Abstract
Steelmaking slag contains considerable amount of manganese (Mn) and phosphorus (P), and recycling them is an important issue. Since Mn is an alloy added to enhance the steel quality, while P is a harmful impurity in the steel product, they need to be separated for recycling. We have already found the possibility of separating Mn and P from steelmaking slag by selectively reducing P2O5 and FeO while suppressing the reduction of MnO. As a fundamental study for the selective reduction of P from steelmaking slag, in this study, we vary the slag basicity, graphite mixing ratio, temperature, and crucible type to find the optimum condition. Artificial steelmaking slag with various compositions is synthesized and mixed with graphite powder in an Al2O3 or a MgO crucible. The reduction is conducted at 1673 K, 1773 K, and 1873 K for 20 to 80 minutes. As a result, carbon saturated metal is formed from the slag, and the reduced metal and slag were separated easily. We find that a decrease in slag basicity suppressed the reduction rate of Mn while enhancing that of P. The activation energy for the reduction rate constant of P is found to be close to that of the diffusion of P in slag.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The integrated steelmaking process generates blast furnace slag and steelmaking slag as byproducts, where most of the blast furnace slag is valuably reused as a material for cement production, whereas the steelmaking slag is used as a material for road base and civil engineering work.[1,2] However, in the future, domestic construction work is expected to decrease and the regulation for environmental suitability to become strict. Therefore, various studies have been conducted to develop another method for valorization of steelmaking slag.[3,4,5] Meanwhile, steelmaking slag contains several percentage of MnO and P2O5, and in Japan, the amounts of these elements in steelmaking slag are close to those of manganese imported as ferro-alloy or manganese ore and phosphorus as phosphate rock.[3,6]
Mn is a key alloying element for various steel products, and P is an essential nutrient in agricultural production. In the last 5 years, the global consumption of Mn and P has not decreased; rather, many experts predict that their global demand will continue to increase along with industrial development and population growth. However, their deposits are substantially biased globally, and the top five countries occupy more than 70 pct of the total deposits in the world.[7,8] Therefore, their stable supply is an important national issue. For these reasons, many researchers have considered steelmaking slag as a secondary resource for these elements and studied their recovery from steelmaking slag using various methods.
For example, Teratoko et al.[9] reported the dissolution behavior of a solid solution with high P content in an acidic solution and proposed the selective leaching process to separate P from steelmaking slag. As a fundamental study of this process, Numata et al.[10] and Du et al.[11] showed the difference in the dissolution behaviors of P- and Fe-rich phases in steelmaking. The magnetic separation process using the difference in the magnetic properties between the P- and Fe-rich phases was proposed by Kubo et al.[12] Using this process, powdered steelmaking slag was separated into Fe- and P-rich powders, and the content of P in the unmagnetized powder was found to be similar to that of the P-rich phase in steelmaking slag. Kim et al.[13] studied the separation of Mn from steelmaking slag by sulfurization using the difference in the thermodynamic stability of sulfide between Mn and P. They considered the process of producing the raw material for ferro-manganese alloy by a combination of sulfurization and oxidation. Besides the abovementioned studies, some other studies have also reported methods such as the gravity separation process[14,15] and capillary action.[16]
Carbothermic and smelting reduction are simply applicable for recovering Fe, Mn, and P from steelmaking slag. Li et al.[17] proposed a waste-free steelmaking process that consists of a conceptual slag regenerator. In this process, steelmaking slag is reduced by smelting reduction in the slag regenerator and the P content in the hot metal is increased. The slag obtained after this treatment is reused in the steelmaking process as a flux, and the hot metal with high P content is used as a raw material to produce fertilizer. Morita et al.[18] reported the recovery of Fe and P from steelmaking slag by carbon reduction using the microwave process. In this study, the FeO and P2O5 present in steelmaking slag were reduced and formed a Fe-P-C (sat.) alloy with high P content. In addition, for separating Fe and P from the alloy, the dephosphorization process was studied using Na2CO3 and K2CO3 as a flux. Matsui et al. studied the effect of temperature on the reduction behavior of FetO and P2O5,[19] and concluded that the reduction ratio of P was strongly influenced by the equilibrium distribution ratio of P between the slag and metal. Recently, Nakase et al.[20] investigated the influence of FetO content in the slag on the reduction behavior of Fe and P by conducting a carbothermic reduction of steelmaking slag. In their study, the reduction and evaporation behaviors of P were significantly influenced by FetO in the slag and about a half of P in the slag could be removed by evaporation under the optimum condition.
Previous research has mainly conducted the removal of P from steelmaking slag through carbothermic or smelting reduction. However, separation of Mn and P has not been considered. In general, Fe, Mn, and P in steelmaking slag are reduced simultaneously, and a Fe-P-Mn-C alloy is formed. To utilize Mn and P in steelmaking slag as a secondary resource, these elements must be recovered separately. Especially, to recover Mn as a raw material of ferro-manganese, since P is a harmful impurity that lowers the toughness and ductility of steel products, it must be removed.
It is well known that MnO is a basic oxide and P2O5 is an acidic oxide, and the standard free energies of formations of these oxides differ widely. In the hot metal dephosphorization process using flux injection, the Mn present in the hot metal is not oxidized during dephosphorization, because of the formation of a highly basic slag with low oxygen potential and its operation at a relatively low temperature. To increase the yield of Mn in basic oxygen furnace refining, a highly basic slag is developed. These operations indicate that the selective oxidation of Mn or P is conducted in the steelmaking process. Therefore, we consider the possibility of selectively reducing Mn and P from steelmaking slag. By controlling the slag basicity and temperature, the separation of Mn and P by selective reduction can be achieved. We have previously shown the thermodynamic calculation of the theoretical background and the results of some simple experiments.[21,22,23] We found the possibility to separate Mn and P from steelmaking slag by reducing P2O5 into metal selectively while suppressing the reduction rate of MnO.
In this study, as a fundamental study for the selective reduction of P from steelmaking slag, the effects of slag basicity (mass pct CaO/mass pct SiO2), graphite mixing ratio, temperature, and crucible type are mainly elucidated to determine the optimum condition.
Experimental Procedure
Reagent-grade CaCO3, SiO2, Fe, Fe2O3, Ca3(PO)4, MnO, MgO, and Al2O3 were used to synthetize the slag. First, FeO was pre-synthesized through a reaction of Fe with Fe2O3 at 1673 K for 1 hour in Ar atmosphere, and CaO was calcined from CaCO3 at 1473 K for 12 hours in air. The reagents with CaO and FeO were mixed homogenously and pre-melted at 1673 K in an Al2O3 crucible for 1 hour under Ar atmosphere (flow rate: 100 mL/min). After a given time, the pre-melted slag was quenched in water. The chemical composition of the slag, analyzed by inductively coupled plasma atomic emission spectroscopy (ICP–AES), is shown in Table I. The contents of P2O5 and MnO were set to about 4 and 6 mass pct, which are similar to that of industrial steelmaking slag. The Al2O3 content was about 10 mass pct, which is higher than that of industrial slag, as an Al2O3 crucible was used. For investigating the effect of slag basicity, the ratio of CaO and SiO2 was varied from 0.5 to 1.5. The synthesized slags were used for each experiment after crushing them into powder (with particle size of smaller than 500 μm).
The graphite powder (with particle size of smaller than 44 μm) was used as a reductant in this experiment. We homogenously mixed 3 g of crushed slag with graphite powder. The amount of mixed graphite powder was set as 100 to 200 pct of the amount required for reducing all FeO, MnO, and P2O5 in the slag, which was stoichiometrically calculated by Eqs. [1] through [3].
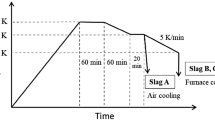
The experiment was performed using a vertical type of resistance furnace equipped with an alumina reaction tube (Inner diameter: 42 mm, Height: 1000 mm). Basically, a dense Al2O3 crucible (Inner diameter: 19 mm, Height: 45 mm) was used, and a dense MgO crucible of the same size was applied for comparison to investigate the influence of the crucible. Before the sample was sent in, the furnace was heated to the target temperature (1673 K, 1773 K, and 1873 K) and Ar gas (purity: 99.9999 pct) was injected (flow rate: 300 ml/min) into the reaction tube for 30 minutes for oxygen removal. Subsequently, the mixture of slag and graphite powder loaded in the crucible was charged into the heated furnace, and this moment was set as the start of the experiment. A typical temperature change of the sample with time is shown in Figure 1. To reach the target temperature, it generally took 7 to 9 minutes. The experimental time was 20, 40, and 80 minutes and the inner atmosphere of the furnace was completely controlled by Ar gas (flow rate: 500 mL/min) during the experiment. After the experiment, the sample with the crucible was unloaded from the furnace and quenched in water immediately. The sample was separated into slag and metal, and the compositions of each phase were analyzed by ICP–AES. The carbon content in the metal was analyzed by the combustion method using a combustion infrared spectrometer.
The mass of the slag was calculated by CaO balance assuming that the mass of CaO in the slag did not change during the experiment. The mass of the metal was determined by the change in FeO content in the slag. The equations for the calculations of the slag and metal are expressed as
where \( W_{\text{slag}} \) and \( W_{\text{Metal}} \) indicate the masses of slag and metal after the experiment (g), \( W_{\text{CaO}}^{\text{Initial}} \) and \( W_{\text{FeO}}^{\text{Initial}} \) indicate the masses of CaO and FeO in the initial slag, (mass pct CaO) and (mass pct FeO) indicates the concentration of each oxide in the slag after the experiment, and \( {\text{M}}_{\text{Fe}} \) and \( {\text{M}}_{\text{FeO}} \) indicate the atomic weight of Fe and the molecular weight of FeO.
Results
Figure 2 shows a cross-section of the sample after 20-minute reduction at 1773 K. As shown in this figure, the metal is easily separated from the slag. Through the shape of the cross-section, the slag and metal are considered to be in the molten state before quenching. When 3 g of slag is used for the reduction, the diameter of the reduced metal reaches 4 to 6 mm (0.5 to 0.7 g). The top of the sample is covered by the residual graphite powder. However, as the graphite powder is lost during the quenching procedure, it is not observed in this figure.
A typical composition change of the slag and metal for Slag B at 1773 K with time is shown in Figure 3. In Figure 3(a), preferential reduction of FeO is observed, and then, P2O5 and MnO are gradually reduced with time during the initial 20 minutes. The content of Al2O3 in the slag is significantly increased with time owing to the dissolution of Al2O3 from the crucible. Corresponding to the change in the slag composition, the contents of Mn and P in the metal phase gradually increase with time (Figure 3(b)). In this figure, the initial value of carbon is plotted as 4 mass pct, considering the saturated value of the hot metal without the presence of Mn and P. The carbon content decreases continuously due to the change in solubility due to an increase in Mn and P contents. The changes in slag composition are shown in Figure 4 on the phase diagram of SiO2-CaO-FetO and SiO2-CaO-Al2O3 systems.
Composition changes of slags in SiO2-CaO-FetO and SiO2-CaO-Al2O3 phase diagrams.[24]
Compared to the actual temperature change of the sample, as shown in Figure 1, the slag is considered to be liquid during the initial 20 minutes. This indicates that except for the initial heating period, the reduction has been occurring at the interface between the molten slag and hot metal, besides the interface of the molten slag and graphite powder floating on the surface.
Figure 5 shows the change in Mn and P contents with reduction time in each phase for the slag with varying basicity at 1773 K. Regarding the reduction rate of MnO shown in Figure 5(a), with an increase in slag basicity, the reduction is enhanced and Slag A (C/S = 1.49) shows the fastest reduction rate. A small increase in MnO content is observed in Slags D and E for 20 minutes, which is attributable to the rapid reduction of other components in the slag. On the contrary, regarding the reduction rate of P2O5, as shown in Figure 5(b), with a decrease in slag basicity, the reduction rate increases and Slag D (C/S = 0.76) and Slag E (C/S = 0.50) show the most rapid decrease. The content of P2O5 in these slags is lower than 0.20 mass pct after 20 minutes. Corresponding to the composition change in the slag phase, Slag A shows the greatest Mn content in the metal phase (Figure 5(c)), while Slags D and E show the greatest P content (Figure 5(d)).
The mass balances of Mn and P are shown in Figure 6. The result shows that a decrease in slag basicity from 1.49 to 0.50 suppresses MnO reduction and enhances P2O5 reduction. At 20-minute reduction of Slags D and E, more than 95 mass pct of Mn remains in the slag, while more than 95 mass pct of P is reduced into metal. The separation between Mn and P is achieved when the slag basicity is lowered below 0.76 at 1773 K. When the reduction time is increased to 40 minutes for Slags D and E, the residual mass of P in the slag is lowered further, while the reduced mass of Mn in the metal increases.
In this experiment, the selective reduction of P, suppressing the reduction of Mn, is achieved using the acid slag. However, the reduction of MnO gradually occurs through the elongation of the reduction time. Therefore, the optimum condition should be determined not only by the equilibrium condition but also by the kinetics of the reduction reaction.
The influence of temperature is investigated using Slag D (C/S = 0.76) at a graphite mixing ratio of 200 pct. Figure 7 shows the changes in Mn and P contents in each phase with reduction time by varying the temperature. Clearly, a rapid reduction in MnO is observed at 1873 K (Figure 7(a)) and the reduction rate of P2O5 at 1673 K is significantly slow (Figure 7(b)). The content of P2O5 in the slag at 1673 K is 1.64 mass pct after 20 minutes, and about 0.5 mass pct of P2O5 is observed in the slag even after 40 minutes. The changes in Mn and P contents in the metal (Figures 7(c) and (d)) exhibit a consistent tendency with the composition change in the slag.
Figure 8 shows the result of mass balances of Mn and P at each temperature. At 1873 K, the reduced mass of Mn gradually increases with time (Figure 8(a)). On the other hand, at 1673 K, a considerable mass of P remains in the slag even after 20 minutes and the reduction rate is remarkably decreased (Figure 8(b)).
Therefore, for the reduction of P2O5, low-temperature treatment is not appropriate. However, when the temperature increases, the reduction rate of MnO is gradually increased. To conduct an efficient separation of Mn and P, the optimum reduction time should be determined for each temperature.
Figure 9 illustrates the effect of graphite mixing ratio on the reduction behavior of Slag D. As the graphite mixing ratio changes, the reduction trend differs significantly. When the graphite mixing ratio decreases from 200 to 150 pct, the reduction of MnO is effectively suppressed during the initial period of reduction, but the reduction rate of P2O5 does not change much. The decreases in FeO and C concentrations in the metal are almost the same. On the other hand, when the graphite mixing ratio decreases to 100 pct, the reduction of FeO does not fully proceed and about 5 mass pct of FeO remains in the slag even after 40 minutes. This indicates graphite powder is partially oxidized during the initial period of reduction, because air exists among the slag particles and graphite powders when charging them into furnace at high temperature. Furthermore, the carbon content is lower than that in the other conditions. Considering the difference of P content in the metal, in this case, the formed hot metal is not saturated with carbon. The content of P2O5 in the slag does not decrease much and the rate of increase of P in the metal is very low. In addition, the content of Mn in the metal does not increase. As the reduction behavior is much influenced by the mixing ratio of graphite, the reaction would mainly occur at the interface of slag and graphite powder.
Since an Al2O3 crucible is used, the Al2O3 content in the slag increases to about 30 mass pct or more in the above experiments. Al2O3 is a neutral oxide in the slag and lowers the melting temperature. Therefore, for retaining the liquid state after FeO reduction, Al2O3 plays an important role. To clarify the effect of Al2O3, the experiments of Slags D and E are conducted using a MgO crucible. The influence of the crucible is summarized in Figure 10. The considerable dissolution of MgO is also observed, and the MgO content in the slag is found to reach about 25 mass pct or more.
Compared to the result of the Al2O3 crucible, when the MgO crucible is applied, the reduction rate of MnO is enhanced, while that of P2O5 is slightly decreased. This behavior is similar to that obtained by increasing the slag basicity. Based on the phase diagram of the CaO-SiO2-MgO system,[24] the liquid slag is formed during the experiment using a MgO crucible. The difference in the crucible is attributed to the slag basicity.
Discussion
In this experiment, although a mixture of solid slag and graphite were loaded in the crucible, the slag and the formed metal were in the liquid state from the early stage of the reduction. Therefore, except for the very initial stage, for most of the experimental period, graphite powders floated on the surface of the molten slag. In this case, a reduction in the slag can occur at the interface of the molten slag and graphite powder floating on the slag. According to the result that shows the influence of the mixing ratio of graphite (Figure 9), the main reaction site can be considered as the interface of graphite powder floating on the slag surface.
The reduction rates of Mn and P (R; mol/s) in the case of Slag C are calculated and shown in Figure 11. Here, slag C is used for kinetic analysis in Figure 11 because the selective reduction of P over Mn has been well achieved. The reduction rate is calculated by Eqs. [6] and [7].
where W indicates the masses of Mn and P in metal (g) and M is atomic weight (g/mol); t is the reaction time (s). In this figure, the relation between the average reduction rates between each experimental time is plotted with the average experimental time. The reduction rate of P decreases with time, but that of Mn does not change much and is lower than that of P. In most of the experiments, the reduction rate of Mn is low and the concentration change during the experiment is small. Thus, for the kinetic analysis, the reduction rate of P is used.
Figure 12 shows the influence of P content in the slag on the reduction rate of P. For this analysis, the results of Slags B and C were used as these slags showed the intermediate reduction rate (RP). With a decrease in P2O5 content in the slag, the reduction rate decreased linearly. As the reduction occurred at the interface of floating graphite powder, mainly, the reduction rate was not affected by the P content in the metal, and the mass transfer of P2O5 in the slag can be considered as a rate-controlling step. The rate of decrease of P in the slag can be expressed as in Eq. [8], where kP (1/min) is a rate constant. Through the relation shown in Figure 12, kP is evaluated and its temperature dependency is discussed.
As most of the reaction finished before 20 minutes, the rate constant for each temperature was evaluated using the P content in the slag during the initial 20 minutes. The results are summarized in the form of Arrhenius equation, as shown in Figure 13, and Eq. [9] was thus obtained.
Temperature dependence of the rate constant calculated by Eq. [8]
Using this equation, the activation energy is calculated to be about 181 kJ/mol. The activation energy of the diffusion of P in a molten slag with compositions of 40 to 43 mass pct CaO—30 to 35 mass pct SiO2—21 mass pct Al2O3 is about 188 kJ/mol.[25] Therefore, the rate-controlling step can be considered as the mass transfer in the slag phase. Figure 14 shows the effect of basicity on the reduction rate of Mn and P.
The distribution ratio of each element (L) between the slag and metal phase is calculated in Eq. [10] using the result at 20 minutes. In these equations, (pct X) and [pct X] indicate the concentrations of element X in the slag and metal phase, respectively. The effect of basicity on each crucible is shown in Figure 15.
In the case of the Al2O3 crucible, regarding the behavior of Mn, with an increase in basicity, the reduction rate increases and the distribution ratio decreases. On the contrary, regarding the behavior of P, the opposite trend is observed. When the MgO crucible is used, the behavior of P does not change much but the reduction rate of Mn increases and the distribution ratio decreases. Therefore, for the selective reduction suppressing the MnO reduction, the crucible made of neutral oxide (Al2O3) is favorable, compared to that made of a basic oxide crucible (MgO).
The target value of distribution ratio for the selective reduction is considered. The assumed composition of a typical industrial steelmaking slag is shown in Table II. After controlling the slag basicity by SiO2 addition, a selective reduction of this slag is calculated as a first step. As a second step, the reduction of the residual slag in the first step is calculated to produce a ferro-manganese alloy.
In the first step, 97 pct of FeO is assumed to be reduced, and the slag composition is calculated by changing the distribution ratio of Mn and P. In the second step, FeO, MnO, and P2O5 in the residual slag are considered to be completely reduced and produce ferro-manganese alloy. In this case, the carbon concentration in the ferro-manganese alloy is assumed to be 7.0 pct. The result is shown in Figure 16. Since LP and LMn obtained in the experiment are 0.02 to 0.085 and 0.2 to 13, respectively, Mn concentration in the ferro-manganese is sufficiently high under this condition, but the concentration of P is estimated to be about 0.9 pct. As the P content in the high-carbon ferro-manganese is generally about 0.2 pct, the target value of LP should be 0.004 or less. This calculation indicates that a further improvement to suppress the reduction of P during the selective reduction is necessary.
The relation between Mn and P obtained from Eqs. [2] and [3] is represented by Eq. [11].
The equilibrium constant K of the reaction is represented by Eq. [12][26,27]:
Because P2O5 is an acidic oxide and MnO is a basic oxide, the activity of P2O5 will increase while that of MnO decreases when the slag becomes acidic. Thus, basing on Eq. [12], the ratio between the activity of Mn and P in metal decreases at a given temperature. This explains the enhancement on the selective reduction of P over Mn by decreasing the slag basicity. If the activity of the oxide in the slag is calculated by a regular solution model[28] and the activity coefficient of the solute element in the molten metal is assumed as unity, the value of [mass pct Mn]2.5/[mass pct P] can be calculated. By using the experimental results of Slag D at 1773 K after 20-minute reduction, the calculated value is about 0.03. However, this value obtained by the experiment is about 190, which does not agree with the theoretical result at all. This means that the activity coefficient in a dilute solution, which is generally used to analyze steelmaking reactions, cannot be used for this system. It is necessary to measure the activity coefficients of Mn and P in a high Mn, high P molten metal.
Conclusion
Here, as a fundamental study for the selective reduction of P from steelmaking slag, the slag basicity, graphite mixing ratio, temperature, and crucible type were varied to determine the optimum condition. The following conclusions were drawn.
-
(1)
Decreasing the slag basicity from 1.49 to 0.50 suppressed the reduction rate of Mn while enhancing that of P. When the slag basicity was lower than 0.76 at 1773 K, the selective reduction of P was achieved.
-
(2)
The effect of temperature on the reduction rates of Mn and P was investigated. From the relation between the rate constant for P2O5 reduction in the slag and temperature, the activation energy was evaluated. The obtained value was close to the activation energy required for the diffusion of P in the slag.
-
(3)
The reduction behaviors of FeO, MnO, and P2O5 were influenced by the mixing ratio of graphite. The reduction reaction was considered to mainly occur at the interface between the slag and graphite powder.
-
(4)
With a change in the crucible type, the reduction rate and distribution ratio of Mn changed, while those of P did not change much.
References
Nippon Slag Association: Production and Uses of Steel Slag in Japan, 2017. http://www.slg.jp/pdf/Steel%20Slag%202017FY.pdf. Accessed 29 August 2018.
2. K. Horii, N. Tsutsumi, T. Kato, Y. Kitano, K. Sugahara: Nippon Steel & Sumitomo Metal Tech. Rep., 2015, vol. 109, pp. 5−11.
3. K. Matsubae‐Yokoyama, H. Kubo, K. Nakajima, T. Nagasaka: J. Ind. Ecol., 2009, vol. 13, pp. 687−705.
4. T. Kato, C. Kosugi, E. Kiso, K. Torii: Nippon Steel & Sumitomo Metal Tech. Rep., 2015, vol. 109, pp. 79−84.
5. X. Gao, M. Okubo, N. Maruoka, H. Shibata, T. Ito, S. Kitamura: minuteser. Process. Extra. Metall., 2015, vol. 124, pp. 116−124.
6. K. Nakajima, K. Yokoyama, T. Nagasaka: ISIJ Int., 2008, vol. 48, pp. 549−553.
U.S. Department of the Interior U.S. Geological Survey: Mineral Commodity Summaries. 2018. https://minerals.usgs.gov/minerals/pubs/mcs/2018/mcs2018.pdf#search=%27MINERAL+COMMODITY+SUMMARIES+2018%27. Accessed 31 Aug 2018
Japan Oil, Gas and Metals National Corporation: Mineral Resources and Materials Flow, 2017. http://mric.jogmec.go.jp/wp-content/ebook/201803/5aa63015/material_flow2017.pdf. Accessed 31 Aug 2018 [Japanese]
9. T. Teratoko, N. Maruoka, H. Shibata, S. Kitamura: High Temp. Mater. Proc., 2012, vol. 31, pp. 329–338.
10. M. Numata, N. Maruoka, S. Kim, S. Kitamura: ISIJ Int., 2014, vol. 54, pp. 1983−1990.
11. C. Du, X. Gao, S. Ueda, S. Kitamura: ISIJ Int., 2017, vol. 57, pp. 487−496.
12. H. Kubo, K. Matsubae-Yokoyama, T. Nagasaka: ISIJ Int., 2010, vol. 50, pp. 59−64.
13. S. Kim, H. Shibata, N. Maruoka, S. Kitamura, K. Yamaguchi: High Temp. Mater. Proc., 2011, vol. 30, pp. 425−434.
14. C. Li, J. Gao, Z. Guo: ISIJ Int., 2016, vol. 56, pp. 759−764.
H. Ono, A. Inagaki, T. Masui, H. Narita, T. Mitsuo, S. Nosaka, S. Gohda: Tetsu-to-Hagané, 1980, vol. 66, pp. 1317–1326.
16. T. Miki, S. Kaneko: ISIJ Int., 2015, vol. 55, pp. 142−148.
17. H. Li, H. Suito, M. Tokuda: ISIJ Int.,1995, vol. 35, pp. 1079−1088.
18. K. Morita, M. Guo, N. Oka, N. Sano: J. Mater. Cycles and Waste Manage., 2002, vol. 4, pp. 93−101.
19. A. Matsui, K. Nakase, N. Kikuchi, Y. Kishimoto, K. Takahashi, K. Ishida: Tetsu-to-Hagané, 2011, vol. 97, pp. 416−422. (In Japanese)
20. K. Nakase, A. Matsui, N. Kikuchi, Miki Y: ISIJ Int., 2017, vol. 57, pp. 1197−1204.
21. D. Shin, X. Gao, S. Ueda, S. Kitamura: Proceeding 5 th International Slag Valorization Symposium, KU Leuven, Belgium, 2017, 161−164.
D. Shin, X. Gao, S. Ueda, S. Kitamura: Proceedings of the 9th International Symposium on High Temperature Metallurgical Processing, TMS Annual Meeting & Exhibition, TMS, USA, 2018, pp. 305–311.
23. D. Shin, X. Gao, S. Ueda, S. Kitamura: CAMP-ISIJ, 2017, vol. 30, pp. 248.
Verein Deutscher Eisenhüttenleute: Slag atlas 2nd Edition., Verlag Stahleisen GmbH, Düsseldorf, Germany, 1995
The Japan Institute of Metals: Metals Handbook (6th edition), Maruzen, Tokyo, 2000 (Japanese).
26. E. Turkdogan, J. Pearson: J. Iron Steel Inst., 1953, vol. 175, pp. 393−401.
The Japan Society for the Promotion of Science, the 19th Committee on Steelmaking: Steelmaking Data Sourcebook (Revised Edition), 1988, Gordon and Breach Science Publications, New York.
28. S. Ban-ya: ISIJ Int., 1993, vol. 33, pp. 2−11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted October 12, 2018.
Rights and permissions
About this article
Cite this article
Shin, D.J., Gao, X., Ueda, S. et al. Separation of Phosphorus and Manganese from Steelmaking Slag by Selective Reduction. Metall Mater Trans B 50, 1248–1259 (2019). https://doi.org/10.1007/s11663-019-01556-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01556-6