Abstract
During ISA copper smelting process, ISASMELT furnace discharges a large amount of matte and slag mixture and the separation of them has an important influence on the recovery of valuable metals. This paper presented a reduction-sulfurization sedimentation process for recovering copper and cobalt from the matte–slag mixture of ISA furnace. Firstly, matte–slag mixture and traditional static sedimentation slag are characterized to determine their mineral composition and occurrence state. It indicates that Cu is primarily lost in slag in the form of sulfide, while Co is mostly lost in the form of oxide. With coke and pyrite as the reducing agent and vulcanizing agent, an orthogonal laboratory experiment was conducted to determine the effects of the smelting temperature and additive dosage on the recovery process. The optimum slag cleaning conditions were found to be: a coke dosage of 2 pct, a pyrite dosage of 2 pct, and a smelting temperature of 1260 °C lasting for 2 hours. In order to improve on the low utilization ratio of additives associated with the industrial sedimentation process, an innovative additive introducing method was put forward which enables the additives to mix and react with slag more adequately. A powder injection device was purpose-made to inject additives into molten slag in dispersion state, and a laboratory experiment was carried out to simulate this process. By injecting 2 pct coke and 2 pct pyrite, the contents of Cu and Co in cleaned slag decreased to 0.46 and 0.01 pct, respectively. It proves that the injection of additives into molten slag is an effective method to recover Cu and Co from the matte–slag mixture of ISA furnace.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ISA process, a typical oxygen-enriched top-blowing bath smelting technology, has been widely used in copper production across the world.[1] A typical ISA process for copper mainly consists of smelting in an ISASMELT furnace, sedimentation in an electric furnace and converting in a Pierce-Smith converter. In the ISA smelting process, a certain amount of oxygen-enriched air is injected through the lance into the molten bath with the addition of copper concentrate, fluxes, and fuel. The molten slag and matte are discharged intermittently from the ISASMLT furnace through a tap hole into an electric furnace.[2] In general, the range of elemental mass percent in matte–slag mixture is as follows: Cu 18 to 26 pct, Fe 30 to 38 pct, S 3 to 10 pct, CaO 1 to 5 pct, and SiO2 10 to 18 pct.[3] The matte–slag mixture is then separated by gravity in the electric furnace at about 1250 °C.[4] However, with the increase of oxygen concentration in the ISA process over these years, the contents of oxides in the matte–slag mixture have increased constantly.[5] Valuable metals such as copper and cobalt in an oxide form can be chemically dissolved in slag, which leads to heavy loss of them.[6] In addition, the slag viscosity is higher due to the increasing content of Fe3O4, which makes separation of matte from slag more difficult in an electric furnace.[7] As Cu and Co contents in depleted slag rise, higher amounts of ores have to be consumed to maintain a stable yield of valuable metals. This causes not only a waste of mineral resource but also a potential severe damage to the ecological environment, such as heavy metal pollution to surrounding soils and water resource. Therefore, proper treatment and metal recovery from the matte–slag mixture is imperative, whether from the perspective of resource preservation or environmental protection.
Much effort has been made to recover valuable metals from copper slag, for instance by using beneficiation process, hydrometallurgical process, or pyrometallurgical process.[8,9,10,11] The beneficiation process is considerable successful to recover copper from slow-cooled copper slag by flotation. However, this method requires a large occupation of land and high investment.[12,13] On the other hand, significant amounts of gangues are present in the matte–slag mixture of ISA furnace, so the acid consumption is large and the purification process is complex in the hydrometallurgical method.
Pyrometallurgy, in particular, is a widely used technology for recovering valuable metals from copper and cobalt slag.[14] Busolic et al.[15] used direct reduction method to recover copper from the slag of flash smelting. The copper content in the final slag dropped to 0.06 pct by using as the reductant 150 pct of the stoichiometric carbon of coke. A similar study was conducted by Zhai et al.,[16] in which activated carbon acted as the reductant and TiO2 as the slag modifier to recover cobalt and copper from converter slag. Under the optimal conditions, the contents of copper and cobalt in depleted slag were 0.97 and 0.025 pct, respectively. Besides the reducing agent, pyrite (FeS2) or some other sulfides were also used as a vulcanizing agent and collector to reduce the content of valuable metals in slag.[1,17,18] For example, pyrite was employed to recover Co, Co and Ni from smelter slag, and these valuable metals were enriched into a sulfide matte.[19]
According to the above-mentioned work, the contents of copper and cobalt in slag can be significantly reduced by introducing reducing and vulcanizing agents. However, the reported investigations focus on the depletion of valuable metals from converter slag or reverberatory furnace slag. There is little work that addresses the matte/slag separation of the mixture from ISA furnace. Furthermore, in those available researches, the additives are premixed with slag and then the mixture heated in a laboratory furnace. However, in the industrial process, additives need to be introduced directly into molten slag. Since these additives have a lower density than slag, most of them float on the surface of slag, impossible to be used adequately.
In the refining process of molten steel, RH desulfurization technology has been used, in which pulverized desulfurization agent is injected into the molten metal through a submerged injection lance.[20,21] The desulfurization agent and the molten metal can be thoroughly mixed, highly accelerating the reaction.[21] This classical steel making process inspired us to introduce, by an injection method, the reducing and vulcanizing agents into the bath of sedimentation electric furnace, which provides satisfactory intermixing and homogeneity of the additives and slag in the sedimentation process.
In this paper, the mineral characterization of matte–slag mixture and traditional static sedimentation slag was performed to identify the mineral composition and occurrence state of Cu and Co. Since experimental variables may potentially produce interactive effect, an orthogonal experiment was carried out to determine the effects of coke, pyrite addition, and smelting temperature on the recovery of copper and cobalt from matte–slag mixture. Finally, an injection simulation experiment was performed to demonstrate the feasibility of the method that injects additives into the molten slag using a designed injection device.
Materials and Methods
Experimental Materials
Pyrite (S 38.6 wt pct) and metallurgical coke (C 85.9 wt pct) used in this experimental study were provided by Hangzhou Iron and Steel Group Company. The matte–slag mixture was provided from an ISA copper plant in Africa. During the discharging in ISA smelting process, the matte–slag mixture was sampled at intervals for five times and 500-mL molten sample was obtained in each sampling. The samples were rapidly cooled by water quenching, then they were broken and mixed. The chemical composition of the matte–slag mixture sample was analyzed by an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 7000 DV, Perkin Elmer instruments), and the result is shown in Table I. As can be seen, the mixture contains 22.57 pct of Cu and 0.44 pct of Co.
Characterization
The crystalline phases of slag and matte were determined by an X-ray diffractometer (XRD, Ultima IV, Rigaku, Japan). An optical microscope (Axiovision, Car Zeiss, Germany) and the scanning electronic microscopy (SEM, MLA250, FEI) equipped with an energy dispersive spectrometer (EDS, MLA250, FEI) were used to analyze the microstructure morphology of slag and matte. In addition, the matte–slag mixture of ISA furnace was chemically investigated for its mineral phases by means of wet-chemical analysis based on the solubility difference between different phases in solvent. The composition and particle size distribution of copper minerals in matte–slag mixture were determined by a mineral liberation analyzer (MLA).
Orthogonal Experiment Design
An orthogonal experiment provides an optimization method to research a target involving multiple factors and levels. This study includes an orthogonal design to investigate the effect of parameters on copper and cobalt contents in cleaned slag. The following three factors were analyzed: smelting temperature (factor A), coke dosage (factor B), and pyrite dosage (factor C). An OA16(43) was employed to assign the considered factors as shown in Table II, which contains an orthogonal array of three factors at four levels. Sixteen trials were conducted in the optimization process according to the OA16(43). These arrays were created using an algorithm developed by Taguchi, allowing for each variable and setting to be tested equally.[22,23,24]
The orthogonal experiment was carried out using a muffle furnace. In an experimental procedure, 200 g matte–slag mixture was mixed a predetermined amount of coke (0.5 to 2 wt pct) and pyrite (1 to 4 wt pct) in a corundum crucible. The crucible was put in the muffle furnace and held for 2 hours at a desired temperature. The orthogonal experiment was studied at temperature range of 1200 °C to 1290 °C, which is the operating temperature range of industrial electric furnace. The crucible was naturally cooled to room temperature in the furnace before being taken out. After separation and crushing, an ICP-OES was used to analyze the Cu and Co contents in the cleaned slag.
Injection Simulation Experiment
Figure 1 shows the schematic diagram of the experimental apparatus for additive injection. An additive tank was specially designed to ensure that all the additives could be sprayed into the molten slag in the dispersion state by flowing gas. In an experimental procedure, 120 g matte–slag mixture was put in a corundum crucible and the high-frequency induction furnace was heated slowly to 1260 °C with the protection of Ar2 atmosphere. The molten slag was stirred for 15 minutes at a 1.5 L/min argon flow and simultaneously the 300-mesh coke and pyrite were sprayed into the bath. After 2 hours of sedimentation, the crucible was naturally cooled to room temperature. The slag and matte in the crucible were manually separated, and the contents of copper and cobalt in the cleaned slag were analyzed by ICP-OES.
Results and Discussion
Mineralogical Analysis of the Matte–Slag Mixture
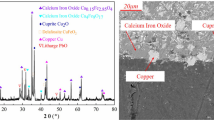
The XRD patterns and mineral contents of the matte–slag mixture are shown in Figure 2 and Table III. As is clear from the results, bornite (Cu5FeS4) is the primary copper phase in the mixture, and its content is 20.3 pct. Major gangue minerals existent in the mixture are fayalite and glassy substance. The latter is an unstable and amorphous solid containing mainly SiO2 and FeO.
Figure 3 shows the optical microscope images of typical mineral phases in matte–slag mixture. Most of the copper minerals occur as aggregates while they are either present as liberated free crystals or entrapped in the gangue minerals as belt shapes. According to the MLA analysis, the distribution of copper mineral aggregates is 77.5 pct and their particle sizes are in the range of 20 to 100 μm. In addition, a small part of copper minerals with micro-fine particles sizes are entrapped in gangues dispersedly. These microparticles are harder to conglomerate and recover in the high-temperature sedimentation process.
Characterization of the Slag by Traditional Static Sedimentation
To analyze the forms in which copper and cobalt lose into the slag, the matte–slag mixture was separated without any additive, in a muffle furnace at 1250 °C for 2 hours, and static sedimentation slag was obtained. Table IV shows the results of copper and cobalt chemical compositions in the static sedimentation slag. The contents of Cu and Co in the static sedimentation slag are 1.20 and 0.42 pct, respectively, much higher than the average contents of Cu and Co in industrial discarded slag (0.5 and 0.1 pct, respectively). Table V shows the contents of copper phases, determined by chemical species analysis, in the static sedimentation slag. It suggests that 71 pct of copper exists in the form of sulfides and 16 pct in the form of metallic, while the content of copper oxides is only 13 pct. So, the copper is lost mainly because it is entrained physically in the static sedimentation slag. Figure 4 shows the result of particle size distribution of copper minerals. It is noted that the particle size of copper minerals is between 20 and 50 μm mostly. Because of their fine particle size, it is difficult to recover these copper from static sedimentation slag by physical separation processes.
Figure 5(a) displays an SEM back-scattered electron (BSE) image of typical phases in the static sedimentation slag. The corresponding EDS analysis results are shown in Table VI. According to the results, fayalite (region 3) is the dominant phase, which is closely associated with glassy substance and magnetite (region 2). Copper mainly associates with sulfur as copper matte (region 1). From EDS mapping results shown in Figures 5(c) and (d), it is apparent that Cu and S are located at similar places (circled in Figure 5(b)), suggesting that copper is lost mostly in the form of sulfide, which is consistent with the findings of chemical species analysis in Table V. For cobalt, its distribution is relatively homogeneous and with no significant segregation (Figure 5(e)). It implies that cobalt dissolves into the static sedimentation slag mainly in the oxidized form.
Results and Discussion for the Orthogonal Experiment
Though above analysis, it is clearly that Cu is primarily lost in slag in the form of sulfide with fine particle size, while Co is mostly lost in the form of dissolved oxide. Therefore, we introduced pyrite and coke as vulcanizing agent and reducing agent during the sedimentation process, in order to decrease the Cu and Co contents in cleaned slag. In addition, an appropriate temperature is helpful to the matte–slag separation, especially to the aggregation and sedimentation of metal particles with fine size.
An orthogonal experiment was then used to investigate the effect of coke, pyrite dosage, and sedimentation temperature on Cu and Co contents in cleaned slag. According to the OA16(43), 16 experiments were carried out and the results are presented in Table VII. Range analysis was made to evaluate the significant level of different factors that may influence the recovery of Cu and Co, with the most significant factors being identified on the basis of the findings of range analysis. These average values of each level for each factor are named ki, which are calculated using Eq. [1]. The differences between the factors are defined by Eq. [2], to estimate the distance between the extreme values of the data. The greater the range (R), the more influential the factor is.[24,25]
where i is the number of levels, j the number of factors, yi,j the performance value for each factor j in level i, and N the total number of levels, N = 4 for this study.
The range analysis result for Cu and Co contents in slag is given in Table VIII. The result suggests that the order of influence on the recovery of Cu and Co is RA > RC > RB and RA > RB > RC, respectively. Factors A, C, and B influencing the recovery of Cu correspond to temperature, pyrite dosage, coke dosage, in descending order of their influence. The order of factors A, B, and C influencing the recovery of Co is temperature > coke dosage > pyrite dosage. RA is always the largest, suggesting that temperature is the most significant factor influencing the recovery of both Cu and Co. Coke dosage is more influential than pyrite dosage in reducing the content of Co in slag, while pyrite dosage is a more important factor than coke dosage in influencing the content of Cu in slag.
The relationship between significant factors and Cu and Co contents in slag is shown in Figure 6. It should be noted that these curves are for showing the trends of each factor only rather than predicting the values which were not investigated by experiments. From Table VIII, the influence of coke dosage on the recovery of Cu (RB 0.71) is not significant. Nor is the influence of pyrite dosage on the recovery of Cu (RC 0.81) and Co (RC 0.03). Their range values are way below the values of significant factors, so this analysis method is not suitable. The following discussion therefore covers, smelting temperature and coke addition, two factors affecting Cu and Co contents and Co content, respectively, in slag.
Figure 6 shows the effect of smelting temperature on Cu and Co contents in cleaned slag. It is apparent that copper and cobalt contents decrease gradually with the increase of temperature, especially in the case of copper content. The contents of Cu and Co in slag are 0.53 and 0.11 pct, respectively, at 1260 °C. A higher temperature allows a better fluidity and lower viscosity of slag, which favors aggregation and sedimentation of matte particles. As a result, less matte particles are entrained in slag. As the temperature increases from 1260 °C to 1290 °C, the contents of copper and cobalt change slightly. Considering the energy consumed in industrial production, the smelting temperature of 1260 °C is the optimal condition.
Table VIII shows that the Co content in slag decreases gradually as coke addition grows from 0.5 to 2.0 pct. The minimum value, Co 0.08 pct, occurs at 2.0 wt pct coke addition. According to mineralogical analysis of the matte–slag mixture, cobalt in slag mainly occurs in oxides. Under a reductive atmosphere, cobalt oxides are reduced to metals and enriched in matte. Copper mainly exists in the form of sulfide in the matte–slag mixture, so coke has little influence on the recovery of copper. Considering the coke dosage has a greater influence on cobalt content, a coke dosage of 2 pct provides the optimal condition.
Considering that the k2 values of factor C (pyrite) are the lowest for both Cu and Co contents in cleaned slag, the optimum pyrite dosage is taken at 2 wt pct of slag. Based on the above analysis, the optimum parameter of orthogonal experiment is determined as below: a coke dosage of 2 pct, a pyrite dosage of 2 pct, and a smelting temperature of 1260 °C. Under this condition, the contents of Cu and Co in cleaned slag are 0.41 and 0.06 pct, respectively.
Injection Simulation Experiment Results
After the optimum parameters were made known by this orthogonal experiment, an injection simulation experiment was conducted. Three methods of additive addition are compared for their recovery efficiency of Cu and Co, and the results are shown in Table IX. Experiments 1 and 2 were compared that by adding the additives on the surface of the molten slag directly, the contents of Cu (0.62 pct) and Co (0.17 pct) in resultant cleaned slag were higher than the contents (Cu 0.41 pct and Co 0.06 pct) in the cleaned slag by premixing additives. It indicates that direct addition of additives produces no favorable effect. In the case of the direct addition method, additives floating on the slag surface were partially burnt by oxygen, leading to waste of additives and insufficient contact between additives and slag. Experiments 1 and 3 were compared that by injecting additives into molten slag, the contents of Cu and Co in the resultant cleaned slag reached 0.46 and 0.01 pct, equivalent to the method of premixing the additives with slag. It suggests that the effect of the injection method is basically the same as the method of premixing additives with slag. As for experiments 2 and 3, the contents of Cu and Co in cleaned slag using injection method were lower than that of the direct adding method, especially for Co. It indicates that the utilization ratio of additives by injection method is much better than the direct addition method. When additives were injected, the reaction interfacial area between the additives and molten slag was significantly increased that the reaction was greatly accelerated. This innovative method maximizes the efficiency of additives and has great potential of application in the industrial sedimentation process.
Characterization of Smelting Products
Cleaned slag and copper–cobalt matte were obtained in the injection simulation experiment with an addition of coke and pyrite both at 2 wt pct. The XRD analysis of the smelting products is shown in Figure 7. It is observed from Figure 7(a) that the cleaned slag primarily comprises fayalite (Fe2SiO4), magnetite (Fe3O4), and augite (Ca(Fe,Mg)Si2O6). Compared with the matte–slag mixture of ISA furnace, bornite phase disappears in cleaned slag, suggesting that adding coke and pyrite by injection provides an efficient way of recovering valuable metals in the matte–slag mixture. According to XRD analysis of copper–cobalt matte in Figure 7(b), valuable metals are primarily enriched in matte in the form of sulfides.
Figure 8 presents the SEM-EDS analysis results of cleaned slag. In Figure 8(b), the spectrum of copper and cobalt is observed at low energy. Figures 8(c) and (d) show the EDS mapping of Cu and Co in cleaned slag. It is obvious that the point density of Cu and Co decreases much when compared to Figures 5(c) and (d). It further indicates that the reduction-sulfurization sedimentation process is an efficient way to minimize Cu and Co contents in sedimentation slag. The cleaned slag can be discarded directly or used as the raw material for production of cement, which reduces land occupation and environmental pollution.
Figure 9 presents the optical microscope images of the copper–cobalt matte. It is observed that Cu5FeS4 and Cu2S occur as a polycrystalline aggregate, the dominant phase in copper–cobalt matte. In addition, large particles of copper metallic are noted in matte. Cobalt mainly combines with iron in the form of sulfide and metallic state around iron sulfide. The copper–cobalt matte is further processed in the continuous converting furnace to produce a blister copper melt and cobalt-rich slag. The metal values in matte can also be separated and recovered using a leaching process.[26]
Conclusions
In this study, the characterization of traditional static sedimentation slag substantiates that Cu lost into slag mainly exists in the form of sulfide with fine particle size, while Co lost into slag mainly in the form of oxides. Therefore, coke and pyrite were used as reducing and vulcanizing agents to lower the contents of Cu and Co in sedimentation slag. The experiments were based on an OA16(43) orthogonal design and statistical analysis was employed to determine the optimal parameters. Higher temperature and a certain addition of coke and pyrite are beneficial to minimize Cu and Co contents in slag. The optimum slag cleaning conditions were found to be as follows: a coke dosage of 2 pct, a pyrite dosage of 2 pct, and a smelting temperature of 1260 °C for 2 hours. In order that additives are reasonably applied with greatest effect in industrial sedimentation process, the additives were injected into the molten slag by a specially designed additives injection device. Under the optimum conditions, Cu and Co present in cleaned slag reached 0.46 and 0.01 pct, respectively. They are almost as much as the Cu and Co contents in cleaned slag obtained by additive-premixing method, but lower than that obtained by adding additives on the surface of molten slag (Cu 0.62 pct and Co 0.17 pct). It proves that the injection of additives into molten slag sedimentation process is an effective method to recover Cu and Co from the matte–slag mixture of ISA furnace.
References
1. R. Matusewicz and E. Mounsey: JOM, 1998, vol. 50, pp. 51-52.
2. P. Vernon and S. Burks: J. S. Afr. Inst. Min. Metall., 1997, vol. 97, pp. 89-102.
3. Z.Z. He, J.Q. Qi: Modern Copper Metallurgy, Science Press, Beijing, BJ, 2003, pp. 289-95.
4. C. Arslan and F. Arslan: Hydrometallurgy, 2002, vol. 67, pp. 1-7.
5. R. Moskalyk and A. Alfantazi: Miner. Eng., 2003, vol. 16, pp. 893-919.
6. P.K. Gbor, V. Mokri, and C.Q. Jia: J. Environ. Sci. Health A, 2000, vol. 35, pp. 147-67.
7. H. Chikashi: Southern Africa Pyrometallurgy, Johannesburg, SA, March 2011, pp. 185-98.
8. E. Rudnik, L. Burzyńska, and W. Gumowska: Miner. Eng., 2009, vol. 22, pp. 88-95.
9. Y. Li, V.G. Papangelakis, and I. Perederiy: Hydrometallurgy, 2009, vol. 97, pp. 185-93.
10. A.H. Kaksonen, L. Lavonen, M. Kuusenaho, A. Kolli, H. Närhi, E. Vestola, J.A. Puhakka, and O.H. Tuovinen: Miner. Eng., 2011, vol. 24, pp. 1113-21.
11. J.H. Heo, B.S. Kim, and J.H. Park: Metall. Mater. Trans B, 2013, vol. 44, pp. 1352-63.
12. H. Shen and E. Forssberg: Waste Manage., 2003, vol. 23, pp. 933-49.
13. A. Sarrafi, B. Rahmati, H. Hassani, and H. Shirazi: Miner. Eng., 2004, vol. 17, pp. 457-59.
14. C. Tang, Y. Li, S. Yang, Y. Chen, L. Ye, and W. Zhang: Nonferr. Met., 2015, vol. 1, pp. 1-5.
15. D. Busolic, F. Parada, R. Parra, M. Sanchez, J. Palacios, and M. Hino: Min. Process. Extr. Metall., 2011, vol. 120, pp. 32-36.
16. X.J. Zhai, N.J. Li, X. Zhang, F. Yan, and L. Jiang: Trans. Nonferrous Met. Soc. China, 2011, vol. 21, pp. 2117-21.
17. S. Hughes: JOM, 2000, vol. 52, pp. 30-33.
18. Y. Li, Y. Chen, C. Tang, S. Yang, J. He, and M. Tang: J. Hazard Mater., 2017, vol. 322, pp. 402-12.
19. S. Mikhail and A. Webster: Canadian metallurgical quarterly, 1992, vol. 31, pp. 269-81.
20. T. Mitsuo, T. Shoji, Y. Hatta, H. Ono, H. Mori, and T. Kai: Trans. Jap. Inst. Metals, 1982, vol. 23, pp. 768-79.
21. Y. Okada, S. Fukagawa, K. Maya, H. Ikemiya, and K. Shinme: Rev. Metall., 1994, vol. 91, pp. 923-30.
22. W.H. Yang and Y.S. Tarng: J. Mater. Process. Technol., 1998, vol. 84, pp. 122-29.
23. X. Wu and D.Y. Leung: Appl. Energy, 2011, vol. 88, pp. 3615-24.
24. R.K. Roy: Design of experiments using the Taguchi approach: 16 steps to product and process improvement, John Wiley & Sons, New York, NY, 2001, pp. 15-29.
25. D. M. Byrne and S. Taguchi: Quality Progress, 1987, vol. 20, pp. 19-26.
26. L. Burzyńska, W. Gumowska, and E. Rudnik: Hydrometallurgy, 2004, vol. 71, pp. 447-55.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51834008, 51874040, 51504022); the Fundamental Research Funds for the Central Universities (Nos. FRF-TP-17-036A2, 230201606500078).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted April 11, 2018.
Rights and permissions
About this article
Cite this article
Yang, X., Zhang, J., Zhang, J. et al. Efficient Recovery of Copper and Cobalt from the Matte–Slag Mixture of ISA Furnace by Injection of Coke and Pyrite. Metall Mater Trans B 49, 3118–3126 (2018). https://doi.org/10.1007/s11663-018-1396-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1396-3