Abstract
This study provides the thermodynamic properties of the Mg-B binary alloys. Experiments were performed to measure the electromotive force (emf) as a function of temperature (773 K to 873 K) using a solid-state electrochemical cells that can be represented as
The activities of Mg in Mg-B alloys were calculated from the experimental emf data. The activity coefficients of Mg in Mg-B alloys were also determined. The integral Gibbs energies of formation (∆G °f ) of alloys were calculated from the activities of the Mg using tangent rule. The integral Gibbs energies of formation (∆G °f ) of MgB2, MgB4, and MgB7 are − 15.48, − 22.03, and − 15.89 kJ/mol-atoms at 873 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
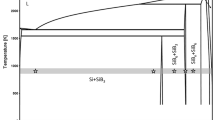
After the discovery of the MgB2 superconducting phase at 39 K,[1] the Mg-B binary system drew attention for further studies. The Mg-B binary system contains intermediate compounds, such as MgB2, MgB4, and MgB7. In this binary system, it has semiconducting phases, like MgB4 and MgB7, which could be good candidates for high-temperature thermoelectric applications. MgB4 has already been reported as a high-temperature thermoelectric material.[2] The other intermediate phases Mg3B2,[3] MgB6, and MgB12[4] are also reported in the early literature. However, they have not been confirmed yet with the further experimental evidence. Low-purity materials in the initial experiments, or the mixture of unstable borides and/or boron, were accountable for these phases.[5] In addition, based on Spear’s[6] assessment, Massalski reported the Mg-B phase diagram,[7] which also confirmed the three intermediate phases MgB2, MgB4, and MgB7. Recently, Liu et al.[8] and Kim et al.[9] used CALPHAD and ab initio calculations to reassess the Mg-B binary phase diagram. Their study did not confirm the Mg3B2, MgB6, and MgB12 phases. The phases present in Mg-B system and their crystallographic structures are summarized in Table I. These theoretical studies reproduced the phases reported by Massalski.[7] Thermo-Calc and PANDAT also corroborated the same in the literature.[10,11] Although all three boride phases are reported as stoichiometric compound,[12] several studies found the small homogeneity of the boride phases.[2,13,14,15,16,17,18] The phase diagram of Mg-B system obtained from Thermo-Calc is shown in Figure 1.[10] The literature has also reported the different decomposition temperatures of these phases, which are summarized in Table II. This might be due to the limited availability of experimental data and different sources of thermodynamic database employed in a CALPHAD method.
Binary Mg-B phase diagram.[10]
Experimental difficulties arise for Mg due to its chemical reactivity and high vapor pressure at high temperature.[19] Thus, the thermodynamic data for Mg-B binary system have been limited to a few experimental phase equilibria studies. Cook et al. carried out vapor pressure and enthalpy measurements using Knudsen effusion vacuum thermogravimetry and calorimetry over the temperature range of 873 K to 1123 K.[20] In their study, they limited their measurements up to 0.8 mole fraction of boron. Thus, the thermodynamic properties of Mg-B alloy beyond 0.8 mole fraction were not reported. In another study, Brutti et al. performed vapor pressure measurements using a Knudsen spectrometry technique over the range of 883 K to 1154 K.[21]
To manage the high vapor pressure of Mg at a high-temperature range of 873 K to 1123 K reported,[20,21] we executed our thermodynamic measurements in the range of 773 K to 873 K in a pure solid-state electrochemical cell using a solid electrolyte. The Mg-B system was studied in the current study using solid-state electrochemical cell:
In this cell, solid Mg served as a reference electrode (RE), solid-state CaF2 as an electrolyte, and solid Mg-B alloys as a working electrode (WE). Due to its superior chemical stability and higher ionic conductivity, CaF2 was used in this study.[22,23] Solid-state CaF2 electrolyte was widely utilized in the recent emf studies in the temperature range of 723 K to 1100 K.[24,25,26,27,28] It needs to be mentioned that a eutectic mixture of CaF2 and MgF2 \( ({\text{X}}_{{{\text{MgF}}_{2} }} = 0.504) \) was chosen to prepare the electrodes to preserve ionic conductivity.[29] Only metal fluoride, i.e., MgF2 could be used for this purpose, keeping in mind that metal fluoride tends to react with solid electrolyte CaF2. The eutectic CaF2-MgF2 mixture was chosen for maintaining ionic conductivity in the electrodes to avoid any interaction with the cell electrolyte (CaF2). It needs to be mentioned that the melting temperature of the CaF2-MgF2 eutectic mixture is 980 °C[30] which is above the experimental temperature range (500 °C to 600 °C).
Since fluoride-ion-conducting electrolyte CaF2 is used, the half-cell reactions for the galvanic cell are
Equations [2] and [3] give the net cell reaction:
The change in the partial molar Gibbs energy of magnesium (\( \Delta \bar{G}_{\text{Mg}} \)) for the proceeding galvanic cell reaction is written as
where \( a_{{{\text{Mg }}({\text{alloy}})}} \) is the activity of Mg in Mg-B alloy, \( a_{{{\text{Mg }}\left( {\text{S}} \right)}} \) is the activity of the pure magnesium, \( \Delta G _{\text{Mg}}^{ \circ } \) is the standard partial molar Gibbs energy, R is the universal gas constant, and T is the absolute cell temperature. The measured equilibrium open-circuit potential, Ecell, is used in the Nernst equation (Eq. [7]) to obtain the partial molar Gibbs energy of Mg and the activity of Mg in Mg-B alloys.
where n (n = 2) is the number of electrons participating in the half-cell reactions, and F is the Faraday constant (F = 96,500 C/mol). In the current study, the magnesium crystal (cr, P63/mmc) at each cell temperature T (773 K to 873 K) and the ambient pressure was selected as the standard state.
The more reliable and accurate thermodynamic data were measured after obtaining an equilibrium state in a solid-state galvanic cell. These equilibrium thermodynamic data can be used in the optimization of the phase boundary calculations for the Mg-B system.
Experimental
Electrode Preparation
Magnesium-boron alloys were prepared using different molar ratios of boron (XB = 0.07, 0.4, 0.73, 0.84, and 0.95) from an elemental mixture of magnesium and boron powder (both 99.99 pct pure, metal basis, purchased from Alfa Aesar). The mixing was done in an argon atmosphere glove box and transferred into a sealed jar for 24 hours jar milling to obtain a homogeneous mixture of boron and magnesium. After that, 13 mm diameter pellets were made from the homogeneous mixture using a Carver cold pressing unit at a pressure of 5000 psi for 5 minutes. The pellets were wrapped in a tantalum (Ta) foil to avoid surface oxidation, transferred into a vacuum-sealed quartz ampoule, and placed in a Fisher Scientific box furnace at 600 °C for 168 hours to get an equilibrium phase composition.
Electrodes (RE or WEs) were made using the mixture of Mg or Mg-B alloys and the eutectic mixture of CaF2-MgF2 (both 99.99 pct pure, metal basis, purchased from Alfa Aesar). The nominal compositions of the electrodes are summarized in Table III. These electrodes were also made into a 13 mm diameter pellet at 5000 psi for 5 minutes using a Carver cold pressing unit. Then, the electrodes were wrapped in a Ta foil and transferred into a vacuum-sealed quartz ampoule with Cu getter to avoid oxidation. Finally, the pellets were placed in a Fischer Scientific box furnace for sintering at 600 °C for 336 hours.[31]
Solid-State Electrochemical Cell
Figure 2 has shown the schematic diagram of the solid-state galvanic cell used in this study. In the previous studies, Reddy et al. had described the detailed experimental procedure for the determination of phase stabilities of different binary systems.[28,31,32,33,34] The solid-state emf cell was in a single compartment alumina tube. This alumina tube was placed in a vertical resistance furnace. A continuous flow of ultrahigh-purity dry argon was maintained in the compartment. A vacuum pump was continuously used to evacuate the chamber. In the argon-purged line, anhydrous calcium sulfate (dierite) and a Cu gettering furnace were utilized to eliminate residual moisture and oxygen from the argon gas, respectively. The Cu gettering furnace was maintained at 985 K. Cu getters were also used in the bottom of the main compartment to prevent any further oxidation during the experiment. The emf cell assembly (inset of Figure 1) was located in the isothermal zone of the vertical resistance furnace to maintain a uniform temperature. A type K thermocouple measured the cell temperature accurately. The emf cell assembly consists of the RE (bottom), the optical grade single-crystal CaF2 (purchased from Sigma Aldrich, 99.99 pct metal basis), and the WEs (top). This emf assembly was sandwiched between the Pt electrodes which were connected through the Pt/Rh wire to the Keithly 2700 multimeter. This sandwiched setup was pushed from both sides by alumina disks with the spring tightened alumina pressing tube. Open circuit potential (OCP) of electrochemical cell was obtained at a constant temperature after holding for 7 to 9 hours during the heating and cooling cycle, using the Keithly 2700 multimeter. The potential variation was within ± 0.01 mV for higher boron concentrations. This small variation was considered as the stable cell potential indicating that the system had reached an equilibrium state.[31]
Schematic cross-sectional diagram of solid-state electrochemical galvanic cell: 1—Pt/Rh wires with alumina sleeve, 2—sample thermocouple (type k), 3—gas outlet, 4—gas inlet, 5—water cooled brass flange, 6—vertical tubular furnace, 7—spring, 8—fire brick, 9—heating coil, 10—alumina pressing tube, 11—galvanic cell assembly, 12—Cu gutter, 13—alumina tube, 14—alumina support disk, 15—Pt disk, 16—working electrode, 17—CaF2 electrolyte, and 18—reference electrode
Electrodes’ (RE and WEs) Phase Equilibria and Phase Analyses
The pure Mg and Mg-B alloys were mixed with the CaF2-MgF2 eutectic mixture to prepare the RE and WEs, respectively. The electrodes’ phases were identified using a Philips X’pert MPD X-ray diffraction (XRD) analyzer, by varying the 2θ values from 10 to 90 deg with a step of 0.001 at 0.05 deg/s. The XRD was performed after the equilibrium measurement to validate the phase stabilities of the RE and WEs. In Figure 3, the XRD analysis confirms that no other phases are present in the RE after the equilibrium. The corresponding phases (Mg, MgF2, and CaF2) are indicated as a line (taken from ICDD) below the RE XRD spectrum. The PDF card of the probable competing phase CaMg2 is also added below the RE XRD spectrum to compare.
The XRD analysis in Figures 4(a) through (d) again confirms that other phases were not present in the WEs after the equilibrium measurements. The corresponding phases (Mg, B, MgB2, MgB4, MgB7, MgF2, and CaF2) are shown as a line (taken from ICDD) below the WEs XRD spectrum.
In addition, a detail thermodynamic phase equilibria study was also done using Fact Sage 7 (Tables IV and V) thermodynamic software.[35] In Table IV, all the competing phases are tabulated for the different nominal composition of the electrodes with their respective activities at 600 °C. As mentioned earlier, equimolar CaF2 and MgF2 were added in all the electrodes. As seen from Table IV, for any particular WE, the activities of any individual phase have the value of one (ai = 1), which represents the stability of that particular phase at 600 °C. In contrast, any phases contributing an activity value less than one (ai < 1) represents the less-stable phase at that temperature. For example, a particular alloying composition (Mg0.16B0.84) with equimolar of CaF2 (1 mole) and MgF2, the phase activities [CaF2, MgF2, MgB4, and Mg(B6)2] are one, which confirms that the four-phase equilibrium exists at 600 °C for this particular composition. It needs to be mentioned that the Mg(B6)2 phase is used alternatively for MgB7 in FactSage 7 (FactPS database). In addition, one of the leading competing phases, i.e., CaMg2 has the activities of less than one in all the electrodes system, which confirms that the formation of CaMg2 phase is thermodynamically not stable at 600 °C. In addition to this, the spontaneities of all the competing phases at the desired working temperature are summarized in Table V using reaction module of Factsage 7. The main competing phases, such as CaMg2 and Ca, are thermodynamically not possible since the Gibbs energies for those reactions are positive. This finding again confirms that the formation of these competing phases is not possible within this temperature range. These findings agree with the XRD analysis of the WEs after emf measurements. This also confirms that no other phases, such as CaMg2, were formed during the emf measurements. Moreover, it has already been discussed in the previous section that the recent literature confirmed that MgB7 is a more stable phase than MgB12 for a higher boride system. Therefore, MgB7− was considered in this study to obtain the equilibrium measurements for higher boride system.
Experimental Errors
Errors in experimental measurements could arise from the high vapor pressure of Mg, which eventually decreases the desired Mg concentration in electrodes. For a low concentration of Mg, the measured emf value varies from 240 to 680 mV for different compositions and temperatures. The measured emf values show up to 0.15 pct error that comes from the experimental setup and the instrumental measurement technique, which is less significant compared to the emf values that are large enough. Besides, for a high concentration of Mg, the measured emf values vary from 0.296 to 0.886 mV for different temperatures and compositions. The measured emf values show up to 0.3 pct error, which is within the acceptable limit. Since the emf values are small enough in high Mg concentration, the percent error has relatively greater significant effect than that observed with low Mg concentration. This uncertainty resulted from the highly volatile nature of Mg.
Results and Discussion
The stable open-circuit potential (emf) of the electrochemical cell represented in Eq. [1] for Mg-B alloys is shown in Figure 5 over the temperature range of 773 K to 873 K. All the emf measurements are also presented in Table VI. As seen in Figure 5, the emf data vary linearly with the temperature. No change in slope indicates the phase stabilities of these alloys over the selected temperature and compositional ranges. For the magnesium-rich solution (XMg ≥ 0.40), the measured emf values were very small. The measured emf values vs T for XMg = 0.93 and XMg = 0.40 are almost invariant. In this region, Mg + MgB2 are present. Pure Mg is the reason for obtaining a very lower emf value, which approaches zero with the increasing Mg concentration. This behavior agrees with the previously observed Ca-Bi, Ca-Mg, and Sr-Bi systems by Kim et al.[24,27] The emf values increased to ~ 0.25 V in the MgB2 + MgB4 two-phase region, and increase further in the MgB4 + MgB7 and B + MgB7 two-phase regions by ~ 0.3 and ~ 0.15 V, respectively. The emf is also shown as a function of mole fraction of boron in Figure 5. As seen in Figure 5, the emf values did not change significantly in the Mg + MgB2 binary region. It changed considerably after the composition crossed the MgB2 stoichiometric line. An increasing trend of emf change was observed with the increasing mole fraction of boron (Figure 6).
In Figure 7, the natural logarithm of activity of Mg, ln aMg, is presented, where the Nernst equation was used to calculate the activity of Mg at 823 K:
As seen from Figure 7, the activity of Mg decreases with the increasing mole fraction of boron. The activity does not change in the two-phase regions. Using this principle, we estimated the activities of Mg in the two-phase boundaries to obtain the activity of Mg close to the MgB2, MgB4, and MgB7 stoichiometric composition. The compositions of the prepared MgB2, MgB4 and MgB7 are varied due to the experimental uncertainties, characterized by energy dispersive X-ray spectroscopy (EDS). In Figure 8, the variation of Mg mole fraction is shown with the error bar. In Table VII, the EDS analyses of the phases are presented and compared to those in the literature. All the activity data (ln aMg) for 773 K to 873 K are reported in Table VIII. Activity coefficients are also calculated for Mg and summarized in Table IX. The integral Gibbs energies of formation (∆G °f ) of MgB2, MgB4, and MgB7 were also estimated using the tangent rule with the measured activities of the Mg. In the two-phase region, the equal chemical potential of each component or species characterizes the equilibrium. Hence, starting from the boron-rich (B + MgB7) equilibrium, \( \frac{{\Delta G_{\text{f}} \left( {{\text{MgB}}_{7} } \right)}}{1/8} = \frac{{RT\ln a_{\text{Mg}} }}{1} \) gives the ∆G °f (MgB7) = − 15.89 kJ/mol-atoms at 873 K. The integral Gibbs energies of formation (∆G °f ) for all the equilibrium phases for the range from 773 K to 873 K are summarized in Table X.
Conclusions
In this study, a solid-state galvanic cell over the temperature range from 773 K to 873 K was used to determine the thermodynamic properties of the magnesium-boron binary system. The emf values for different alloys of the Mg-B system (XB = 0.07, 0.6, 0.73, 0.84, and 0.95) were measured using a solid-state CaF2-based emf cell. The activities of Mg in Mg-B alloys were evaluated, and the activity coefficient of Mg at each alloy was also reported. The integral Gibbs energies of formation (∆G °f ) of MgB2, MgB4, and MgB7 were also estimated using tangent rule and reported for different temperatures. The integral Gibbs energies of formation (∆G °f ) of MgB2, MgB4, and MgB7 are − 15.48, − 22.03, and − 15.89 kJ/mol-atoms at 873 K, respectively.
References
J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani and J. Akimitsu, Nature, 2001, vol. 410, pp. 63-64.
A. Pediaditakis, M. Schroeder, V. Sagawe, T. Ludwig and H. Hillebrecht, Inorganic Chemistry, 2010, vol. 49, pp. 10882-10893.
W.C. Johnson, Journal of Chemical Education 1934, vol. 11, p. 256.
Y. Markovskii, Y.D. Kondrashev and G.V. Kaputovskaya, Zh. Obshch. Khim., 1955, vol. 25 pp. 433-444.
R. Naslain, A. Guette and P. Hagenmuller, Journal of the Less Common Metals, 1976, vol. 47, pp. 1-16.
K.E. Spear: Boron and Refractory Borides, V.I. Matkovich, ed., Springer, Berlin, 1977, pp. 439–456.
T.B Massalski, H. Okamoto, P.R. Subramanian, L. Kacprzak and W.W. Scott: Binary alloy phase diagrams, American Society for Metals, Metals Park, OH, 1986.
Z.K. Liu, Y. Zhong, D.G. Schlom, X.X. Xi and Q. Li, Calphad,2001, vol. 25, pp. 299-303.
S. Kim, D.S. Stone, J.I. Cho, C.Y. Jeong, C.S. Kang and J.C. Bae, Journal of Alloys and Compounds, 2009, vol. 470, pp. 85-89.
J.O. Andersson, T. Helander, L. Höglund, P. Shi and B. Sundman, Calphad, 2002, vol. 26, pp. 273-312.
S.L. Chen, S. Daniel, F. Zhang, Y.A. Chang, X.Y. Yan, F. Xie, R. S. Fetzer and W.A. Oates, Calphad, 2002, vol. 26, pp. 175-188.
S.D. Bohnenstiehl, M.A. Susner, S.A. Dregia, M.D. Sumption, J. Donovan and E.W. Collings, Thermochimica Acta, 2014, vol. 576, pp. 27-35.
B. Birajdar, N. Peranio and O. Eibl, Microscopy and Microanalysis, 2007, vol. 13, pp. 290-291.
D. G. Hinks, J. D. Jorgensen, Hong Zheng and S. Short, Physica C: Superconductivity 2002, vol. 382, pp. 166-176.
E. I. Kuznetsova, S. V. Sudareva, T. P. Krinitsina, Yu V. Blinova, E. P. Romanov, Yu N. Akshentsev, M. V. Degtyarev, M. A. Tihonovskiy and I. F. Kislyak, The Physics of Metals and Metallography, 2014, vol.115, pp. 175-185.
P. Bordet, M. Mezouar, M. N. Regueiro, M. Monteverde, M. D. Regueiro, N. Rogado, K. A. Regan, M. A. Hayward, T. He, S. M. Loureiro and R. J. Cava, Physical Review B, 2001, vol. 64, p. 172502.
X. H. Chen, Y. S. Wang, Y. Y. Xue, R. L. Meng, Y. Q. Wang and C. W. Chu, Physical Review B, 2001, vol. 65, p. 024502.
T. A. Prikhna, W. Gawalek, Y. M. Savchuk, T. Habisreuther, M. Wendt, N. V. Sergienko, V. E. Moshchil, P. Nagorny, C. Schmidt, J. Dellith, U. Dittrich, D. Litzkendorf, V. S. Melnikov and V. B. Sverdun, Superconductor Science and Technology, 2007, vol. 20, p. S257.
P. W. Gilles, Journal of the American Chemical Society, 1964, vol. 86, pp. 5702-5703.
L.P. Cook, R. Klein, W. W. Ng, Q. Huang, R. A. Ribeiro and P.C. Canfield, IEEE transactions on applied superconductivity, 2005, vol. 15, pp. 3227-3229.
S. Brutti, A. Ciccioli, G. Balducci, G. Gigli, P. Manfrinetti and A. Palenzona, Applied Physics Letters, 2002, vol. 80, pp. 2892-2894.
N. I. Sorokin and B. P. Sobolev, Crystallography reports, 2007, vol. 52, pp. 842-863.
J. Delcet, R. J. Heus and J. J. Egan, Journal of The Electrochemical Society 1978, vol. 125, pp. 755-758.
H. Kim, D.A. Boysen, D.J. Bradwell, B. Chung, K. Jiang, A.A. Tomaszowska, K. Wang, W. Wei and D. R. Sadoway, Electrochimica Acta, 2012, vol. 60, pp. 154-162.
S. Poizeau, H. Kim, J. M. Newhouse, B. L. Spatocco and D. R. Sadoway, Electrochimica Acta, 2012, vol. 76, pp. 8-15.
J.M. Newhouse, S. Poizeau, H. Kim, B.L. Spatocco and D.R. Sadoway, Electrochimica Acta, 2013, vol. 91, pp. 293-301.
N.D. Smith, T. Lichtenstein, J.Gesualdi, K. Kumar and H. Kim, Electrochimica Acta, 2017, vol. 225, pp. 584-591.
P. George, S. C. Parida and R.G. Reddy, Metallurgical and Materials Transactions B 2007, vol. 38, pp. 85-91.
S. C. Hu and L. C. D. Jonghe, Ceramics International, 1983, vol. 9, pp. 123-126.
P. Villars, K. Cenzual and R. Gladyshevskii: Handbook. (Walter de Gruyter GmbH & Co KG, Berlin, 2015).
M.A. Imam and R.G. Reddy, Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies, Springer, Cham, Switzerland, 2017, pp 457-464.
S.C. Parida and R.G. Reddy, The Journal of Chemical Thermodynamics, 2007, vol. 39, pp. 888-892.
R.G. Reddy, A.M. Yahya and L. Brewer, Journal of alloys and compounds, 2001, vol. 321, pp. 223-227.
S.G. Kumar, R.G. Reddy and L Brewer, Journal of phase equilibria 1994, vol. 15, pp. 279-284.
C. W. Bale, P. Chartrand, S. A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A. D. Pelton and S. Petersen, Calphad, 2002, vol. 26, pp. 189-228.
Acknowledgments
The authors gratefully acknowledge the financial support, Grant No. DMR-1310072, provided by the National Science Foundation (NSF). The authors thank Dr. M.R. Bogala and J.S. Young for proof reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 12, 2017.
Rights and permissions
About this article
Cite this article
Imam, M.A., Reddy, R.G. Thermodynamic Properties of Magnesium-Boron Binary Alloys Determined Using Solid-State Electrochemical Measurements. Metall Mater Trans B 49, 3504–3512 (2018). https://doi.org/10.1007/s11663-018-1392-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1392-7










 estimated from experimental data
estimated from experimental data
 )
)