Abstract
The effect of Al2O3 concentration on the density and structure of CaO-SiO2-Al2O3 slag was investigated at multiple Al2O3 mole percentages and at a fixed CaO/SiO2 ratio of 1. The experiments were conducted in the temperature range of 2154 K to 2423 K (1881 °C to 2150 °C) using the aerodynamic levitation technique. In order to understand the relationship between density and structure, structural analysis of the silicate melts was carried out using Raman spectroscopy. The density of each slag sample investigated in this study decreased linearly with increasing temperature. When the Al2O3 content was less than 15 mole pct, density decreased with increasing Al2O3 content due to the coupling of Si (Al), whereas above 20 mole pct density of the slag increased due to the role of Al3+ ion as a network modifier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CaO-SiO2-Al2O3 is one of the important silicate melts in the iron- and steel-making processes. Thermo-physical properties of the slag such as density, viscosity, surface tension, and thermal conductivity are crucially important to optimize the high temperature processes. Notably, these properties are highly dependent on the silicate structure.[1] Therefore, advanced knowledge of structural information is essential to develop a thorough comprehension of the slag system.
In the CaO-SiO2-Al2O3 slag system, CaO is a basic oxide that acts as a modifier in silicate melts, i.e., it depolymerizes the silicate structure and provides non-bridging oxygen (NBO). The structural units of silicate melts are monomer (SiO4 4−), dimer (Si2O7 6−), chain (SiO3 2−), sheet (Si2O5 2−), and fully polymerized structures (SiO2).[2] These structural units are denoted by Q0, Q1, Q2, Q3, and Q4, respectively (Qn notation indicates n bridging oxygens per tetrahedron). Mysen et al. suggested that these structural units are equilibrated according to the quantity of non-bridging oxygen atoms per cation (NBO/T).[3,4]
In this study, NBO/T is estimated according to Eq. [4].[5,6]
On the other hand, Al2O3 is an amphoteric oxide whose equilibrium forms are suggested by Eqs. [5] through [7].[2]
In a silicate system, alumina forms Al3+ ions that balance the free oxygen ions and also combine with free oxygen ions to form AlO4 5− ions. Accordingly, the relation between Al3+ and AlO4 5− ions will depend on the slag basicity (or the activity of oxygen ions); Al3+ is more stable in the acidic region, whereas AlO4 5− in the basic region.[2] If the metal cation Ca2+ balances the local charge, Al3+ may fit into the tetrahedral sites of the silicate structure. Accordingly, Si(Al) coupling occurs upon substitution of Si by Al in the tetrahedral structure of the silicate melt.[3,7,8,9] If there are insufficient metal cations, Al3+ may dissociate from the tetrahedral sites and act as a network modifier.
This structural modification may yield changes in the thermo-physical properties of silicate melts. For example, Grundy et al. reported the relationship between viscosity and structure of the CaO-SiO2-Al2O3 system at 1773 K (1500 °C).[10] They investigated the variation of viscosity with respect to the (wt pct Al2O3)/[(wt pct Al2O3) + (wt pct CaO)] at constant SiO2 concentrations (40, 50, 60, and 70 wt pct). The viscosity exhibited maximum values when this ratio equaled to 0.7 to 0.8, regardless the SiO2 content. They asserted that the transition of the composition dependence in the viscosity was due to the amphoteric structure modification by Al2O3. Kang and Morita measured the thermal conductivity of the CaO-SiO2-Al2O3 system at 1773 K and 1873 K (1500 °C and 1600 °C) at fixed CaO/SiO2 ratios of 0.4 and 0.9, and obtained maximum thermal conductivity values when the Al2O3 content was at around 15 wt pct.[11] From nuclear magnetic resonance (NMR) analysis, they confirmed that AlO4 5− was dominant when the Al2O3 content was below 15 wt pct, while Al3+ was high when Al2O3 content was above 15 wt pct.
Although density provides direct information about the silicate structure and allows observation of the amphoteric behavior of Al2O3, it has not been examined in conjunction with structural analysis. In this study, the relationship between density and structure of the CaO-SiO2-Al2O3 slag system is investigated as a function of Al2O3 content at a constant ratio of CaO/SiO2 = 1.
Experimental
Sample Preparation
For density measurements, six samples were prepared. The composition ranged from 4 to 24 mole pct of Al2O3 at a fixed CaO/SiO2 ratio of 1. After weighing and mixing chemicals, the slag sample was pre-melted in a platinum crucible at 1853 K (1580 °C), which was above the liquidus temperature of the sample. In this study, CaO was prepared in advance by the calcination of CaCO3 at 1373 K (1100 °C) for two hours in a furnace under air atmosphere. Pre-melting was performed twice in a vertical electric resistance furnace, and each time the sample was ground to fine powder to ensure homogeneity. Finally, the sample was charged in a platinum crucible and placed in the furnace for an additional 2 hours to remove internal bubbles. The vitrescence nature of the prepared samples was confirmed by X-ray diffraction (D/Max-2500V/PC, Rigaku) as shown in Figure 1. The sample composition was analyzed using scanning electron microscopy (S-4300, Hitachi) equipped with energy dispersive X-ray spectroscopy (6853-H, Horiba).
Aerodynamic Levitation Method
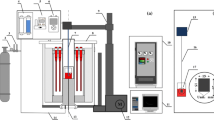
The aerodynamic levitation facility was used for the density measurements.[12] With this technique, the temperature range of the measurements can be higher compared to the conventional Archimedean method.[13] Figure 2 shows a schematic illustration of the experimental facility. Once a sample weighing 35 to 50 mg was placed in the sample holder, the sample was heated rapidly with a CO2 laser (100 W, Firestar-t series, Synrad Inc., USA) with blowing O2 gas through a conical nozzle (2.5 mm diameter) at a flow rate of 400 ml/min STP. Temperature was controlled in the range of 2154 K to 2423 K (1881 °C to 2150 °C), which was measured with a pyrometer (IR-SAS2SN, Chino, Japan). The temperature calibration was carried out by measuring the melting point of the CaO-SiO2 compound. It was assumed that the emissivity of the slag was the same as that of the CaO-SiO2 slag at the melting point and did not change with temperature and composition.
During the experiments, the sample images were captured with a high-speed video camera (FASTCAM MC2, Photron, Japan). Figure 3 shows typical images of the sample. The surface contour of the sample image and the corresponding diameter and volume were determined using lab-made MATLAB software. Figure 4 shows the radius of a typical sample as a function of the rotation angle ϕ. Differences between the average radius and the maximum and the minimum radii are 1.37 and 1.58 pct, respectively. If it is assumed that the vertical radius has a value between the maximum and the minimum, the maximum error in volume would be −1.80 to +1.15 pct.
Raman Spectroscopy Analysis
The structure of the silicate melts was investigated using Raman spectroscopy. For the structural analysis, two additional samples were examined together (1.87 and 32.79 wt pct Al2O3). The Raman spectra were recorded with a Horiba Jobin-Yvon LabRam Aramis spectrometer. A 514.5 nm beam from an Ar-ion laser was used as the excitation source. The Raman scattered light signal was collected in a backscattering geometry using a 50x microscope objective lens. The Raman excitation beam spot size was ~1 μm in diameter.
Results
Density
Density of each slag sample is plotted as a function of temperature in Figure 5. A linear temperature dependence of density was observed: the density decreased with increasing temperature. For comparison, density values obtained from the Archimedean method are plotted with the experimental results.[13,14] The extrapolated value from the present results of the sample containing 4.23 mole pct of Al2O3 is slightly higher than that reported in reference 13 (the difference being ~3.7 pct). The results of the sample containing 11.73 mole pct Al2O3 show a much steeper temperature dependence than the reported data in Reference 14. They have almost the same value at 2073 K. With the Archimedean method, inaccurate data for thermal expansion of the bob might cause experimental errors. On the other hand, with the aerodynamic levitation method, the assumption of a constant emissivity might cause experimental errors. In Figure 6, the slag density is plotted with respect to mole pct of Al2O3. Regardless of experimental temperature, the density initially decreased with increasing Al2O3 content up to 15 pct, and then increased from 20 pct. The transition composition region is close to that observed in thermal conductivity measurements by Kang and Morita.[11]
Raman Spectra
Figure 7 shows the Raman spectra of the CaO-SiO2-Al2O3 (CaO/SiO2 = 1) slag samples at multiple Al2O3 mole percentages. In the spectra, two broad peaks appeared in the spectral range of 800 to 1200 cm−1 when the Al2O3 concentration was less than ~5 mole pct. When the Al2O3 content was higher than ~5 mole pct, one broad peak was observed. The maximum intensity peak position gradually shifted to the lower frequency side as Al2O3 concentration increased. However, at Al2O3 concentrations above ~15 mole pct, the peak position shifted to the higher frequency side. This transition range coincides with the results from density measurements. For a better understanding of the structural modification, deconvolution of the Raman spectra is necessary. The deconvolution of the Raman spectra was carried out using the Peakfit program (Figure 8). The peak deconvolution provided Q0, Q2, BO, and Q3 units for Al2O3 mole percentages between 1.87 and 7.89. On the other hand, above 11.73 mole pct Al2O3, peak deconvolution showed the Q2, BO, Q3, and Q4 units.
Discussion
Figure 9 shows the Raman bands frequency evolution of the silicate structure units in the samples. When Al2O3 content was less than 15 pct, all frequencies except that for Q0, decreased with increasing Al2O3 content. As addressed before, this may be the result of the Si(Al) coupling due to the incorporation of Al-O0 units into Si-O0 units. On the other hand, no significant change in frequency was observed at concentrations above 20 pct. This may be the result of no additional Si(Al) coupling due to the depletion of Ca cations.
The relative areas of deconvoluted peaks correspond to the ratios of the individual silicate structure units.[15,16] In Figure 10, ratios of each structure are presented with respect to Al2O3 concentration. Below 15 pct Al2O3, the amount of Q0 and Q2 decreased with increasing Al2O3 content, while the amount of BO, Q3, and Q4 increased. Therefore, it is determined that Si(Al) coupling occurred and Al behaved as a network former, reinforcing the polymerization of silicate melts. On the other hand, when Al2O3 content was higher than 20 pct, the amount of Q2 and Q3 increased, while BO and Q4 decreased as the Al2O3 concentration increased. The increase of Q3 structure is much higher than that of Q2. Considering the equilibrium of the silicate structure was determined according to Eq. [3], depolymerization of Q4 mostly resulted in Q3 structure enhancement. Consequently, dissociated Al3+ ions acted as network modifiers due to the lack of charge-balancing cations.
Liu et al. investigated the local atomic structure of the CaO-SiO2-Al2O3 system (CaO/SiO2 = 1 at various Al2O3 mole percentages) by using the neutron diffraction experiments coupled with density functional theory.[17] This study found that regardless Al2O3 content, Si cations were tetrahedrally coordinated. However, the coordination number of Al cations was 4 or 5. The predominant form of Al cations was AlO4 (~70 pct), while AlO5 was ~30 pct. The amount of AlO5 slightly increased with increasing Al2O3 content to 40 pct, while that of AlO4 slightly decreased. The results obtained by Liu et al. coincide with the present results that the addition of Al2O3 first enhances the silicate network structure with the charge compensation effect. Consequently, when the Al2O3 content is less than 15 pct, the density decreased with increasing Al2O3 content because the bond length of Al-O (~0.177 nm) is higher than that of Si-O (~0.160 nm).[18] However, when the Al2O3 content is higher than 20 pct, with the depletion of Ca cations, Al cations are surrounded by non-bridging oxygen ions, yielding the AlO5 structure. Takehashi et al. reported that the formation of AlO5 was related to the increased ionic packing factor.[19] In this composition range, therefore, density of the slag would increase with increasing the Al2O3 content. This behavior was confirmed in the present density measurements.
Conclusions
The effect of the Al2O3 content on the density of CaO-SiO2-Al2O3 slag was investigated at a fixed CaO/SiO2 ratio of 1 using the aerodynamic levitation method. The slag density decreased as the Al2O3 content increased to 15 mole pct, while the density increased at concentrations greater than 20 mole pct. The structural analysis of the slag was carried out with Raman spectroscopy. When the composition of Al2O3 was less than 15 mole pct, Si(Al) coupling dominantly occurred, resulting in increased Al-O bond length. As a result, the slag density decreased with increasing Al2O3 concentration. When the composition of Al2O3 is greater than 20 mole pct, Al3+ was destabilized and became a network modifier, causing the formation of the AlO5 structure and enhancing the ionic packing factor. As a result, the slag density increased with increasing Al2O3 concentration.
References
[1] K. Mills: ISIJ Int., 1993, vol. 33, pp. 148-155.
[2] N. Sano, W. K. Lu, P. V. Riboud and M. Maeda: Advanced Physical Chemistry for Process Metallurgy, Academic Press, San Diego, 1997, p.48.
[3] B. O. Mysen, D. Virgo and I. Kushiro: Am. Min., 1981, vol. 66, pp. 678-701
[4] B. O. Mysen, R. J. Ryerson and D. Virgo: Am. Min., 1980, vol. 65, pp. 690-710.
[5] K. Mills: Slag Atlas 2nd edition, Verlag Stahleisen GmbH, Dusseldorf, 1995, p. 6.
[6] B. O. Mysen and P. Richet: Silicate Glasses and Melts-Properties and Structure, Elsevier, Amsterdam, 2005, pp. 108-109
[7] D. Virgo, F. Seifert and B. O. Mysen: Carnegie Inst. Wash. Yearb., 1979, vol. 78, pp. 506-511.
[8] S. A. Brawer and W. B. White: J. Non-Cryst. Solids, 1977, vol. 23, pp. 261-278.
[9] B. O. Mysen, A. Lucier and G. D. Cody: Am. Min., 2003, vol. 88, pp. 1668-1678.
[10] A. N. Grundy, I. Jung, A. D. Pelton and S. A. Decterov: Int. J. Mat. Res., 2008, vol. 99, pp. 1195-1209.
[11] Y. Kang and K. Morita: ISIJ Int., 2006, vol. 46, pp. 420-426.
[12] K.J. Lee, M.S.V. Kumar, S.K. Jung, C.H. Lee, C.H. Lee, S. Yoda, and W.S. Cho: J. Ceram. Process. Res., 2012, vol. 13, pp. 476-479.
[13] J. Lee, L. T. Hoai, J. Choe and J. Park: ISIJ Int., 2012, vol. 52, pp. 2145-2148
[14] P. Courtial and D. B. Dingwell: Geo. Acta., 1995, vol. 59, pp. 3685-3695.
[15] J. H. Park: Metall. Mater. Trans. B, 2013, vol. 44, pp. 938-947.
[16] J. H. Park: Met. Mater. Int., 2013, vol. 19, pp. 577-584.
[17] M. Liu, A. Jacob, C. Schmetterer, P. J. Masset, L. Hennet, H. E. Fischer, J. Kozalily, Ss. Jahn and A. G. Weale: J. Phys.: Condens. Matter, 2016, vol. 28, 135102.
[18] D. R. Lide: CRC Handbook of Chemistry and Physics 86th edition, Taylor & Francis, Boca Raton, 2005, pp. 9-54.
[19] S. Takahashi, D. Neuville, and H. Takebe: J. Non. Cryst. Solids, 2015, vol. 411, pp. 5-12.
Acknowledgments
This work was supported by the Industrial Strategic technology development program (10063488, Development of earthquake resisting reinforced concrete using grade 700 MPa reinforcing bars for enhancement of seismic safety) funded by the Ministry of Trade, Industry & Energy (MI, Korea), and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. NRF-2014R1A2A2A01007011). One of the authors (RR) was supported by the Korea University Grant. The authors are grateful to valuable comments from Prof. Joo-Hyun Park, Hanyang University on structure analysis with Raman spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 29, 2016.
Rights and permissions
About this article
Cite this article
Rajavaram, R., Kim, H., Lee, CH. et al. Effect of Al2O3 Concentration on Density and Structure of (CaO-SiO2)-xAl2O3 Slag. Metall Mater Trans B 48, 1595–1601 (2017). https://doi.org/10.1007/s11663-017-0964-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-0964-2