis of P-Rich Phase Morphologies and Areas in Dephosphorization Slag at Different Basicities by SEM-EDS
The typical morphologies, areas and map scanning results of P-rich phase in dephosphorization slag at different basicities are shown in Figure 6. When the dephosphorization slag basicity is in the range of 1.26 to 1.37, the P-rich phases are in long strip shape, and their area is between 4 and 36 μm2. The content of P2O5 in the P-rich phase is less than 10 pct and it contains high contents of Ca and Si. The map scanning result is not obvious for the identification of phosphorus element in each phase. Therefore, it is inferred that the solid solution containing phosphorus is not formed in the basicity range of 1.26 to 1.37.
When the dephosphorization slag basicity is 1.50, the P-rich phases are dispersed into several small block P-rich phases, which are mainly distributed in dendrite shape, and their areas are mainly concentrated in 8 to 25 μm2. When the dephosphorization slag basicity is further increased to the range of 1.71 to 2.20, the dispersed small block P-rich phase grows rapidly, irregular blocks gradually grow into the blocks with smooth edge, and the area of P-rich phase grows from 142 μm2 at basicity of 1.71 to 8000 μm2 at the basicity of 2.20. It can be seen from Figure 6 that the Ca, Si and P are mainly concentrated in the P-rich phase. Therefore, according to the results of Figures 5 and 6, the solid solution containing phosphorus is formed in the basicity range of 1.90 to 2.20.
Analysis of P-Rich Phase at Different Basicities
In order to determine the existing form of phosphorus containing solid solution (CiS-CjP) in P-rich phase of dephosphorization slag, five groups of P-rich phase composition data were obtained by SEM-EDS analysis under each basicity, and the results were averaged to reduce the element content differences caused by different points in the P-rich phase. Because phosphorus mainly exists in the form of C3P in the solid solution,[16,17] and the sum of the masses of CaO, SiO2 and P2O5 in the P-rich phase is equal to the sum of CiS and C3P, the average mass fraction of CiS and C3P can be calculated. According to the mass fraction ratio of CaO and SiO2 in the P-rich phase, it can be seen that the calcium silicate existing in the P-rich phase is mainly the mixture of CS and C2S. Table VII shows the average mass fractions of different compositions in P-rich phase of dephosphorization slag and the average mass fractions of C3P and CiS at the different basicities.
Figure 7(a) shows the Gibbs free energy changes of CiS(i=1, 2) and CjP(j=1,2,3) under different basicities of dephosphorization slag, Figure 7(b) shows the effect of dephosphorization slag basicity on C3P content, basicity, CiS content in P-rich phase of dephosphorization slag and Figure 7(c) shows the determination of coefficient n in nC2S-C3P of the P-rich phase at different basicities. The Gibbs free energy of each substance in Figure 7(a) is calculated according to Table VI. With increasing dephosphorization slag basicity, the content of C3P and basicity of P-rich phase increase, the content of CiS decreases as a whole. The basicity in P-rich phase increases from 1.21 to 1.97, which corresponds to the actual basicity change of dephosphorization slag. It also shows that calcium silicate in P-rich phase is a mixture of CS and C2S. By comparing the free energy of formation of CiS(i = 1, 2) and CjP(j = 1,2,3) at different basicities, CiS is stabler than CjP at 1370 °C to 1420 °C, and the combination ability of CaO with P2O5 is stronger than that with SiO2.[38,39,40] With the increase of P2O5 content in the P-rich phase, CaO will first combine with P2O5 to form C3P. Therefore, with increasing dephosphorization slag basicity, the content of C3P increases and the content of CiS decreases in P-rich phase.
(a) Gibbs free energy changes of CiS (i=1,2) and CjP (j=1,2,3) under different basicities of dephosphorization slag; (b) Effect of dephosphorization slag basicity on C3P content, basicity, CiS content in P-rich phase of dephosphorization slag; (c) Determination of coefficient n in nC2S-C3P of the P-rich phase at different basicities
The phosphorus containing solid solution in dephosphorization slag may exist in the form of C2S-C3P, 2C2S-C3P, 4.8C2S-C3P, 6C2S-C3P and 20C2S-C3P.[30] Therefore, taking nC2S-C3P (n = 1, 2, 4.8, 6, 20) as the judgment standard, the coefficient n in nC2S-C3P of P-rich phase under different basicities is roughly determined, as shown in Figure 7(c). When the dephosphorization slag basicity is 1.26 to 1.37, the values of n are 6 to 20. When the basicity is 1.50 to 1.90, the values of n are 2 to 4.8. When the basicity further increases to 2.20, the values of n are 1 to 2. The results show that with increasing the basicity, the coefficient n of nC2S-C3P presents a decreasing trend, which indicates that the phosphorus content in nC2S-C3P increases gradually with increasing basicity, and the phosphorus-enrichment ability of the dephosphorization slag increases.
Analysis and Discussion of IMCT Thermodynamic Calcuation Results
Effect of Dephosphorization Slag Basicity on Mass Action Concentration Ni of Components in Dephosphorization Slag
According to the IMCT-Ni thermodynamic calculation model, the mass action concentration Ni of each component in Table VI was calculated. The mass action concentration Ni in IMCT can be used to characterize the reaction capacity of the composition like the activity ai in molecular theory.[18,19,20,21,25] In order to study the relationship between mass action concentration Ni and the basicity of dephosphorization slag, the best fitting method was selected according to the data changing rule, and the goodness of fit was expressed by the fitting coefficient r2.
In order to analyze the phosphorus-enrichment capacity of calcium silicate in dephosphorization slag, the effect of dephosphorization slag basicity on mass action concentration of CaO , SiO2 and the their ratio are analyzed. Figure 8 shows the effect of dephosphorization slag basicity on \( N_{\text{CaO}}\), \( N_{{{\text{SiO}}_{2} }} \) and \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \). With increasing dephosphorization slag basicity, \( N_{{{\text{CaO}}}} \) increases exponentially and \( N_{{{\text{SiO}}_{2} }} \) decreases exponentially. The fitting coefficient r2 of both is greater than 0.97, which indicates that there is a good mathematical relationship between the dephosphorization slag basicity and \( N_{{{\text{CaO}}}} \) and \( N_{{{\text{SiO}}_{2} }} \).
With increasing dephosphorization slag basicity, \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \) increases exponentially, and its fitting coefficient is greater than 0.999, reaching the fitting convergence. The results show that both basicities of B and \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \) have the similar effect in dephosphorization slag at temperature of 1370 °C to 1420 °C and basicity of 1.26 to 2.20. The relationship between B and \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \) is Eq. [23]. It should be noted that the range of \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \) has large values of 4 to 1202, so that Eq. [23] can only reflect the their change trends.
Four kinds of calcium silicate complex molecules CS, C2S, C3S, C3S2 formed by CaO and SiO2 are further considered. The effect of dephosphorization slag basicity on the mass action concentration of calcium silicates were analyzed under the conditions of lower temperature range of 1370 °C to 1420 °C and lower basicity range of 1.26 to 2.20.
Figure 9 shows the effect of dephosphorization slag basicity on the mass action concentration of calcium silicates. With increasing dephosphorization slag basicity, \(N_{{{\text{CS}}}}\) decreases exponentially, \(N_{{{\text{C2S}}}}\) increases first and then decreases, \(N_{{{\text{C3S}}}}\) increases exponentially and \(N_{{{\text{C3S2}}}}\) shows a decreasing trend as a whole. The variation trend of \(N_{{{\text{CS}}}}\), \(N_{{{\text{C2S}}}}\) and \(N_{{{\text{C3S}}}}\) is consistent with the results obtained by Jingyan Li et al.[28] At the conditions of CaO-15 pct SiO2-FeO-Fe2O3-5 pct P2O5 slag, 1450 °C to 1600 °C, basicity of 1.8 to 3.7. The increase of dephosphorization slag basicity can reduce \(N_{{{\text{C3S2}}}}\), and there is no obvious mathematical relationship between them.
Figure 9 shows that \(N_{{{\text{CS}}}}\) and \(N_{{{\text{C2S}}}}\) are one order of magnitude higher than \(N_{{{\text{C3S}}}}\) and \(N_{{{\text{C3S2}}}}\), indicating that the former two have strong reaction ability in the dephosphorization slag, being the main components in the dephosphorization slag. Between \(N_{{{\text{CS}}}}\) and \(N_{{{\text{C2S}}}}\), the value of \(N_{{{\text{C2S}}}}\) is always higher than that of \(N_{{{\text{CS}}}}\), which indicates that C2S has the strongest reaction ability in the dephosphorization slag, and the phosphorus in hot metal is easier to combine with C2S to form phosphorus containing solid solution.
With increasing dephosphorization slag basicity from 1.26 to 2.20, it is calculated that \(N_{{{\text{C2P}}}}\) varies from 7.52 × 10−7 to 2.88×10−6, \(N_{{{\text{C3P}}}}\) varies from 0.012 to 0.026, and \(N_{{{\text{C4P}}}}\) varies from 2.14 × 10−5 to 2.20×10−4. In addition, the variation range of \(N_{{{\text{F3P}}}}\) is 2.57 × 10−14 to 3.29 × 10−12, the variation range of \(N_{{{\text{F4P}}}}\) is 4.32 × 10−14 to 6.90 × 10−12, the variation range of \(N_{{{\text{M2P}}}}\) ranging from 3.13 × 10−10 to 8.43 × 10−9 and that of \(N_{{{\text{M3P}}}}\) from 3.58 × 10−9 to 5.82 × 10−8. Considering the above range value, the variation range of \(N_{{{\text{C2P}}}}\), \(N_{{{\text{C4P}}}}\), \(N_{{{\text{F3P}}}}\), \(N_{{{\text{F4P}}}}\), \(N_{{{\text{M2P}}}}\) and \(N_{{{\text{M3P}}}}\) is less than 10−4 in the dephosphorization slag with the basicity from 1.26 to 2.20, so it is reasonable to ignore the effect of the above components on the phosphorus-enrichment effect. According to the above description, in the low temperature range of 1370 °C to 1420 °C and the low basicity range of 1.26 to 2.20, the P2O5 containing components in the dephosphorization slag mainly combined with calcium silicate are C3P.
Effect of Dephosphorization Slag Basicity on Phosphorus-Enrichment Contribution Ratio \(R_{{Ci}}\) of Calcium Silicate
The phosphorus-enrichment contribution ratio \(R_{{Ci}}\) can well indicate the enrichment degree of P2O5 containing solid solution by different calcium silicate in slag.[27] According to Eq. [16], the phosphorus-enrichment contribution ratios of four kinds of calcium silicate of \(R_{{{\text{CS}}}}\), \(R_{{{\text{C2S}}}}\), \(R_{{{\text{C3S}}}}\) and \(R_{{{\text{C3S2}}}}\) are calculated. The effect of dephosphorization slag basicity on phosphorus-enrichment capacity of different calcium silicate in dephosphorization slag is further studied under the conditions of low temperature range of 1370 to 1420 °C and low basicity range of 1.26 to 2.20.
Figure 10 shows the changes in the phosphorus-enrichment contribution ratio \(R_{{Ci}}\) under different basicities of dephosphorization slags. From the proportion of different strip areas in Figure 10(a), it can be seen that the phosphorus-enrichment contribution ratio of \(R_{{{\text{C2S}}}}\) is the largest. With increasing dephosphorization slag basicity from 1.26 to 2.20, the \(R_{{{\text{C2S}}}}\) increases from 0.56 to 0.84, the \(R_{{{\text{CS}}}}\) significantly reduces, and the value of \(R_{{{\text{CS}}}}\) decreases from 0.41 to 0.11, while the proportions of \(R_{{{\text{C3S}}}}\) and \(R_{{{\text{C3S2}}}}\) have very small values. This indicates that with increasing dephosphorization slag basicity, phosphorus tends to combine with C2S to be enriched. It can be seen from Figure 10(b) and (c) that the change trend of \(R_{{{\text{C2S}}}}\) is the same as dephosphorization ratio, but the change trend of \(R_{{{\text{CS}}}}\) is opposite to dephosphorization ratio. Therefore, the increase of dephosphorization slag basicity mainly will increase the phosphorus-enrichment capacity of C2S and decrease the phosphorus-enrichment capacity of CS.
Changes in the phosphorus-enrichment contribution ratio \(R_{{Ci}}\) under different basicities of dephosphorization slags. (a) Effect of dephosphorization slag basicity to the phosphorus-enrichment contribution ratio \(R_{{Ci}}\); Effect of dephosphorization slag basicity on (b) \(R_{{{\text{CS}}}}\), (c) \(R_{{{\text{C2S}}}}\), (d) \(R_{{{\text{C3S}}}}\), (e) \(R_{{{\text{C3S2}}}}\)
The mathematical relationship between dephosphorization slag basicity and phosphorus-enrichment contribution ratio of calcium silicate are further studied. Figure 10(b-e) shows that with increasing dephosphorization slag basicity, \(R_{{{\text{CS}}}}\) and \(R_{{{\text{C3S2}}}}\) decrease exponentially, \(R_{{{\text{C2S}}}}\) and \(R_{{{\text{C3S}}}}\) increase exponentially. The regression coefficients, r2, of \(R_{{{\text{CS}}}}\), \(R_{{{\text{C2S}}}}\) and \(R_{{{\text{C3S}}}}\) against dephosphorization slag basicity are greater than 0.99. There is an obvious exponential relationship between dephosphorization slag basicity and \(R_{{{\text{CS}}}}\), \(R_{{{\text{C2S}}}}\) and \(R_{{{\text{C3S}}}}\), which can be expressed by Eqs. [24] through [26]:
Effect of Dephosphorization Slag Basicity on Phosphorus-Enrichment Behavior
It has been shown that C2S is the main phosphorus-enrichment component in the dephosphorization slag. Therefore, the phosphorus-enrichment behavior of C2S in the dephosphorization slag can represent the phosphorus-enrichment behavior in the dephosphorization slag. Based on the enrichment degree \(R_{{Ci - Pj}}\) of P2O5 containing solid solution, the effect of the dephosphorization slag basicity on the phosphorus-enrichment behavior of C2S in dephosphorization slag was analyzed under the conditions of low temperature range of 1370 °C to 1420 °C and low basicity range of 1.26 to 2.20.
In the dephosphorization reaction of slag, CaO reacts with SiO2 to generate C2S. FeO and CaO react with P2O5 to form F3P and C3P, respectively, which initially fixes phosphorus in dephosphorization slag.[41] Further, P2O5 in F3P is easy to react with CaO to form more stable C3P,[27] the chemical reaction is expressed as Eq. [27]. According to the standard Gibbs free energy of the above reactions summarized in Table VI, the actual Gibbs free energy of F3P reacting with CaO to produce C3P is derived, which is expressed as Eq. [28]. The substituted FeO diffuses into CaO solid through the reaction interface and forms CaO-FeO layer.
Under the conditions of lower temperature and lower basicity in the dephosphorization stage of the NDSP, the existence state of C2S is α’H-C2S state,[42] and α’H-C2S mainly exists in the form of 2Ca2+ and [SiO4]4-. C3P mainly exists in the form of 3Ca2+ and 2[PO4]3-. The radii of [SiO4]4- and [PO4]3- are 2.79 Å and 2.76 Å, respectively, and their crystal structures are tetrahedral.[43] This shows that the structure and size of [SiO4]4- and [PO4]3- are very similar and can replace each. In the range of 1370 °C to 1420 °C, the Gibbs free energy of the reaction of CaO with P2O5 to produce C3P is much smaller than that of the reaction of CaO with SiO2 to produce C2S. The affinity of CaO with P2O5 is much stronger than that with SiO2. P can replace part of Si in the tetrahedral structure, resulting in the change of its coordination structure, which shows that [PO4]3- replaces [SiO4]4- in the solvent C2S lattice.
Furthermore, \( \Delta {\text{G}}_{{{\text{F3P}} - {\text{C3P}}}} \) is always less than 0 in the NDSP. C2S-F3P can not exist stably, and F3P will spontaneously transform into C3P. Therefore, it can be considered that the formation of C2S-C3P solid solution is through the replacement of [SiO4]4- in C2S phase by [PO4]3- in slag, or transformation of the unstable C2S-F3P phase into the stable C2S-C3P.
Figure 11 shows the effect of dephosphorization slag basicity on \(R_{{{\text{C2S - C3P}}}}\), \(R_{{{\text{C2S - C4P}}}}\) and \(R_{{{\text{C2S - F3P}}}}\). With increasing dephosphorization slag basicity, \(R_{{{\text{C2S - C3P}}}}\), \(R_{{{\text{C2S - C4P}}}}\) both increase exponentially, while \(R_{{{\text{C2S - F3P}}}}\) decreases exponentially. This is because the increase of basicity will promote the formation of C2S-C3P solid solution in slag, and the coefficient n of nC2S-C3P solid solution will decrease with increasing basicity, thus improving the enrichment degree of phosphorus in C2S-C3P solid solution in dephosphorization slag. In addition, under the conditions of lower temperature and lower basicity in the dephosphorization stage of the NDSP, F3P spontaneously transforms to C3P, and with increasing dephosphorization slag basicity, this change trend will become more obvious. Therefore, it can be concluded that increasing dephosphorization slag basicity is beneficial to the enhancement of phosphorus-enrichment degree in dephosphorization slag, and the phosphorus-enrichment ability of C2S-C3P and C2S-C4P will be significantly improved.
Comparison Between the Measurement Results of Industrial Experiment and the Calculated Results of IMCT
In Section IV–C, the mass fraction of CaO, SiO2 and P2O5 in the P-rich phase is analyzed by SEM-EDS. The sum of the mass fraction of CaO, SiO2 and P2O5 represents the mass fraction of phosphorus containing solid solution in the P-rich phase, expressed as (pct CiS-CjP). According to the conclusion in Section V–C, C2S is the main component of phosphorus enrichment in the dephosphorization slag. Therefore, the product of the mass fraction of phosphorus containing solid solutions (pct CiS-CjP) and the phosphorus-enrichment contribution ratio \(R_{{{\text{C2S}}}}\) of C2S is regarded as the mass fraction of C2S-CjP solid solution in P-rich phase(IMCT-(pct C2S-CjP)), which is calculated by Eq. [29]. The areas of P-rich phase in dephosphorization slag with different basicities can be calculated using Image Pro Plus software under three viewing fields of SEM at the magnification of 1500 times, and Eq. [30] is used to calculate the average area fraction of P-rich phase(AP-rich phase). The calculated results of IMCT-(pct C2S-CjP) and \(R_{{{\text{C2S}}}}\) can illustrate the phosphorus-enrichment degree. Both the calculation results are compared with the measurement results of AP-rich phase, and (pct P2O5)P-rich phase to verify the rationality of the IMCT calculation results.
Figure 12 shows the comparison between the calculated results of IMCT-(pct C2S-CjP) and \(R_{{{\text{C}_{2}{\text{S}}}}}\), and the measurement results of AP-rich phase, and (pct P2O5)P-rich phase of industrial experiment. With increasing dephosphorization slag basicity, IMCT calculated results and experimental results are all increased. The trends of the enrichment degree of phosphorus in dephosphorization slag are well consistent among the results characterized by different ways. Therefore, the IMCT calculated results can correctly express the phosphorus-enrichment degree of dephosphorization slag, and further reveal the phosphorus-enrichment degree of the different calcium silicates in dephosphorization slag.
Conclusions
In the percent work, the dephosphorization experiments using new double slag converter steelmaking process(NDSP) has been carried out in a 180 ton top-bottom combined blowing converter under the low temperature range of 1370 °C to 1420 °C and low basicity of 1.26 to 2.20. Based on the ion-molecule coexistence theory (IMCT) and phase analysis of dephosphorization slag, the effect of dephosphorization slag basicity on the hot metal dephosphorization and the phosphorus-enrichment behavior in slag is studied, and the following conclusions are obtained:
-
1.
With increasing basicity, the phosphorus distribution ratio LP between hot metal and slag increases. The dephosphorization ratio and the decarbonization ratio both increase, while the demanganization ratio decreases. The desiliconization ratio has no obvious change and remains at a high level. When the basicity is 2.20, the phosphorus content in hot metal decreases from 0.142 to 0.048 pct, the dephosphorization ratio increases to 66.2 pct and the phosphorus distribution ratio increases to 81.33.
-
2.
With increasing basicity from 1.26 to 2.20, the morphologies of P-rich phase change from long strip shape (B = 1.26 to 1.37) to dendritic shape (B = 1.50) and to massive shape(B = 1.71 to 2.20). The area of P-rich phase increases from about 4 μm2 to about 8000 μm2. The content of P2O5 in the P-rich phase increases and the value of the coefficient n in nC2S-C3P of P-rich phase decreases from 6-20 to 1-2.
-
3.
With increasing basicity, \( N_{\text{CaO}}\) and \( N_{{{\text{SiO}}_{2} }} \) increases and decreases exponentially, respectively, and \( N_{{{\text{CaO}}}} /N_{{{\text{SiO}}_{2} }} \) also increases exponentially. The phosphorus-enrichment contribution ratio of calcium silicate is in the order of \(R_{{{\text{C2S}}}}\)>\(R_{{{\text{CS}}}}\)>\(R_{{{\text{C3S}}}}\)>\(R_{{{\text{C3S2}}}}\). C2S is the main phosphorus-enrichment calcium silicate in dephosphorization slag, and \(R_{{{\text{C2S}}}}\) has the value of 0.56 to 0.84. The phosphorus-enrichment degree in dephosphorization slag is enhanced mainly by C2S-C3P.
-
4.
In the dephosphorization stage of the NDSP, C2S is in the existence state of α’H-C2S state. The formation of C2S-C3P solid solution is through the replacement of [SiO4]4- in C2S phase by [PO4]3- in slag, or transformation of the unstable C2S-F3P phase into the stable C2S-C3P.
-
5.
With increasing basicity, the calculated results of IMCT-(pct C2S-CjP) and \(R_{{{\text{C2S}}}}\) are well consistent with the measurement results of AP-rich phase and (pct P2O5)P-rich phase of industrial experiment, indicating that the IMCT calculated results can correctly express the phosphorus-enrichment degree of dephosphorization slag. It is possible to use the enrichment contribution ratio \(R_{{{\text{C}}i}}\) to qualitatively predict the phosphorus-enrichment degree of calcium silicate in dephosphorization slag.
-
6.
From the present results, it is reasonable to deduce that the method combining laboratory phase analysis and IMCT thermodynamic calculation can facilitate optimizing the dephosphorization process parameters of NDSP under low temperature and low basicity, which has a guiding significance for industrial production.
Abbreviations
- a i, b i :
-
Initial mole numbers of component i in 100 g dephosphorization slag, (mol)
- ∑n i :
-
Total equilibrium moles of all structural units in 100 g dephosphorization slag based on IMCT, (mol)
- N i :
-
Mass action concentration of structural unit i or ion couple i in dephosphorization slag based on IMCT, (−)
- \(\Delta _{{\text{r}}} G_{{{\text{m,}}ci}}^{\Theta }\) :
-
Change of standard molar Gibbs free energy of reaction for structural unit i or component i, (J/mol)
- \(K_{{ci}}^{\Theta }\) :
-
Standard equilibrium constants of chemical reactions for forming component i or structural unit i, (−)
- M i :
-
Relative atomic mass of element i or the relative molecular mass of component i, (−)
- \(N_{{Ci-Pj}}\) :
-
Defined-enrichment possibility of containing P2O5 solid solution based on the calculated mass action concentration Ni of complex molecule Ci and Pj, (−)
- \(R_{{Ci - Pj}}\) :
-
Enrichment degree of solid solution containing P2O5 based on IMCT, (−)
- \(R_{{Ci}}\) :
-
Phosphorus-enrichment contribution ratio of calcium silicate based on IMCT, (−)
- \(\eta _{{\text{i}}}\) :
-
Removal ratio of elements in hot metal, (pct)
- T :
-
Temperature, (°C)
- B:
-
Binary basicity, (−)
- r2 :
-
Fitting regression coefficient, (−)
- \(n{\text{(}}i{\text{)}}^{{{\text{ P - rich phase}}}}\) :
-
Mole number of i in P-rich phase, (mol)
- \( \Delta {\text{G}}_{{{\text{A{-}B}}}} \) :
-
Actual Gibbs free energy of A to B reaction, (J)
- IMCT-(pct C2S-CjP):
-
Mass fraction of C2S-CiP solid solution in P-rich phase by IMCT, (pct)
- AP-rich phase :
-
Average area fraction of P-rich phase at 1500 times under 3 fields of view, (pct)
References
Y. Ogawa, M. Yano, S. Y. Kitamura and H. Hirata: Tetsu-to-Hagané., 2001, vol. 87 (1), pp. 21-28.
Y. Ogawa, M. Yano, S. Y. Kitamura and H. Hirata: Steel Res. Int., 2003, vol. 74 (2), pp. 70-76.
N. Sasaki, Y. Ogawa, S. Mukawa and K. I. Miyamoto: Nippon Steel Tech. Rep., 2012, vol. 394, pp. 26-32.
Y. Wang, S. F. Yang, J. S. Li, J. Feng and F. Wang: High Temp. Mater. Proc., 2018, vol. 37(7), pp. 625-33.
W. K. Yang, J. Yang, Y. Q. Shi, Z. J. Yang, F. B. Gao, R. H. Zhang and G. F. Ye: Ironmaking Steelmaking., 2020. https://doi.org/10.1080/03019233.2020.1731256.
Y. R. Fang, B. C. Huang, Z. Y. Lai, B. Y. Chen and X. F. Zeng: Steelmaking., 2014, vol. 30(3), pp. 1-4.
F. Y. Liu, G. C. Wang, Y. Zhao, J. W. Tan, C. M. Zhao and Q. Wang: Ironmak. Steelmak.., 2019, vol. 46(4), pp. 392-403.
M. L. Wang and W. Y. Yang: Ironmak. Steelmak., 2019. https://doi.org/10.1080/03019233.2019.1673546.
G. F. Ye, J. Yang, R, H, Zhang, W. K. Yang and H. Sun: Int. J. Miner. Metall. Mater., 2021, vol. 28(1), pp. 66-75.
S. L. Xie and W. L. Wang: Steel Res. Int., 2016, vol. 87(3), pp. 376-85.
S. L. Xie, W. L. Wang, Z. C. Luo and D. Y. Huang: Metall. Mater. Trans. B., 2016, vol. 47B, pp. 1583-93.
S. Kakimoto, A. Kiyose and R. Murao: ISIJ Int., 2017, vol. 57(10), pp. 1710-17.
C. M. Du, N. N. Lv, C. Su, W. M. Liu, J. X. Yang and H. C. Wang: J. Iron Steel Res. Int., 2019, vol. 26, pp. 1162-70.
P. K. Son and Y. Kashiwaya: ISIJ Int., 2008, vol. 48(9), pp. 1165-74.
S. Fukagai, T. Hamano and F. Tsukihashi: ISIJ Int., 2010, vol. 47(1), pp. 187-89.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46(2), pp. 174-79.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46(2), pp. 180-87.
X. C. Ma, G. G. Cheng and J. Zhang: J. Univ. Sci. Technol. Beijing., 2011, vol. 33(11), pp. 1337-40.
G. G. Cheng and J. Zhang: Iron Steel Vanadium Titanium, 1994, vol. 15(3), pp. 1-2.
G. G. Cheng and J. Zhang: Iron Steel Vanadium Titanium, 1994, vol. 15(2), pp. 1-4.
G. G. Cheng and P. Zhao: J. Univ. Sci. Technol. Beijing., 1995, vol. 17, pp. 52-56.
X. M. Yang, J. P. Duan, C. B. Shi, M. Zhang, Y. L. Zhang and J. C. Wang: Metall. Mater. Trans. B., 2011, vol. 42B, pp. 738-70.
X. M. Yang, C. B. Shi, M. Zhang, J. P. Duan and J. Zhang: Metall. Mater. Trans. B., 2011, vol. 42B, pp. 951-76.
N. M. Chuiko: Ferrous Met., 1959, vol. 5, pp. 3-10.
J. Zhang: Computational Thermodynamics of Metallurgical Melt and Solutions, Metallurgical Industry Press, Beijing, China, 2007.
P. C. Li,X. M. Yang and J. Zhang: J. Univ. Sci. Technol. Beijing., 2014, vol. 36(12), pp. 1608-14.
S. L. Xie, W. L. Wang, D. Y. Huang, H. C. Li and Y. Du: Steel Res. Int., 2018, vol. 89, pp. 1700317.
J. Y. Li, M. Zhang, M. Guo and X. M. Yang: Metall. Mater. Trans. B., 2014, vol. 45B, pp. 1666-82.
J. Y. Li, M. Zhang, M. Guo and X. M. Yang: Int. J. Miner. Metall. Mater., 2016, vol. 23(5), pp. 520-33.
W. K. Yang, J. Yang, Y. Q. Shi, Z. J. Yang, F. B. Gao, R. H. Zhang and G. F. Ye: Steel Res. Int., 2021, vol. 92. https://doi.org/10.1002/srin.202000438.
W.K. Yang, J. Yang, Y.Q. Shi, Z.J. Yang, F.B. Gao, R.H. Zhang and H. Sun: Metals, 2021, vol. 11. https://doi.org/10.3390/met11030417.
X. Han, C.G. Zhou, J. Li, C.B. Shi, J.P. Ge and K.S. Cai: J. Iron Steel Res., 2016, vol. 28(9), pp. 40-49.
W.L. Dong, H.W. Pan, L. Luo, C.X. Ji, H.B. Li and Z.H. Tian: Iron and Steel, 2020, vol. 55(2), pp. 75-81, 94.
X. H. Huang: Principles of Iron and Steel Metallurgy, Metallurgical Industry Press, Beijing, China, 2002.
W. Xiong, J.Wang and F. Yang: Mining Metallurgy, 2013, vol. 22(4), pp. 47-50.
C. Li, H. J. Guo and G. G. Cheng: Special Steel, 2009, vol. 30(5), pp. 4-6.
Y. Liu, S. H. Peng, Y. J. Xia and X. B. Zhou: J. Univ. Sci. Technol. Anhui., 2019, vol. 36(1), pp. 7-12.
D. Z. Wang, Y. P. Bao and M. Wang: Chinese Journal of Engineering, 2018, vol. 40(1), pp. 65-72.
M. Timucin and A. Muan. J. Am. Cream. Soc., 1922, vol. 75, pp. 1399-1406.
I. Barin, O. Knacke and O. Kubaschewski. Thermochemical Properties of Inorganic Substances. Springer, Berlin. 1977.
S. Y. Kitamura, S. Saito, K. Utagawa, H. Shibata and D. G. C. Robertson: ISIJ Int., 2009, vol. 49(12), pp. 1838-44.
W. S. Zhang, J. T. Zhang, J. Y. Ye, J. S. Qian, W. G. Shen and Z. Y. Wang: J. Chin. Ceram. Soc. 2019, vol. 47(11), pp. 1664-69.
B. Dikens and W. E. Brown: Tsehermaks Min. Petr. Mitt., 1971, vol. 16, pp. 1-27.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (U1960202).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted 9 April 2021; accepted 23 June 2021.
Rights and permissions
About this article
Cite this article
Sun, H., Yang, J., Zhang, RH. et al. Effect of Slag Basicity on Dephosphorization at Lower Basicity and Lower Temperature Based on Industrial Experiments and Ion-Molecular Coexistence Theory. Metall Mater Trans B 52, 3403–3422 (2021). https://doi.org/10.1007/s11663-021-02270-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02270-y