Abstract
Phase equilibria studies on ZnO-“FeO”-SiO2-Al2O3 system have been carried out in the temperature range between 1523 K and 1573 K (1250 °C and 1300 °C) at Po2 10−8 atm. Experimental techniques applied in the present study include high temperature equilibration, quenching, and electron probe X-ray microanalysis (EPMA). The compositions of the phases present in the quenched samples were measured by EPMA and used to construct phase diagrams of the pseudo-ternary sections at fixed Al2O3 content. The experimental results show that, spinel, SiO2, and willemite are the major primary phase fields in the composition range investigated. With 2 wt pct Al2O3 content in the liquid phase, the liquidus temperature can be increased by 35 K in the spinel primary phase in comparison with Al2O3-free system. The partitioning of ZnO and Al2O3 between the spinel and liquid phases is also discussed in the paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Slagging is critically important in the pyrometallurgical processes to produce metals which closely relates to the slag chemistry under the operational conditions.[1,2] In order to optimize the operational parameters, e.g., concentrates, flux compositions, and temperature for pyrometallurgical processes with high efficiency, less energy consumption, and high metal recovery, it is imperative to obtain accurate information of phase equilibria for the slag system.
Zn-Fe-Al-Si-O system forms the basis of the slags in non-ferrous metal pyrometallurgical processes, such as Imperial smelting furnace (ISF), Kivcet, QSL, Ausmelt, and Isamelt processes,[3] as well as oxygen bottom blowing copper smelting process.[4] The oxide components are introduced into the slag phase from the concentrates and fluxes. Meanwhile, zinc-bearing slags are also produced in the secondary metal production and in the zinc-bearing waste recycling processes.[5,6] However, these processes are generally operated at varied temperatures and oxygen partial pressure (Po2), e.g., the operating temperature is 1423 K (1150 °C) with Po2 close to 10−11 to 10−13 atm in lead blast furnace,[7] while operating temperature is around 1453 K (1180 °C) with Po2 close to 10−8 atm in a Fangyuan oxygen bottom blowing copper smelting furnace,[4,8] which both have a profound effect on the phase equilibria behavior and physicochemical properties of the slag.[9]
Previous studies on the ZnO-containing copper smelting slag systems had been carried out under metallic iron saturation,[3,10–19] of which the composition range investigated was relevant to the lead and zinc blast furnace slags. By applying “pie-type” sample of which inner part was ZnO-containing master slag wrapped by metallic iron foil approach, Zhao et al.[3,12,15–18] studied ZnO-“FeO”-Al2O3-CaO-SiO2 and higher order systems with varied CaO/SiO2 and (CaO+SiO2)/Al2O3 ratios under the metallic iron saturation. The results showed that Al2O3 content increases the liquidus temperature in the spinel primary phase field. Yamaguchi et al.[6] measured the activities of ZnO in the CaO-SiO2-FeOx-Al2O3 slag with Po2 fixed at around 10−11 atm. They found that the ZnO activities were increased with the addition of Al2O3 in the slag, and no phase relations were reported in their study. Our previous work on the ZnO-“FeO”-SiO2 system at 10−8 atm[20] indicated major difference on the phase equilibria behavior under varied Po2. The results at iron saturation cannot be directly applied to the copper smelting slags due to the significant difference of Po2 between these processes.
Meanwhile, no phase equilibria work has been reported on the ZnO-“FeO”-SiO2-Al2O3 system at Po2 10−8 atm. With an aim to further improve the understanding of the ZnO-containing copper smelting slag, the phase equilibria studies on the ZnO-“FeO”-SiO2-Al2O3 system with Al2O3 concentration up to 6 wt pct have been carried out at Po2 10−8 atm.
Experimental
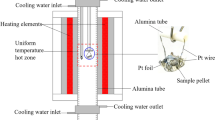
The detailed experimental procedure used in the present study is similar to that reported in the previous work.[4,20] Briefly, two master slags, SiO2-FeO and ZnO-SiO2-Al2O3 were firstly prepared. The zinc-silicate master slag was prepared by preheating the mixture of ZnO, SiO2, and Al2O3 at 1773 K (1500 °C) in air to obtain homogeneous liquid. The iron-silicate master slag was obtained by pre-equilibration of the mixture of Fe2O3 and SiO2 in the target temperature and Po2. The final mixture was prepared by mixing the desired proportions of iron-silicate and zinc-silicate master slags with the three components Fe3O4, SiO2, or ZnO. Primary phase substrate technique was applied for the experiments in the primary phase fields of spinel and SiO2. A platinum basket was used for the experiments in the willemite primary phase field. After pelletising, the mixture (around 0.15 g) was placed in a suitable container and suspended in the hot-zone of the reaction tube with the CO2/CO mixture passing through to maintain Po2 at 10−8 atm to achieve final equilibration. After equilibration, the sample was quenched into ice water. The obtained sample was then mounted and polished for EPMA.
Optical microscopy was firstly applied to examine the phase assemblages present in the sample. Carbon coating was performed on the QT150TES (Quorum Technologies) prior to electron probe microanalysis (EPMA). A JXA 8200 Electron Probe Microanalyzer equipped with wavelength-dispersive X-ray spectroscopy (Japan Electron Optics Ltd) was applied to microstructures and phase composition analyses. The working voltage and probe current were 15 kV and 15 nA, respectively. The standards used for the analyses were from Charles M. Taylor Co. (Stanford, California): Fe2O3 for Fe, Al2O3 for Al, and CaSiO3 for Si, and Micro-Analysis Consultant Ltd (Cambridge, UK): ZnO for Zn. The ZAF correction procedure supplied with the EPMA was applied. Although Fe2+ and Fe3+ are both present in the quenched samples, only metal cation concentrations can be measured by EPMA. Therefore, all the iron was recalculated to “FeO” for presentation purpose only.
Results and Discussions
In the present study, phase equilibria in the ZnO-“FeO”-SiO2-Al2O3 system have been determined between 1523 K and 1573 K (1250 °C and 1300 °C) under Po2 fixed at 10−8 atm with Al2O3 content up to 6 wt pct. The EPMA results are presented in Tables I, II, and III. The phase diagrams are presented in the form of pseudo-ternary at constant Al2O3 concentration as indicated in Figure 1. Three primary phase fields were identified in the composition range studied, including spinel [(Fe2+, Zn)O·(Fe3+, Al)2O3], tridymite (SiO2), and willemite [(Fe2+, Zn)2SiO4]. The typical microstructures from the quenched samples are presented in Figure 2 that include the equilibrium of liquid with spinel (Figure 2a), tridymite (Figure 2b), willemite (Figure 2c), and with both spinel and willemite (Figure 2d), respectively. The extensive solid solution was measured for spinel and will be further discussed in later sections.
The liquidus surfaces with 2, 4, and 6 wt pct Al2O3 on the ZnO-“FeO”-SiO2-Al2O3 system were constructed based on the data listed in Tables I, II, and III, as shown in Figures 3 through 5. In the Figures 3 through 5, the thin solid lines are experimentally determined isotherms, while the thick solid lines represent the experimentally determined boundaries and the thick dash lines indicate the phase boundaries in the areas without experimental data. It can be seen that, the liquidus temperatures mainly decrease in the spinel primary phase field and increase in the tridymite primary phase field with increasing SiO2 concentration. In the willemite primary phase field, the liquidus temperatures increase with increasing ZnO concentration.
Effects of Al2O3 and ZnO on the Primary Phase Fields and Liquidus Temperatures
Alumina (Al2O3), commonly present in the copper smelting slag, is introduced by copper concentrate, silica flux, or fuel. Using the current experimental data, liquidus surfaces at 1523 K (1250 °C) with varied Al2O3 contents are constructed as shown in Figure 6. The liquidus temperatures predicted by FactSage[21] are also presented in the figure for comparison. It can be seen from the figure that up to 6 wt pct, Al2O3 does not have a significant effect on the size of the fully liquid area surrounded by the isotherms in the spinel and tridymite primary phase fields. Both isotherms move towards high SiO2 direction. The fully liquid area at 1523 K (1250 °C) determined from the present study is much smaller than that from FactSage prediction[21] at Po2 10−8 atm.
For the industrial implication purposes, the effect of ZnO on the liquidus temperatures in the spinel primary phase field is presented in pseudo-binary sections with Fe/SiO2 (mass ratio) relevant to the copper smelting slags.[4] It can be seen from Figure 7 that, liquidus temperatures in spinel phase field continuously increased with increasing ZnO concentration regardless of Al2O3 concentration. However, it can be seen that the spinel liquidus is more sensitive to ZnO concentration if Al2O3 is present. In Al2O3-free slag the liquidus temperature is increased by approximately 45 K with 10 wt pct ZnO addition. When 6 wt pct Al2O3 is present, the liquidus temperature will be increased by approximately 70 K with 10 wt pct ZnO addition. It also can be seen that, FactSage predictions[21] of the spinel liquidus are much lower than the experimental results. For example, at 2 wt pct Al2O3 and 10 wt pct ZnO, the liquidus temperature predicted by FactSage is 70 K lower than that determined in the present study.
Comparison of the liquidus temperature between the present study and FactSage predictions[21] in spinel primary phase field as a function of ZnO concentration at fixed Fe/SiO2 = 1.5 and Po2 10−8 atm
Figure 8 shows the effect of Al2O3 on the liquidus temperatures in the spinel primary phase field at Fe/SiO2 weight ratio of 1.5 in the liquid phase. It can be seen that liquidus temperatures in the spinel primary phase field increase with increasing Al2O3 concentration at fixed ZnO concentrations. Addition of 6 wt pct Al2O3 in the slag can increase the liquidus temperature by 20 K and 50 K depending on the ZnO concentrations in the liquid.
Silica is commonly used as a flux in the copper smelting process to control the slag properties. It is convenient for the industry to use the correlation between liquidus temperature and SiO2 concentration. Figure 9 shows the liquidus temperature in spinel phase field as a function of SiO2 concentration (Fe/SiO2 weight ratio) at fixed 5 wt pct ZnO. It can be seen that spinel and SiO2 are the major primary phases in the composition range investigated (Fe/SiO2 weight ratio 1.0 to 3.0). In spinel primary phase field, the liquidus temperatures increase with increasing Fe/SiO2 weight ratio. In contrast, the liquidus temperatures in the SiO2 primary phase field decrease with increasing Fe/SiO2 weight ratio. The minimum liquidus temperature (eutectic between the primary phase fields of spinel and silica) at a given Al2O3 concentration increases with increasing Al2O3 and move towards low Fe/SiO2 weight ratio. Clearly it can be seen from the figure that liquidus temperatures predicted by FactSage[21] are much lower than that determined in the present study in both spinel and silica primary phase fields. The above results suggest that it is an effective way to control the operating temperature by tuning the Fe/SiO2 ratio in the industrial copper smelting operation to offset the effects of Al2O3 and ZnO in the slag.
Solid–Liquid Equilibria
One of the advantages using the present experimental approach is that the compositions of solid phase that in equilibrium with the liquid phase can be measured in the same quenched sample. In the present study, the spinel phase forms an extensive solid solution [(Fe2+, Zn)O·(Fe3+, Al)2O3] as shown in Tables I, II, and III. In other words, using the current experimental scheme, it is able to provide accurate data on the solid solutions that are very important for the development of thermodynamic modeling.
Copper smelting slag is usually located in the spinel primary phase field which is a solid solution of Fe3O4, ZnFe2O4, FeAl2O4, and ZnAl2O4.[18] As an example, the partitioning of ZnO between spinel and liquid is shown in Figure 10. The solid symbols in the figure are the present results and the results from Al2O3-free system at Po2 10−8 atm[20] and Al2O3-ZnO-“FeO”-SiO2-CaO at metallic iron saturation[3,15–18] are also shown in the figure for comparison. It appears that Al2O3 in liquid can influence the ZnO partitioning and the effect of temperature is not significant. The line passing the symbols represents approximately 5 wt pct Al2O3 in the liquid. It can be seen that the ZnO in the spinel is approximately 66 pct of that in the corresponding liquid in the system ZnO-“FeO”-SiO2-Al2O3 at Po2 10−8 atm. This is slightly higher than that in the system ZnO-“FeO”-SiO2 at Po2 10−8 atm,[21] but much lower than that in the system Al2O3-ZnO-“FeO”-SiO2-CaO[3,15–18] at metallic iron saturation. It appears that the ZnO concentration in the spinel is increased with the presence of Al2O3 in the slag which suggests that Al2O3 can stabilize the ZnO-containing spinel. As shown in Table IV, the Gibbs free energy of formation (ΔG f) of ZnAl2O4 is lower than other spinels which indicates the higher stability of ZnAl2O4. Moreover, a much higher ZnO content in the spinel can be observed in the Al2O3-ZnO-“FeO”-SiO2-CaO system[3,15–18] at metallic iron saturation as compared to that from higher Po2. This phenomenon can be explained by the fact that at more reducing condition, e.g., under metallic iron saturation, the Fe3O4 and ZnFe2O4 will be much less stable compared to ZnAl2O4 and ZnFe2O4. The results indicate that both Al2O3 and Po2 can affect the ZnO partitioning behavior and the influence of Po2 is more significant.
Industrial Implications
The optimization of pyrometallurgical process is largely depending on the improved understanding of the slag properties. Present study has been carried out to fill the knowledge gap of the phase equilibria for the ZnO-containing copper smelting slag. The results in the present study show that the introduction of Al2O3 in the slag phase increases the liquidus temperatures in spinel primary phase field resulting in the participation of spinel solid phase. Presence of the spinel phase in the slag has been proven to be beneficial to a longer campaign life of the smelting furnace.[22,23] However, the addition of Al2O3 in the slag could lead to a sharp increase of the slag liquidus temperature if ZnO is also present in the slag. Precipitation of the spinel phase may not be easily controlled and it may cause significant operation difficulty.[23] On the other hand, the liquidus temperatures in the spinel primary phase field can be adjusted by Fe/SiO2 ratio and the proportion of the spinel solid can be managed to be at a proper level.
FactSage[21] is a useful tool for industry to predict the liquidus temperatures and proportion of the solid phase at given temperatures. The present results show that the differences of the liquidus temperatures between FactSage predictions and experimental data are significant. The discrepancies are due to the lack of the accurate data in the ZnO-containing systems under the copper smelting conditions. Experimental results determined in the present study will be used to improve the thermodynamic model to be used for copper industry.
Conclusions
Phase relations and liquidus temperatures in the ZnO-“FeO”-SiO2-Al2O3 system have been determined at Po2 10−8 atm relevant to copper smelting slags. The results show that, spinel, SiO2, and willemite are the major primary phase fields in the composition range investigated. The liquidus temperatures increase with the increasing of Al2O3 concentration in the liquid phase, accompanying with an extension of the spinel primary phase field. A detailed analysis on partitioning of ZnO between liquid and the conjugated spinel phase shows that ZnO in the spinel is lower than that in the liquid phase. Present study fills the gap of phase equilibria in zinc-containing system at conditions relevant to the copper smelting process. The results will be applied to guide the industrial operation and optimize the thermodynamic database for the zinc-containing system.
References
P. J. Mackey, Can. Metall. Q., 1982, vol. 21, pp. 221–60.
T. Barry, A. Dinsdale and J. Gisby, JOM, 1993, vol. 45, pp. 32–38.
B. Zhao, P. C. Hayes and E. Jak, Int. J. Mater. Res., 2011, vol. 102, pp. 134–42.
B. Zhao, Z. Cui and Z. Wang, In 4th International Symposium on High-Temperature Metallurgical Processing, Wiley, 2013, pp. 1–10.
K. Verscheure, M. Van Camp, B. Blanpain, P. Wollants, P. Hayes and E. Jak, Metall. Mater. Trans. B, 2007, vol. 38, pp. 21–33.
K. Yamaguchi, M. Kudo, Y. Kimura, S. Ueda, and Y. Takeda, (Minerals, Metals & Materials Society: 2006), pp. 199–208.
J. Matousek, JOM, 2011, vol. 63, pp. 63–67.
E. Jak: in Ninth International Conference on Molten Slags, Fluxes and Salts (MOLTEN12), The Chinese Society for Metals, 2012.
K. C. Mills, L. Yuan and R. T. Jones, J. South. Afr. Inst. Min. Metall., 2011, vol. 111, pp. 649–58.
E. Jak, S. Degterov, A. D. Pelton and P. C. Hayes, Metall. Mater. Trans. B, 2001, vol. 32B, pp. 793–800.
E. Jak, B. Zhao and P. Hayes, Metall. Mater. Trans. B, 2000, vol. 31B, pp. 1195–1201.
B. Zhao, P. C. Hayes and E. Jak, Metall. Mater. Trans. B, 2011, vol. 42, pp. 490–99.
E. Jak, B. Zhao and P. C. Hayes, Metall. Mater. Trans. B, 2002, vol. 33B, pp. 865–76.
E. Jak, B. Zhao and P. C. Hayes, Metall. Mater. Trans. B, 2002, vol. 33B, pp. 877–90.
B. Zhao, P. C. Hayes and E. Jak, Int. J. Mater. Res., 2011, vol. 102, pp. 269–76.
B. Zhao, P. C. Hayes and E. Jak, Metall. Mater. Trans. B, 2011, vol. 42, pp. 50–67.
B. Zhao, P. C. Hayes and E. Jak, Metall. Mater. Trans. B, 2010, vol. 41, pp. 386–95.
B. Zhao, P. C. Hayes and E. Jak, Metall. Mater. Trans. B, 2010, vol. 41, pp. 374–85.
B. Zhao, Mineral Processing and Extractive Metallurgy (Trans. Inst. Min Metall. C), 2014, vol. 123, pp. 86–92.
H. Liu, Z. Cui, M. Chen and B. Zhao, Metallurgical and Materials Transactions B, 2015, DOI:10.1007/s11663-015-0480-1.
C. W. Bale, E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I. H. Jung, Y. B. Kang, J. Melançon, A. D. Pelton, C. Robelin and S. Petersen, Calphad, 2009, vol. 33, pp. 295–311.
P. Coursol, N. Tripathi, P. Mackey, T. Leggett and A. S. d. Friedberg, Can. Metall. Q., 2010, vol. 49, pp. 255–62.
P. Coursol, P. Mackey, Y. Prevost, M. Zamalloa and A. Warner: in The Carlos Díaz Symposium on Pyrometallurgy, Toronto Canada, (2007), pp 79–92.
Acknowledgments
The authors wish to thank Dongying Fangyuan Nonferrous Metals Co., Ltd. for providing the financial support to enable this research to be carried out. The University of Queensland International Research Tuition Award (UQIRTA) and China Scholarship Council (CSC) for providing scholarships for Hongquan Liu. Mr. Ron Rasch and Ms Ying Yu of the Centre for Microscopy and Microanalysis at the University of Queensland, who provided technical support for the EPMA facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 19, 2015.
Rights and permissions
About this article
Cite this article
Liu, H., Cui, Z., Chen, M. et al. Phase Equilibria Study of the ZnO-“FeO”-SiO2-Al2O3 System at Po2 10−8 atm. Metall Mater Trans B 47, 1113–1123 (2016). https://doi.org/10.1007/s11663-016-0596-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0596-y