Abstract
Samples of INCONEL 718 were levitated and melted in a slag by the application of an electromagnetic field. The effects of temperature, time, and slag composition on the inclusion content of the samples were studied thoroughly. Samples were compared with the original alloy to study the effect of the process on inclusions. Size, shape, and chemical composition of remaining non-metallic inclusions were investigated. The samples were prepared by Standard Guide for Preparing and Evaluating Specimens for Automatic Inclusion Assessment of Steel (ASTM E 768-99) method and the results were reported by means of the Standard Test Methods for Determining the Inclusion Content of Steel (ASTM E 45-97). Results indicated that by increasing temperature and processing time, greater level of cleanliness could be achieved, and numbers and size of the remaining inclusions decreased significantly. It was also observed that increasing calcium fluoride content of the slag helped reduce inclusion content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Superalloys have been used in a variety of applications. These include gas turbines, steam turbines, military appliances, medical devices, offshore piping, and heat exchangers.[1,2] The nature of these applications requires materials that can withstand harsh operating conditions. Non-metallic inclusions are among factors that can limit operation conditions of superalloys.
Non-metallic inclusions are particles dispersed throughout the matrix of the alloy. Inclusions are usually compounds of metals (iron, silicon, aluminum, etc.) with non-metallic elements such as oxygen, sulfur, and nitrogen.[3,4,5] Inclusions can adversely affect mechanical and physical properties of superalloys. Mechanical properties such as fracture toughness, impact resistance, fatigue resistance, and hot working conditions are greatly reduced by the presence of inclusions.[6,7,8,9] Incoherency between lattice planes of the matrix and the inclusion results in stress concentration and appearance of cracks that could ultimately lead to failure of the component. Larger inclusions are generally more detrimental to the mechanical properties of superalloys. It has also been reported that hard and brittle inclusions are more harmful than soft inclusions.[3,5,6] It is therefore of great interest to reduce inclusion content and size in superalloys.
Three main sources of non-metallic inclusions have been identified in superalloys.[10] These include:
-
1.
Impurities originating from the starting material(s) and carried over to the final product.
-
2.
Impurities and inclusion content from scrap material used in superalloy production.
-
3.
Inclusions formed during the processing of the alloy.
Standard methods employed to process and refine cast superalloys include secondary vacuum induction melting (VIM), electroslag refining (ESR), and vacuum arc remelting (VAR). A combination of these techniques is used in the processing of most superalloys and special steels.[11] VIM process serves to remove some of the inclusions by filtering and/or slag–metal reactions, extract dissolved gases such as oxygen and nitrogen, and evaporate harmful impurities such as lead and bismuth. In addition to decreasing gas content and enhancing mechanical properties, the ESR process is particularly useful in removing non-metallic inclusions. VAR helps to remove nitrogen and other dissolved gases. The alloy is also homogenized while some of the inclusions are removed. Electron beam cold hearth refining (EBCHR) has been used to some extent for refining some alloys.[12] It must, however, be mentioned that this technique is costly, and some researchers have expressed doubt about its usefulness.[13] Electron beam button melting has been used to produce superalloy samples with enhanced cleanness.[14]
While these methods can effectively remove most of the inclusion content of superalloys, a significant amount of inclusions remain in the bulk of the metal. Researchers studying cleanness of steel and superalloys have reported substantial amounts of post treatment inclusion content.[15,16,17,18,19]
One reason for the above observation could be explained by the mechanism of inclusion removal in methods currently employed. None of these techniques can cause sufficient stirring and turbulence in molten droplets. As the sample melts, molten droplets pass through molten slag or vacuum. If an inclusion particle happens to float to the surface of the droplet, it can be absorbed into the slag. No provision is made in ESR and VAR for removal of inclusion particles that do not come to the surface of the particle. Thermocapillary effect can cause stirring in Electron beam processes. This effect, however, diminishes as the depth of melt increases. VIM offers an important first stage refining, which requires further operations for acceptable results.
If a mechanism is adopted, where, melt droplets are stirred while passing through a slag layer, it should be possible to obtain alloys with close to zero inclusion contents. This idea can be tested by melting thin sections of a superalloy and letting molten drops pass through a slag while under the effect of the electromagnetic field. The field both keeps the metal molten, and induces turbulence and stirring within the drop. Thus, every inclusion particle will at some point come to the surface and be exposed to the slag layer surrounding the drop. Electromagnetic levitation melting technique was adapted for this purpose. Using this method, in addition to the process itself, the effect of time on cleanliness may be studied.
Electromagnetic levitation melting (ELM) is an interesting phenomenon which allows the researcher to study molten samples of metals / alloys in a containerless state. It is readily deduced that the samples under study will practically remain as pure as they were at the beginning of the runs. This technique has the potential to be used in many applications to study physical properties of metals. Researchers have employed ELM to measure surface tension,[20,21] thermophysical properties,[22] specific heat capacity,[23] emissivity,[24] thermal expansion,[25] dissolution of impurity gases,[26] and many other properties of metals and alloys. ELM has also been used to observe the behavior of non-metallic inclusions in superalloys.[27] Recently, researchers have adopted this method to produce high purity metallic nanopowders.[28,29] While some researchers have tried to model ELM,[30,31,32] many aspects of this technique remain unexplained.
Electromagnetic levitation melting of metallic samples in a slag can serve to investigate the behavior of inclusions in metallic samples subjected to an electromagnetic field. The inherent nature of this technique allows the sample to be kept in the experimental condition for an indefinite length of time; therefore the effect of time on the process may be studied. Samples levitated in a gas experience oscillations which can lead to the molten drop sticking to the reaction tube. A molten slag has a damping effect on these oscillations. The samples remain stable. This method has not been used before for removal of non-metallic inclusions.
Samples of INCONEL 718 were levitated and melted in a slag by the application of an electromagnetic field. The effects of temperature, time, and slag composition on the inclusion content of the samples were studied thoroughly. Samples were compared with the original alloy to study the effect of the process on inclusions. Size and shape of remaining non-metallic inclusions were investigated. The samples were prepared by Standard Guide for Preparing and Evaluating Specimens for Automatic Inclusion Assessment of Steel (ASTM E 768-99) method and the results were reported by means of the Standard Test Methods for Determining the Inclusion Content of Steel (ASTM E 45-97).
Experimental Procedure
INCONEL 718 samples weighing 1 to 2 g were studied in this research. Chemical composition of the alloy is indicated in Table I.
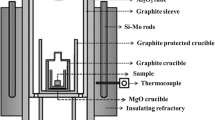
Figure 1 exhibits a detailed drawing of the levitation apparatus. Prior to melting runs, slag samples were prepared. High purity (> 99.9 pct) lime, alumina, and fluorite powders were weighed and thoroughly mixed in the predetermined ratios. CaO serves to control the basicity of the refining slag, while CaF2 lowers melting point and viscosity. Since the melting point of the refining slag should be lower than the superalloy, a slag with a low melting point was prepared and used as the initial flux.[33] The mixture was placed in platinum crucibles and melted in an electric furnace. Molten slags of the desired composition were poured in copper crucibles and left to solidify in air. Solidified slags were subsequently crushed and placed in the silica reaction tube. Finding the correct amount of slag to put in the silica tube was a matter of trial and error. After a couple of initial runs it was found that the level of crushed slag in the tube should not be higher than the top (stabilizing) levitation coil.
Table II indicates the chemical compositions of the prepared slag samples. In order to find the effect of process temperature, process time, and slag composition on the inclusion content in the refined samples, 8 experiments were conducted. Experiment details are listed in Table III.
Superalloy samples were placed on top of the slag, the top lid was closed. The top lid had been connected to a vacuum pump and an argon source by a three-way valve. The chamber was initially put under vacuum for one minute and then argon was allowed into the chamber. Atmospheric pressure of argon was maintained during the runs for safety reasons. A thin sheet of mica refractory was wrapped round the silica tube, but was initially lowered to the bottom of the tube so that the operator could see where the sample was.
The induction current was turned on, and the sample was allowed to melt. The starting time of each run was assumed to be the moment that the sample melted. The molten sample would melt the surrounding slag. Using the power control, position of the sample was fixed so that only a small portion of the metal would protrude from the top of the molten slag. The rest of the molten metal was surrounded by the slag. The power control also served to control the temperature of the sample. In levitation melting, increasing the power lifts the sample away from the electromagnetic field generated by the bottom (heating and levitating) coil. As a result, the temperature is reduced by increasing power and vice versa. When the sample was steady and stable, the mica sheet was lifted upwards to cover the sides of the reaction tube. Temperature of the sample was taken using a disappearing filament pyrometer. The light from the sample was reflected towards the pyrometer by a mirror that was placed above the top window, making an angle of 45 deg with the axis of the tube. Previous calibrations had revealed that the combination of top window and mirror resulted in an error of 30 °C. All the readings were corrected accordingly. The mica sheet served to make the system resemble a black body so that the emissivity of the sample could be assumed to be unity. Unlike what was initially expected, very few runs were complicated by the localized melting and fusion of the silica reaction tube to the molten slag. These runs were repeated using smaller samples which did not cause the slag to fuse into the silica tube. In most runs, due to the refractory nature of the slag, some parts of the slag remained solid (unmelted) making it easy to extract the sample at the conclusion of the test.
Figure 2 is a photograph taken from the side of the apparatus while the sample is molten. The mica sheet was taken away for this photograph. Figure 3 exhibits a photograph taken from the top window while a sample is solidifying at the end of a run. Figure 4 is a schematic drawing of a levitated sample surrounded by molten slag in an electromagnetic field.
At the end of each run, the sample was allowed to cool, and was subsequently extracted from the reaction tube by a pair of tweezers. The sample was then sliced and mounted for microscopy.
Results and Discussion
The following methods have been employed to report the results; method A (Worst Fields) and method D (Low Inclusion Content). These methods require a survey of a 160 mm2 polished surface area of the specimen at ×100. The field size shall equal an area equivalent to 0.50 mm2 on the specimen surface as defined by a square with 0.71 mm long sides. Each 0.50 mm2 field is compared to the standard square field in a search for the worst field that is the highest severity rating, of each inclusion type A, B, C, and D for both thin and heavy series. The severity level of these worst fields will be reported for each sample (method A). On the other hand, in method D the severity level number for each inclusion type is recorded. In contrast with method A, this is an every field rating method. Figure 5 indicates suggested overlay grid for methods A and D.
It should be mentioned that the samples in this study have not been deformed and as a result no A, B, and C type inclusions were detected. Therefore, the minimum inclusion number (of type D) determines the Severity Level Numbers listed in Table IV. It is important to note that if a field of inclusions falls between two severity levels it is rounded down to the nearest severity level.
Effect of Temperature
Three sets of experiments were conducted to find the effect of processing temperature on the inclusion content in the samples. Samples were melted at three different temperatures of 1923 K, 1973 K, and 2003 K (1650 °C, 1700 °C, and 1730 °C) (sample 4, 8, and 1, respectively). Time allocated for each test was 10 minutes and slag composition 1 was adopted in all runs. Extracted data using methods A and D are plotted in Figures 6 and 7, respectively.
Figure 6 indicates that the worst severity level for inclusion type A has been decreased from 2 in the original sample to 1 in sample 4. This value was found to be 0.5 for samples 8 and 1. Observed results clearly indicate the effect of temperature on lowering inclusion content in samples. The worst severity level for D type Heavy inclusion type was decreased to 0.5 in sample 4 from the original value of 1.5. No D type Heavy inclusion was found in samples 8 and 1. No D type Oversize inclusions were detected in any of the samples either. Samples refined at higher temperatures not only showed lower content of inclusions, but also the remaining inclusions were smaller in size, compared to the original sample. Method D was employed to report the frequency of all severity levels for all inclusion types. Figures 7(a) through (e) show the severity level frequency for inclusion types D, D Heavy, D Oversize 25 μm, D Oversize 37.5 μm, and D Oversize 50 μm, respectively. Analyzing extracted data of method D illustrate a decrease in the severity level values for all inclusion types according with any increase in the processing temperature.
Effect of Time
In order to find the effect of time on inclusion removal, four tests were conducted for different times of 2, 5, 10, and 20 minutes (samples 2, 3, 4, and 7, respectively). All the experiments were conducted at a constant temperature of 1923 K (1650 °C), and slag composition 1. Figures 8 and 9 show the results obtained by Method A and Method D, respectively.
Figure 8 shows the effect of processing time on inclusion content. Compared with the original sample, no oversize inclusions (type D) can be detected in any of samples with 2, 5, 10, and 20 minutes refining time. The severity level of D type inclusion decreases from 2.0 to 0.5 during 20 minutes of processing. The severity level of heavy inclusion type D, which was 2.0 for the original sample, is observed to have dropped to values of 0.5 and 0 during processing times of 10 and 20 minutes, respectively. All the Oversize inclusions are eliminated immediately after 2 minutes experiment time. The frequency of all severity levels is shown in Figure 9(a) through (e) for Inclusion types D, D Heavy, D Oversize 25 μm, D Oversize 37.5 μm, and D Oversize 50 μm, respectively. Lower amounts of Heavy and Oversize inclusions have been found in samples which were processed for longer times. Once again, the decreasing trend in inclusion content along with processing time can be observed in Figure 9. As expected, this is consistent with the observed result, the longer refining process time results in cleaner samples.
Figure 10 indicates the effect of slag on inclusion content observed in the microstructures of different samples. While non-metallic inclusions are readily detectible in an optical micrograph, samples treated in slag for various lengths of time seem much cleaner.
Effect of Slag Composition
Three different slag compositions (Table II) were considered in this study. Heats were conducted at 1923 K (1650 °C) for 10 minutes. Samples 4, 6, and 5 were melted with slag compositions 1, 2, and 3, respectively. Slag 1 has the lowest melting point. In slag compositions 2 and 3 the ratios CaF2:CaO:Al2O3 were 6:2:2 and 8:1:1, respectively. So, while the ratio of lime to alumina remains constant, the fraction of calcium fluoride in slag 3 is increased.
Figure 11 indicates that samples 5 and 6 have the same worst severity level for all inclusion types.
However, sample 5 has a smaller value for frequency of the severity levels for inclusion types D and D Heavy, as illustrated in Figure 12. Sample 5, which also has the lowest amount of remaining inclusion content, was refined using slag composition 3, that is, the slag composition with highest ratio of Fluorite. This is clearly an indication of the importance of CaF2 content in refining slags. CaF2 lowers melting point and viscosity of the slag and hence improves its ability to remove inclusions from melt.
Comparison with Existing Practices
Inclusion removal mechanism in this method is quite similar to the ESR process. Qualitatively, inclusions that come to the surface of the melt are absorbed into the slag. The absorption mechanism is a complex function of temperature, slag composition, inclusion type, and surface energies between these phases. No systematic study has been conducted to identify and formulate parameters that are most influential in this respect. There is however one major difference between this method and ESR (or other methods based on slag such as EBCHR). In this technique, the electromagnetic field induces severe stirring in the molten sample. As a result, any inclusion inside the bulk of the melt will at some point come to the surface and make contact with the slag. The slag has thus a chance to absorb all inclusions.
In contrast, in ESR and EBCHR processes inclusion particles can only come in contact with the slag if they happen to be on the surface of the metal when it melts. There is no mechanism to drive inclusions from bulk of the melt to the surface where it can be absorbed by the slag. In electron beam (EB) techniques, a fluid flow is generated on the melt surface in the vicinity of the incident beam. This flow is caused by thermocapillary effects but is not as effective as the stirring caused by an electromagnetic field.
It is needless to emphasize the crucial role of cleanness in superalloys. Lower inclusion content can indeed enhance operation conditions and increase life cycle of industrial and machinery parts. This method has the potential to increase operating temperature of gas turbines. Work is now focused on devising a technique for continuous processing of superalloys using this method for ultimate cleanness.
Conclusions
-
1.
Application of an electromagnetic field to molten droplets of alloy in a slag can substantially reduce inclusion content.
-
2.
By increasing temperature of the melt, the extent of inclusion removal is increased.
-
3.
Inclusion removal is enhanced by increasing residence time of the melt inside the slag.
-
4.
Increasing CaF2 content of the slag can improve inclusion removal.
References
C. Briant: Materials and Manufacturing Processes, 2000, vol. 15, pp. 155-156.
S. Zhao, X. Xie, G. D. Smith, and S. J. Patel: Materials Science and Engineering: A, 2003, vol. 355, pp. 96-105.
R. Kiessling: Met. Sci., 1980, vol 14 (5), pp. 161-172.
T. Lund and J. Åkesson: Effect of Steel Manufacturing Processes on the Quality of Bearing Steels, ed. J.J.C. Hoo, ASTM International, West Conshohocken, 1988, pp. 308–29.
Y. Tomita: Materials Science and Technology, 1995, vol. 11, pp. 508-513.
H. Atkinson and G. Shi: Progress in Materials Science, 2003, vol. 48, pp. 457-520.
J. Lankford: International Metals Reviews, 1977, vol. 22, pp. 221-228.
S. Paul and A. Ray: Journal of Materials Engineering and Performance, 1997, vol. 6, pp. 27-34.
S. Yamaguchi, H. Kobayashi, T. Matsumiya, and S. Hayami: Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 1980, vol. 295, pp. 122.
A. Bromley and R. Parker: Metals Technology, 1984, vol. 11, pp. 419-427.
C.T. Sims, N.S. Stolof and W.C. Hagel: Superalloys II: High Temperature Materials for Aerospace and Industrial Power, Wiley, New York, 1987.
Y. Haruna: Theses Dissertations, University of British Colombia, 1994.
P. Quested, M. McLean, and M. Winstone: Proceedings of Superalloys 1988, TMS/AIME, Warrendale, pp. 387–96.
M. Halali, M. McLean, and D.R.F. West: Materials Science and Technology, 2000, vol. 16, pp. 457-462.
S. Ahmadi, H. Arabi, A. Shokuhfar, and A. Rezaei: Journal of Materials Science & Technology, 2009, vol. 25, pp. 592-596.
F. Binczyk, J. Cwajna, S. Roskosz, and P. Gradoń: Archives of Foundry Engineering, 2012, vol. 12, pp. 5–10.
[17] H. Matysiak, J. Michalski, A. Balkowiec, K. Sikorski, and K. Kurzydłowski: Materials Science-Poland, 2009, vol. 27 (4/1), pp. 1103-1110.
H. Matysiak, M. Zagorska, J. Andersson, A. Balkowiec, R. Cygan, M. Rasinski, et al., Materials, 2013, vol. 6, pp. 5016-5037.
L. Zhang, B. G. Thomas, K. Cai, J. Cui, and L. Zhu: ISS Tech 2003 Conference Proceedings, ISS-AIME, Warrnedale, pp. 141–56.
H.P. Wang, B.C. Luo, T. Qin, J. Chang, and B. Wei: J. Chem. Phys. 2008, 129(12), 124.
M. Przyborowski, T. Hibiya, M. Eguchi, I. Egri: Journal of Crystal Growth, 1995, vol. 151 (1-2), pp. 60-65.
G. Betz, M.G. Frohberg: High Temp. High Press., 1980, vol. 12, pp. 169-178.
K. Ohsaka, J.C. Holzer, E.H. Trinh, and W.L. Johnson: Proceedings of the 4th International Conference Experimental Methods for Microgravity Materials Science Research, 1992, ed. R.A. Schiffman, San Diego, pp. 1–6.
G.P. Hansen, S. Krishnan, R.H. Hauge, J.L. Margrave: Appl. Opt. 1989, 28(10), 1885–1896.
B. Guo: MSc thesis, Auburn University, 2006.
M. Halali: Solubility of Nitrogen in UDMET720, unpublished work.
M Halali, D.R.F. West, and M. McLean: Proceedings of the 8th International Symposium Super Alloys, Seven Springs, 1996.
M. Malekzadeh, M. Halali: Chemical Engineering Journal 2011, vol. 168, pp. 441–445.
A. Vahid Mohammadi, M. Halali: RSC Adv. 2014, vol. 4, pp. 7104-7108.
J. Szekely, E. Schwartz, R. Hyers: JOM 1995, vol. 47 (5), pp. 50–53.
Z.A. Moghimi, M. Halali, M. Nusheh: Metallurgical & Materials Transactions B 2006, vol. 37B (6), pp. 997-1005.
A.A. Roy, V. Bojarevics, and K.A. Pericleous: Proceedings of the COMSOL Conference 2011, Stuttgart, Germany.
A. Zaitsev, N. Korolyov, and B. Mogutnov: Journal of Materials Science, 1991, vol. 26, pp. 1588-1600.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 21, 2017.
Rights and permissions
About this article
Cite this article
Manjili, M.H., Halali, M. Removal of Non-metallic Inclusions from Nickel Base Superalloys by Electromagnetic Levitation Melting in a Slag. Metall Mater Trans B 49, 61–68 (2018). https://doi.org/10.1007/s11663-017-1137-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1137-z