Abstract
Enhancement of heat-affected zone toughness of a weight percentage of 0.014 pct Ti-bearing low carbon steel by TiN particle was investigated. An increase in nitrogen weight percentage from 0.0031 to 0.0083 pct results in increasing of number density of TiN precipitates from 4 × 103 to 3 × 105/mm2, and reduces prior austenite grain size from 850 to 350 μm with a soaking of 1673 K (1400 °C) for 2000 seconds. Effective refinement of austenite grain prohibits formation of ferrite side plate and/or upper bainite, and densely distributed TiN particles promote intra-granular ferrite formation, which is accompanied by an increase of 40 K to 60 K (40 °C to 60 °C) in austenite decomposition temperature during continuous cooling process. The changes in transformed products improved impact toughness of heat-affected zone efficiently, ex., increase absorbed energy of less than 42 J to more than 320 J with a simulated t 8/5 of 550 seconds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, high heat input welding methods, such as sub-merged arc welding, electro-gas welding, and electro-slag welding, have gained engineering applications due to construction efficiency improvement and cost reduction.[1–3] However, increasing welding heat input usually leads to deteriorated impact toughness of weld joints, especially in heat-affected zone (HAZ).[4–6]
Two distinct groups of methods[7–13] were put forwarded to improve HAZ toughness. The first one adopts low carbon, low carbon equivalent chemistry design along with TiN pinning austenite grain growth to refine HAZ grain structure, and hence to improve low-temperature toughness. The second one is fabrication of densely distributed fine oxide particles with high-temperature stability to improve HAZ impact toughness through oxide metallurgy, such as the use of strong de-oxidant Ti, Mg, Zr, and Ca. The latter relates to oxide metallurgy, which requires delicate control of steel-making process with narrow operating window. This is difficult for mill production. From the point of industrial production, the first one is more reliable for quality control.
Positive effect of TiN particles on steel weldability is frequently reported.[14–19] But the fact is that TiN particles dissolve with exposure to temperature higher than 1623 K (1350 °C), and the pinning effect on austenite grain boundary loses.[20,21] Therefore, high-temperature stability of TiN particles is critical for weldability improvement. Increasing nitrogen and nitrogen/titanium ratio improves high-temperature stabilities of TiN and hence heat-affected zone toughnesses were reported.[22–25] However, effect of TiN on microstructural and toughness of HAZ are not well understood. The objective of this study is to clarify the mechanism of HAZ toughness changes resultant from TiN particles.
Experimental Procedures
Two 150-mm-thick steel ingots were prepared by 80 kg vacuum induction melting furnace, and then were hot rolled into 30-mm-thick plates on a rolling machine with a force of 1000 kN. Prior to hot rolling, the ingots were reheated to 1473 K (1200 °C) with a soaking time of 3 hours. Two-stage thermo-mechanically controlled processing and accelerated cooling technique was employed for rolling, and cooling rate is about 15 K/s (15 °C/s). Chemical composition of the rolled plates is shown in Table I.
The plate A# shows a microstructure of polygonal ferrite (PF) plus bainite, while a mixture of PF and pearlite (P) was observed in the plate B#, as shown in Figure 1. The plate A# exhibits a yield strength of 465 MPa, a tensile strength of 564 MPa, and an elongation ≥25 pct. In contrast, the plate B# shows a yield strength of 372 MPa and a tensile strength of 487 MPa. Both plates of A# and B# exhibit an absorbed energy at 253 K (−20 °C) larger than 200 J.
Cylinder samples with a size of Φ5 × 4 mm were cut from the rolled plates, and then the edge surface was grinded and polished. The in situ observation experiment was conducted on a Con-focal Scanning Laser Microscopy (CSLM) machine, and argon is chose as the protecting gas. The samples were heated from room temperature to 1673 K (1400 °C) with a rate of 100 K/s (100 °C/s), and then stayed 10, 60, 100, 400, 1000, and 2000 seconds (s), and finally cooled to room temperature with a rate of 0.5 K/s (0.5 °C/s) and 0.2 K/s (0.2 °C/s), respectively. The austenite grain growth and decomposition process during thermal cycle were recorded real time, and the decomposition temperature is determined by dilatation temperature curves. The samples were grinded, polished and etched with a natal solution, and then observed with optical microscopy and field emission scanning electron microscopy (SEM). Thin foils for transmission electron microscopy examination (TEM) were subjected to twin jet electro-polishing using an electrolyte of 5 pct perchloric acid, 35 pct butoxy ethanol, and 60 pct methanol.
Samples with a size of 11 × 11 × 55 mm were prepared for HAZ simulation on a Gleeble 3800 machine. The peak temperature of 1673 K (1400 °C) and a dwell time of 60 seconds were chosen, and varied cooling times from 1073 K to 773 K (800 °C to 500 °C; t 8/5) of 150, 300, 550, 700, 1000, and 1400 seconds were used. A heating rate of 150 K/s (150 °C/s) was used for simulation, and the thermal cycles were described by employing the Rykalin-3D hear transfer model. Standard samples with a size of 10 × 10 × 55 mm were machined for Charpy impact toughness evaluation.
Results
Effect of N and Soaking Time on Austenite Grain Growth
Figure 2 shows in situ observed austenite grain structure with a dwell time of 10, 40, and 2000 seconds at a soaking temperature of 1673 K (1400 °C). It is obvious that the steel B# has a smaller austenite grain size than the steel A#. The average austenite grain size was calculated by using linear intercept method, and is shown in Figure 3. With an increase in soaking time from 10 to 2000 seconds, steel A# shows an austenite grain growth from 200 to 650 μm as compared to the growth from 90 to 350 μm for steel B#.
TEM study was conducted to clarify the discrepancy in austenite grain growth behavior at high temperatures between the two steels. Figure 4(a) shows a typical structure of the tested sample with 2000 seconds soaking time of the steel B#, and large amounts of densely distributed particles with a size range of 15 to 40 nm embedded in the AF matrix were observed, as indicated by circles.
Electron dispersive spectrum mappings confirmed that these cuboidal particles are TiN, as shown in Figure 4(b). In contrast, the steel A# shows a low density of TiN particles with a size range of 20 to 60 nm, which is coarser than those in the steel B#. The number density of the TiN particle in the steel A# and B# was calculated to be 4 × 103 and 3 × 105/mm2, respectively.
Effect of N Content on Austenite Decomposition
Figure 5 shows typical microstructures of the steel A# resultant from varied soaking times at 1673 K (1400 °C) and cooling rates. Cooling rate of 0.2 K/s (0.2 °C/s) produces a microstructure of ferrite and ferrite side plate (FSP), and longer soaking time decreases the volume fraction of ferrite. While a mixture of ferrite, FSP, and upper bainite (UB) was observed with a cooling rate of 0.5 K/s (0.5 °C/s), and longer soaking time increases volume fraction of UB. The UB having a size of hundreds of microns is nucleated from prior austenite boundaries, and contains parallel lath ferrites with second phases inset between them.
Figure 6 shows the effect of soaking time and cooling rates on austenite decomposition of the steel B#. Cooling rate of 0.2 K/s (0.2 °C/s) produces a microstructure of PF and P (20 to 30 μm) despite of soaking times, and longer soaking times increase PF size. With soaking time of 10 to 100 seconds, an increase in cooling rate from 0.2 K/s to 0.5 K/s (0.2 °C/s to 0.5 °C/s) decreases the size and fraction of the P (Figures 6(a) and (c)). With the cooling rate of 0.5 K/s (0.5 °C/s), an increase in soaking time between 400 and 2000 seconds results in a change from the PF and P type to a mixture of PF, P, and acicular ferrite (AF). Longer soaking time increases size and fraction of the AF.
Austenite decomposition temperatures calculated from dilation curves are shown in Table II. The start and stop temperature of austenite decomposition for the steel A# is between 1028 K and 1067 K (755 °C and 794 °C) and 927 K and 1009 K (654 °C and 736 °C), and an increase in soaking time decreases both the start and stop temperatures. The steel B# shows a start temperature of 1087 K to 1101 K (814 °C to 828 °C) and stop temperature of 999 K to 1076 K (726 °C to 803 °C) during decomposition process, which is about 30 K to 60 K (30 °C to 60 °C) and 50 K to 70 K (50 °C to 70 °C) higher than the steel A#.
Effect of N on Microstructure and Impact Property of Simulated HAZs
Figure 7 shows typical microstructures of simulated HAZs produced with a t 8/5 of 40, 300, and 550 seconds of the tested steels. For the steel A#, t 8/5 ≥ 300 seconds produces an microstructure of UB with GBF embedded in prior austenite grain boundary in HAZ, and the bainitic ferrite laths coarsen with increasing t 8/5. In addition t 8/5 of 40 seconds results in a finer bainitic structure in HAZ. The HAZ of steel B# consists of PF plus P, and PF grains show a large scattered size range, and coarsen with increasing t 8/5. Moreover, coarser pearlites were observed with larger t 8/5.
Table III shows impact absorbed energies of simulated HAZs at 253 K (−20 °C). The HAZs of the steel A# exhibit an absorbed energy ≥234 J with t 8/5 between 40 and 300 seconds, and an absorbed energy ≤44 J with t 8/5 ≥ 550 seconds. Figures 8(a) and (b) show clearly the ductile and brittle fracture of the HAZs produced with t 8/5 of 300 and 550 seconds, respectively. For the steel B#, the HAZs show an absorbed energy ≥285 J with t 8/5 between 40 and 1000 seconds, and an absorbed energy ≤47 J with t 8/5 ≥ 1400 seconds. Figures 8(c) and (d) show clearly the ductile and brittle fracture of the HAZs produced with t 8/5 of 300 and 1400 seconds, respectively. Figure 9(c) shows deeper dimples than the Figure 9(a), which implies that the steel B# has a higher HAZ impact value than the steel A# with t 8/5 of 300 seconds.
Discussion
The results presented in section III clearly show that for a weight percentage of 0.014 pct Ti-bearing low carbon steel, an increase in nitrogen weight percentage from 0.003 to 0.008 pct results in significant changes in size and volume fraction of TiN particles, austenite grain growth behavior during high temperature, austenite decomposition process and transformed microstructures, and impact toughness of heat-affected zone resultant from longer t8/5. For steels with a fixed Ti content, higher nitrogen content promotes TiN formation and refinement of TiN particles can be explained by thermodynamic calculations.
The number density of TiN in the plate A# and B# was calculated to be 5 × 104 and 7 × 106/mm2, respectively. The TiN particle in the steel A# has a size range of 20 to 100 nm, as compared to a size range of 20 to 50 nm of TiN particles in the steel B#. Occasionally larger TiN particles with a size of 0.5 to 2.0 μm were found in both of the steels A# and B#. The large particles were believed to be formed in the solidification process, and not counted due to minor content.
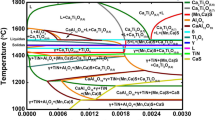
Thermo-Calc calculation shows that the steel A# and B# have a TiN formation temperature of 1709 K and 1762 K (1436 °C and 1489 °C), respectively, which is about 66 K and 13 K (66 °C and 13 °C) lower than their solidus temperature, which was calculated to be 1775 K (1502 °C). The TiN dissolution occurs in both the heating and soaking stage for the steels. Because the soaking temperature is lower than formation temperature, certain amount of TiN particles will remain after the soaking process. Higher TiN formation temperature means high TiN stability in the steel B#. After the completion of soaking at 1673 K (1400 °C), more TiN particles in the steel A# will dissolve than the steel B#. During cooling stage, fresh precipitation of TiN will occur, which is controlled by chemistry and cooling conditions. These precipitates are assumed to have a smaller size than the surviving TiN particles, and have a size smaller than 20 nm. The fresh and surviving particles are not discerned further by size variations, because the narrow size range and small size variation probably make a misleading counting. The TiN particles shown in Figure 4 are actually the mix of the surviving and fresh ones.
The steel B# has a higher volume fraction of finer TiN particles than the steel A#, and higher nitrogen content leads to enhanced thermal stability of TiN particles. Works from Choo[26] and Takashi[27] also show beneficial effect of high nitrogen and nitrogen/titanium ratio for TiN formation. Choo reported that an increase of nitrogen weight percentage from 0.003 to 0.009 pct leads to an evident increase in number density and thermal stability of TiN particles on simulated HAZs of a 0.005 to 0.015 pct Ti-bearing steel with soaking temperature of 1673 K (1400 °C). Takahashi et al.[27] found that an increase in Ti weight content from 0.008 to 0.025 pct resulted in increasing size and decreasing number density TiN particle for a weight percentage of 0.006 pct nitrogen bearing low carbon steel.
The sluggish austenite grain growth behavior at high temperatures in the steel B# relative to the steel A# indicated from Figures 2 and 3 can be explained by Zenner’s equation,[28] which states that finer particles with higher volume fraction are more effective for pinning effect. In consideration of the fact, the steel B# has a finer and higher volume fraction of TiN particles than the steel A#. Thus, it is safe to say that the retarded austenite grain growth behavior for the steel B# during high temperatures is due to effective pinning effect of thermally enhanced TiN particles on grain boundary.
Figures 5 and 6 certify that the change in austenite grain structure leads to changes in austenite decomposition process and transformed products. Table II clearly indicates that the steel A# has a higher austenite decomposition temperature than the steel B#. Comparison between Figures 5 and 6 shows that FSP and/or UB are always transformed products of steel A# despite soaking time and cooling rate, while not in the steel B#. Microstructural constituents like FSP and UB are preferential nucleation site of cracks during impact test, and thus deteriorates toughness. The avoidance of FSP and/or UB partially contributes to enhanced HAZ toughness of the steel B# relative to the steel A#, as shown in Table III.
It is known[29,30] that both austenite grain size and cooling rate affect phase transformation of a structural steel with a given composition during cooling stage. With a fixed composition and cooling rate, smaller austenite grain size is favorable for ferrite nucleation due to the increase in the fraction of grain boundary, which is regarded as preferred nucleation sites at beginning stage of transformation. Favorable nucleation site of TiN for intra-granular ferrite due to its high coherency with ferrite from low carbon Ti-bearing steel was reported,[31–33] although a contradictory opinion[34] also exists.
Figure 9 shows in situ observation of austenite decomposition process during cooling stage, which clearly implies that (1) transformation occurs preferentially at smaller austenite grains, and then shifted to larger austenite grains; and (2) intra-granular ferrite nucleation from TiN particle occurred just shortly after the transformation at grain boundary. This confirmed the role of TiN particles as preferential nucleation site for intra-granular ferrite.
In the present study, higher nitrogen content in the steel B# produces higher volume fraction and finer TiN particles; these particles inhibit austenite grain growth efficiently during exposure to high temperature. On one hand, the refinement of prior austenite grain avoids formation of FSP and/or UB during subsequent cooling stage. On the other hand, the dispersed finer TiN particles promote intra-granular ferrite formation. The prohibition of FSP and/or UB in combination of intra-granular ferrite formation together contributes to the enhanced impact toughness of simulated HAZs.
Conclusions
The basic conclusions that can be drawn from this work are as follows:
-
1.
Increasing nitrogen weight percentage from 0.0031 to 0.0083 pct of a weight percentage of 0.014 pct Ti-bearing low carbon steel increases number density of TiN particles from 4 × 103 to 3 × 105/mm2, and thus effectively inhibits austenite grain growth with exposure to high temperatures as high as 1673 K (1400 °C).
-
2.
Austenite refinement leads to a 40 K to 60 K (40 °C to 60 °C) increase in austenite decomposition temperature, and an avoidance of ferrite side plate and/or upper bainite formation. Densely distributed finer TiN particles promote intra-granular ferrite nucleation and formation.
-
3.
The inhibition of ferrite side plate and/or upper bainite and promotion of intra-granular ferrite formation together contributes to the enhance impact toughness of heat-affected zone of a Ti-bearing low carbon steel plate.
References
C.M. Kim, J.B. Lee, and W.Y. Choo: Proc. 13th Int. Conf. Offshore and Polar Eng., Honolulu, Hawaii, 2003, pp. 90–96.
A. Kojima, K. I. Yoshii, and T. Hada: Nippon Steel Technol. Rep., 2004, vol. 90, pp. 39-44.
S. Okano, T. Koyama, Y. Kobayashi, and M. Yamauchi: Kobe Steel Eng. Rep., 2002, vol. 52(1), pp. 20-24.
B.C. Kim, S. Lee, N.J. Kim, and D.Y. Lee: Metall Trans. A, 1991, vol. 22(1), pp.139-149.
N. Yurioka: Weld. World, 1995, vol. 35(6), pp. 375-390.
R.E. Dolby: Weld. Res. Int., 1977, vol. 7(4), pp. 298-313.
I. Rak, V. Gliha, and M. Kocak: Metall. Mater. Trans. A., 1997, vol. 28(1), pp.199-206.
P. Verrier, T. mauricks, R. Taillard, and G. Garrigues: Proc. 8th Int. Conf. Offshore Mechanics & Arctic Eng., 1989, vol. 3, pp. 641–647.
H. Ikawa, H. Oshige, and T. Tanoue: Trans. J. W. S., 1980, vol. 11(2), pp. 87-96.
M. Yonemoto, T. Miki, and M. Hino: ISIJ Int., 2008, vol. 48(6), pp. 755-759.
A. V. Karasev and H. Suito: ISIJ Int., 2008, vol. 48(11), pp. 1507-1516.
C. van der Eijk, Q. Grong, and J. Walmsley: Mater. Sci. Technol., 2000, vol. 16(1), pp. 55-64.
N. Kikuchi, S. Nabeshima, Y. Kishimoto, T. Matsushiba, and S. Sridhar: ISIJ Int., 2007, vol. 47(9), pp. 1255-1264.
M. Minagawa, K. Shida, Y. Funatsu, and S. Imai: Nippon Steel Technol. Rep., 2004, vol. 90, pp. 7-10.
S. Suzuki, K. Ichimiya, and T. Akita: JFE Technol. Rep., 2005, vol. 5, pp. 24-29.
H. Kawano, M. Shibata, S. Okano, Y. Kobayashi, Y. Okazaki, and H. Hatano: Kobe Steel Eng. Rep., 2004, vol. 54(2), pp. 110-113.
J. S. Park, B. Jung, and J.B. Lee: POSCO Techn. Rep., 2007, vol. 10(1), pp. 46-49.
K. Zhu and Z. G. Yang: Metall. Mater. Trans. A, 2011, vol. 42(8). pp. 2207-2213.
M. Van Ende, M. Guo, R. Dekkers, M. Burty, J. Van Dyck, P.T. Jones, B. Blanpain, and P. Wollants: ISIJ Int., 2009, vol. 49(8), pp. 1133-1140.
Mabuchi H, Uemori R, and Fujioka M: ISIJ Int., 1996, vol. 39(11), pp. 1406-1412.
S. Ueda, M. Ishikawa, K. Kobayashi, and T. Funakoshi: Tessu-to-hagane, 1975, vol. 61, pp.598.
Bang K, Park C, and Liu S: J. Mater. Sci., 2006, vol. 41(8), pp. 5994-6000.
Kitani Y, Ikeda R, Yasuda K, and Ichimiya K: Weld. World, 2007, vol. 51(1-2), pp. 31-36.
S. Suzuki, K. Ichimiya, and Y. Kitani: Proc. Asia Steel Int. Conf., Fukaoka, 2006, pp. 746–751.
Y. Komizo: Trans. JWRI, 2007, vol. 36(1), pp. 1-6.
H.C. Jeong, Y.H. An, and W.Y. Choo: Proc. 5th Workshop on the Ultra-Steel, Tokyo, 2001, pp. 224–225.
Y. Takahashi, M. Izumi, K. Abe, H. Takaoka, and Y. Okazaki: Kobe Steel Eng. Rep, 2008, vol. 58(1), pp. 42-46.
E Nes, N Ryum, and O Hunderi: Acta Metall., 1985, vol. 33(1), pp. 11-22.
A.J. DeArdo: ISIJ Int., 1995, vol. 35(8), pp. 946-954.
M. Militzer: ISIJ Int., 2007, vol. 47(1), pp. 1-15.
Y. Morikage: CAMP-ISIJ, 1997, vol.10, pp. 1309-1310.
S. Zhang, N. Hattori, M. Enomoto, and T. Tarui: ISIJ Int., 1996, vol. 36, pp. 1301-1308.
O.D.S. Sarma and P.G. Jonsson: CAMP-ISIJ, 2008, vol. 21, pp. 883–885.
J.M. Gregg and H.K.D.H. Bhadeshia: Acta Mater., 1997, vol. 45(1), pp.73-81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 9, 2015.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, X. & Ma, H. Enhancement of Heat-Affected Zone Toughness of a Low Carbon Steel by TiN Particle. Metall Mater Trans B 47, 2148–2156 (2016). https://doi.org/10.1007/s11663-015-0534-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0534-4