Abstract
Addition of CaF2 to the CaO-SiO2-MnO (CaO/SiO2 = 0.5) system, which corresponds qualitatively to a silicomanganese ferroalloy smelting slag, affected not only the critical (crystallization) temperature (T CR) but also the viscosity at high temperatures, and its influence on slag properties was strongly dependent on the content of MnO in the slag. The viscosity of CaF2-free 10 mass pct MnO slag was relatively high, i.e., about 10 dPa s at 1773 K (1500 °C), but decreased continuously upon addition of CaF2 to the system. In contrast, the viscosity of the 40 pct MnO system was very low, i.e., 1 dPa s at 1773 K (1500 °C), and CaF2 did not have a large effect. This indicates that Mn2+ is a strong network modifier in manganese ferroalloy smelting slags. Nevertheless, CaF2 addition was very effective at decreasing the viscosity of low MnO slags at low temperatures. The activation energy for the viscous flow of silicate melts decreased linearly in response to CaF2 addition, but this tendency was less pronounced in the more basic composition of the slag. The effect of CaF2 on the viscosity and activation energy for viscous flow of melts was analyzed quantitatively using micro-Raman spectra of quenched glass samples and the silicate polymerization index, i.e., Q3/Q2 ratio. The polymerization index decreased continuously with increasing CaF2 content in less basic (10 pct MnO or C/S = 0.5) slags, whereas it was not affected by CaF2 content in highly basic (40 pct MnO and C/S = 1.0) slags. Bulk thermophysical properties of the CaO-SiO2-MnO-CaF2 slags were quantitatively correlated with the structural information of the slags.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viscosity of silicate melts at high temperatures has been widely investigated because of the close relationship between the macroscopic thermophysical properties of slags and their microscopic molecular structure, which strongly affects reaction efficiency in process metallurgy.[1–26] Recently, we thoroughly evaluated the novel structure–viscosity relationship of the MnO-CaO-SiO2 slag system using Raman spectroscopy due to the importance of this system in smelting and refining processes for the production of manganese ferroalloys.[21,22]
Enhancement of slag-metal reaction kinetics is desirable to increase the production rate and decrease the cost of smelting and refining processes for Mn ferroalloys. Fluorspar (CaF2) addition may improve slag-metal reaction kinetics of MnO-containing slags at high temperatures by decreasing the viscosity of the slags, because CaF2 is well known to decrease the viscosity of silicate melts.[3–12,15,19,20,24,25] The effect of CaF2 on the viscosity of various silicate melts has been investigated experimentally by several researchers for CaO-SiO2 (-MgO, -MnO)-CaF2, [3–5,10–12,24,25] FeO(-CaO)-SiO2-CaF2,[6–8] CaO-SiO2-Na2O-Li2O-CaF2, [19] and CaO-Al2O3-SiO2-MgO-CaF2 systems.[20]
The above studies showed that viscosity itself and the activation energy of Newtonian flow of silicate melts generally decreased with increasing CaF2 content due to depolymerization reactions of silicate networks, indicating that the addition of CaF2 is more effective at higher silica concentrations. However, the effect of CaF2 on the viscosity of the FeO-SiO2 slags was not significant, in contrast to its effects on the CaO-SiO2 slags.[6] In MO (M = Mg, Na2)-containing slags, the addition of CaF2 had a limited effect on viscosity within a specific range of CaF2 concentration due to the contribution of basic oxides such as MgO and Na2O in addition to CaO to silicate depolymerization.[10–12,19] Furthermore, for highly basic slags with a silica content lower than about 10 mass pct, CaF2 suppressed the precipitation of solid phases at lower temperatures, whereas the effect of CaF2 on viscosity itself was less significant when the slags were completely liquid at high temperatures.[20,26]
Little experimental work has been done to determine the effect of CaF2 on the viscosity of MnO-containing silicate melts,[25] even though there is some experimental data regarding the viscosity of the CaO-MnO-SiO2 ternary slags.[1,2,7] Therefore, in the current study, we measured the viscosity of the CaO-SiO2-MnO-CaF2 slags with the composition of CaO/SiO2 = 0.5 (mass pct ratio) to clarify the effect of CaF2 on the viscous flow of molten slags at high temperatures. In addition, the Raman spectra of quenched glass samples were quantitatively analyzed to investigate the structural role of CaF2 in depolymerization of silicate networks with reference to our previous studies. [21–23,25–27]
Experimental
All slag samples were prepared using reagent grade chemicals such as MnO, SiO2, CaF2, and CaO calcined from CaCO3 at 1273 K (1000 °C) for 10 hours, and pre-melted slags were used in experiments. Pre-melting was carried out using a Pt-10 mass pct Rh alloy crucible (diameter 40 mm, depth 60 mm) under a purified Ar atmosphere in a Super Kanthal electric furnace.
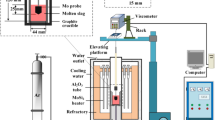
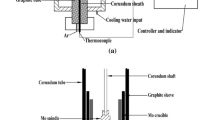
The rotating cylinder method was used in the current study and a schematic diagram of the experimental apparatus is shown in Figure 1. A rotating viscometer (Brookfield, model LV-DV II+ Pro) was set on the Super Kanthal electric furnace. The temperature was measured using an R-type (Pt/Pt-13 mass pct Rh) thermocouple. The experimental temperature range was about 1373 K to 1873 K (1100 °C to 1600 °C).
Viscosity was measured in the current study using a Pt-10 mass pct Rh alloy spindle, crucibles, and suspending wire, the dimensions of which are listed in Table I. The experiment was initiated by placing the crucible, containing pre-melted slag, inside the reaction chamber at 1873 K (1600 °C). Then, the spindle, rotating at a speed of 100 rpm, was lowered into the slag. The tip of the bob was placed about 5 mm above the base of the crucible and about 5 mm of the shaft was immersed in the melt. The equilibration time was approximately 20 minute at each temperature. Standard oil samples with a viscosity of 0.0486, 0.0952, 0.50, and 0.985 Pa s (0.486, 0.952, 5.0, and 9.85 poise) were used to calibrate the spindle.
In addition, the CaF2 content of the slag samples was analyzed using X-ray fluorescence spectroscopy (Bruker, model S4 Explorer) after experiments, because evaporation of fluoride species from the melts was expected.[28] We confirmed that the weight loss of CaF2 by fluoride emission during viscosity measurements was within about 5 pct point of the initial content, which is similar to what was reported in previous studies.[4,10–12,19,25,26] Hence, the composition in the present article refers to initial content.
The procedures used to prepare glass samples, and quantitative analysis of Raman spectra have been described in detail in previous articles.[21–23,25–27]
Results and Discussion
Effect of CaF2 Addition on the Viscosity and Critical Temperature of MnO-CaO-SiO2 Slags
The viscosities of the MnO-CaO-SiO2 (-CaF2) slags (CaO/SiO2 = 0.5) are shown as a function of temperature in Figure 2 for the 10 mass pct MnO system and in Figure 3 for the 40 mass pct MnO system. The viscosity values of the CaF2-free system at 1673 K, 1773 K, and 1823 K (1400 °C, 1500 °C, and 1550 °C) measured by other researchers are also shown in Figures 2 and 3 as well as are listed in Table II for comparison.[1,2,7] Previously reported values and our present results are in good agreement and within the experimental scatter.
The viscosity of the 10 mass pct MnO system was significantly higher than that of the 40 mass pct MnO system at a given CaO/SiO2 (=0.5) ratio and CaF2 content. Viscosity gradually increased with decreasing temperature, and this tendency was emphasized as the CaF2 content decreased when the MnO content was about 10 mass pct (Figure 2). In contrast, the viscosities were generally lower than 5 dPa.s as temperature decreased to about 1523 K (1250 °C) irrespective of the CaF2 content when the MnO content was 40 mass pct. For the latter in Figure 3, the viscosity drastically increased at a specific temperature, which is referred to as the critical (or crystallization) temperature (T CR).[10–12,18,19,25]
The viscosities of the 10 mass pct MnO system at various temperatures are plotted against the content of CaF2 in Figure 4. The viscosity of slags decreased continuously as the content of CaF2 increased at a given temperature, and the effect of CaF2 addition was more pronounced at lower temperatures. The same results were obtained in the 40 mass pct MnO system (Figure 5), even though the viscosity value of the latter was about a tenth of the former at a given temperature.
The results shown in Figures 4 and 5 confirmed that CaF2 improves the fluidity of the MnO-CaO-SiO2 slags with CaO/SiO2 = 0.5. Furthermore, the effect of CaF2 on the fluidity of slags was highly significant in the high SiO2 and low MnO systems at low temperatures. The viscous flow behavior of MnO-based slags shown in Figures 4 and 5 is important for several operating stages during Mn ferroalloys production. For example, interfacial reaction kinetics between the FeMn slag and SiMn melt can be improved by addition of small amounts of CaF2, resulting in the production of a high purity FeMn alloy by desiliconization.[29]
Activation Energy for the Viscous Flow of MnO-CaO-SiO2-CaF2 Slags
The viscosity of the silicate melts is strongly dependent on the degree of polymerization, which is a function of temperature and composition. The relationship between them has generally been discussed by taking the activation energy for viscous flow into account. The activation energy for the viscous flow of the silicate melts can be calculated using the following Arrhenius equation:
where η, η o, E η , R, and T are viscosity, a pre-exponent constant, the activation energy, a gas constant, and an absolute temperature, respectively. Hence, it is possible to calculate E η using a plot of ln η vs 1/T. The results are listed in Table III.
The Arrhenius plot for the MnO-CaO-SiO2 (-CaF2) slags (C/S = 0.5) is shown in Figure 6 as a function of 1/T and the contents of MnO and CaF2, respectively. Here, the viscosities of the partially crystallized melts in non-Newtonian regions were excluded; that is, only the viscosity of melts that exhibited Newtonian flow was taken into account. The viscosity, ln η, of the melts increased linearly with increasing 1/T and the viscosity of the 40 mass pct MnO system was unambiguously lower than that of the 10 mass pct MnO system at a given temperature and CaF2 content.
The slope of the line generally decreased with increasing CaF2 content in both systems, indicating that F− ions contributed to effective depolymerization of the silicate networks. From the phase diagram of the MnO-CaO-SiO2 ternary slag, the 10 mass pct MnO system with C/S = 0.5 is on the tridymite-type silica primary area, which has a highly polymerized three-dimensional tectosilicate framework structure. This fully polymerized network can be further depolymerized into more simple [Si2O4F]-sheets and/or [SiO2F]-chain structures by addition of CaF2, for example, as follows:[10–12,15,25]
However, the 40 mass pct MnO system with C/S = 0.5 is on the wollastonite (CaSiO3) primary area in the CaO-SiO2-MnO phase diagram. The main structural unit of wollastonite (inosilicate or metasilicate subclass) melt is a [SiO3]-chain structure,[21,30–35] which can be further depolymerized into more simple [Si2O6F]-dimers and/or [SiO3F]-monomers by addition of CaF2, for example, as follows:[10–12,15,25]
The depolymerization reactions mentioned above for 10 pct and 40 pct MnO systems are illustrated schematically in Figures 7 and 8, respectively, from the perspective of structural similarity among silicate minerals and melts concluded by Mysen et al.[21,35–39] There were far fewer bond-breaking events that occurred due to supply of F− ions when the MnO content was relatively high in the system, which consisted predominantly of two-dimensional chain structures. This provides us with very important insights into the structural role of F− in silicate melts. Some studies have reported that F− ions are very effective at depolymerizing silicate networks, while other studies have reported that F− ions behave only as a diluent. This inconsistency in the reported role of F− ions is due to the distribution of silicate structural units as discussed above.
The activation energy for the viscous flow of the CaO-SiO2-MnO-CaF2 slags is plotted against the content of CaF2 at different C/S ratios and MnO contents in Figure 9. Activation energy decreased linearly with increasing content of CaF2, irrespective of C/S ratio and MnO content. Furthermore, the effect of CaF2 on activation energy became less significant as the C/S ratio and MnO content increased. This is not only because the dominant fractions of silicates were depolymerized into simpler forms due to the network-modifying roles of Ca2+ and Mn2+ ions before F− ions were introduced, but also because the absolute amounts of silicates broken into flow decreased as the basicity increased. [21]
Structural Changes in Silicate Melts Due to CaF2 Addition: Micro-Raman Spectra Analysis
Raman spectroscopy has been used to probe the local anionic structure of silicates.[21,35,40] The bands assigned to antisymmetric stretching of Si-O− (non-bridging oxygen; NBO) and Si-O0 (bridging oxygen; BO) bonds occur in the 850 to 1200 cm−1 region, whereas Si-O-Si bending modes are found between about 500 and 700 cm−1.[21,35,41] The frequencies of the stretching modes decrease with decreasing degree of polymerization, viz. increasing NBO/Si. There are several types of units; SiO2 (fully polymerized), Si2O5 (sheet), SiO3 (chain), Si2O7 (dimer), and SiO4 (monomer) groups.[35–45] In a nuclear magnetic resonance (NMR) study of silicates,[46,47] the stoichiometric notations for each unit were replaced by the so-called Qn concept, wherein the groups described above correspond to Q4 (NBO/Si = 0), Q3 (NBO/Si = 1), Q2 (NBO/Si = 2), Q1 (NBO/Si = 3), and Q0 (NBO/Si = 4), respectively. We use the Qn concept in the present paper.
The Raman spectra of the CaO-SiO2-CaF2-10 pct MnO system (C/S = 0.5) as a function of wavenumbers (Raman shifts) ranged from 700 to 1300 cm−1 are shown in Figure 10. The intensity of the high frequency Q3 (1050 to 1080 cm−1) band was relatively strong in the CaF2-free high silica system and decreased continuously as the content of CaF2 increased. However, the relative intensity of the Q2 (960 to 970 cm−1) band, which was initially a weak shoulder in the CaF2-free high silica system, increased significantly with increasing CaF2 content. A similar tendency was observed for the Q0 (860 to 870 cm−1) band, even though it was much less dominant compared with Q2 band. A more quantitative analysis of structural changes induced by CaF2 addition is provided below.
Relative fractions of silicate anionic units obtained from Gaussian deconvolution of Raman bands shown in Figure 10 are listed in Table IV and plotted against CaF2 content in Figure 11. Details of the Gaussian deconvolution process are reported in our previous publications.[21–23,25–27] The fraction of the Q3 (Si2O5-sheet) unit decreased continuously from about 55 pct to 35 pct as the CaF2 content increased, whereas the fraction of Q2 (SiO3-chain) and Q0 (SiO4-monomer) units increased from about 40 pct to 52 pct and from about 2 to 12 pct, respectively, as the CaF2 content increased. This indicated that the large Q3 unit was depolymerized into smaller Q2 and Q0 units by addition of CaF2. The relative fraction of the Q1 (Si2O7-dimer) unit was not affected by CaF2 content.
Raman spectra of the CaO-SiO2-CaF2-40 mass pct MnO system as a function of wavenumbers (Raman shifts) ranged from 700 to 1300 cm−1 are shown in Figure 12. The intensity of the high frequency Q3 (1050 to 1100 cm-1) band was negligible through the composition range investigated. The relative intensity of the Q0 [850(±5) cm−1] band increased slightly, whereas that of the Q2 [970(±5) cm−1] band decreased as the CaF2 content increased, but not significantly. There was no remarkable change in the relative intensity of Q1 [910(±5) cm−1] band at CaF2 contents ranging from 0 to 15 mass pct. However, a more quantitative analysis of the structural changes induced by CaF2 addition is provided below.
The relative fractions of silicate anionic units obtained from Gaussian deconvolution of Raman bands shown in Figure 12 are listed in Table IV and plotted against CaF2 content in Figure 13. The fraction of the Q3 (Si2O5-sheet) unit was very small for the entire composition range investigated, i.e., C/S = 0.5 and MnO = 40 mass pct, and decreased slightly from about 5 to 1 pct as the CaF2 content increased. Interestingly, the relative fraction of the Q2 (SiO3-chain) unit decreased slightly from about 75 to 60 pct, while the fractions of Q1 (Si2O7-dimer) and Q0 (SiO4-monomer) units increased from about 5 pct to 10 pct and 15 pct to 30 pct, respectively, as the content of CaF2 increased. In Figures 11 and 13, the fractions of each Qn unit for the CaF2-free ternary system exhibited good agreement with the previous measurements,[21] indicating that the Raman analysis performed in this study was highly consistent.
The Q3/Q2 ratio, which was proposed as a quantitative polymerization index for silicates,[21–23,25,27] was plotted against CaF2 content (Figure 14). The Q3/Q2 ratio on a logarithmic scale decreased continuously with increasing CaF2 content in less basic (C/S = 0.5 or 10 pct MnO) systems, whereas it did not change as CaF2 content increased in the highly basic (C/S = 1.0 and 40 pct MnO) systems.[25] This means that CaF2 contributed effectively to depolymerization of silicate networks in the former system, while it did not in the latter system, as discussed in the previous section.
In our earlier study,[21,25] we were able to predict the activation energy of the CaO-SiO2-MnO (-CaF2) slags as a linear function of the polymerization index, ln (Q3/Q2). The activation energy of the viscous flow of silicate melts in a Newtonian flow region is shown in Figure 15 as a function of ln (Q3/Q2). The activation energy required for the CaO-SiO2-MnO-CaF2 slags can be expressed as a linear function of ln (Q3/Q2) within some experimental scatter by linear regression analysis as follows:
Our Raman spectroscopy results for viscosity measurements and structural analysis of the CaO-SiO2-MnO-CaF2 (C/S = 0.5) slags, which correspond qualitatively to SiMn smelting slags, are consistent with those we reported previously for the CaF2-free ternary system as well as for the CaO-SiO2-MnO-CaF2 (C/S = 1.0) slags, which correspond to FeMn smelting slags.[21,25]
Conclusions
The addition of CaF2 to the CaO-SiO2-MnO (CaO/SiO2 = 0.5) system, which corresponds qualitatively to SiMn smelting slag, affected not only the critical (crystallization) temperature (T CR) but also viscosity at high temperatures, and the influence of CaF2 on slag properties was strongly dependent on the content of MnO in the slag. The viscosity of the CaF2-free slag was relatively high, i.e., about 10 dPa s at 1773 K (1500 °C) and 30 dPa s at 1673 K (1400 °C), and decreased continuously by CaF2 addition in the 10 mass pct MnO system, whereas the viscosity of the 40 pct MnO system was very low, viz. approximately 1/10 of the viscosity of the 10 pct MnO system at a given CaF2 content. This indicates that Mn2+, like Ca2+, is a strong network modifier in manganese ferroalloys smelting slags. Nonetheless, CaF2 addition was very effective at decreasing the viscosity of low MnO slags at low temperatures. For example, the viscosity of 10 pct MnO slag decreased from about 95 dPa.s to 7 dPa s at 1623 K (1350 °C) in response to addition of 15 pct CaF2.
The activation energy for the viscous flow of silicate melts decreased linearly in response to CaF2 addition, though less significantly the more basic composition of the slag, i.e., in the 40 pct MnO and C/S =1.0 system, which is consistent with the experimental results reported for various fluorosilicate melts by several research groups. This tendency can be understood using a structural model for the depolymerization process, e.g., “three-dimensional network → sheet → chain → dimer → monomer” in response to CaF2 addition.
We quantitatively analyzed the effect of CaF2 addition on the viscosity and activation energy for viscous flow of melts using micro-Raman spectra of quenched glass samples and the concept of a silicate polymerization index, the Q3/Q2 ratio. The polymerization index decreased continuously with increasing content of CaF2 in less basic (10 pct MnO or C/S = 0.5) slags, whereas it was not affected by CaF2 content in highly basic (40 pct MnO and C/S = 1.0) slags. Consequently, the bulk thermophysical properties of the CaO-SiO2-MnO-CaF2 slags are quantitatively correlated to the microscopic molecular (or ionic) structure of the slags.
References
L. Segers, A. Fontana, and R. Winand: Electrochim. Acta, 1979, vol. 24, pp. 213-18.
M. Kawahara, K. Mizoguchi, and Y. Suginohara: Bull. Kyushu Inst. Technol., 1981, vol. 43, pp. 53-59.
T.S. Tribe, P.W. Kingston, and W.F. Caley: Can. Metall. Q., 1997, vol. 36, pp. 95-101.
F. Shahbazian, D. Sichen, K.C. Mills, and S. Seetharaman: Ironmaking & Steelmaking, 1999, vol. 26, pp. 193-99.
T. Yasukouchi, K. Nakashima, and K. Mori: Tetsu-to-Hagane, 1999, vol. 85, pp. 571-77.
F. Shahbazian, Du Sichen, and S. Seetharaman: ISIJ Int., 1999, vol. 39, pp. 687-96.
F.Z. Ji: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 181-86.
F. Shahbazian: Scand. J. Metall., 2001, vol. 30, pp. 302-08.
J.H. Park, D.J. Min, and H.S. Song: ISIJ Int., 2002, vol. 42, pp. 344-51.
J.H. Park, D.J. Min, and H.S. Song: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 723-29.
J.H. Park, D.J. Min, and H.S. Song: Metall. Mater. Trans. B, 2002, vol. 35B, pp. 269-75.
J.H. Park and D.J. Min: J. Non-Cryst. Solids, 2004, vol. 337, pp. 150-56.
S. Sukenaga, N. Saito, K. Kawakami and K. Nakashima: ISIJ Int., 2006, vol. 46, pp. 352-58.
M. Hayashi, T. Watanabe, H. Nakada and K. Nagata: ISIJ Int., 2006, vol. 46, pp. 1805-09.
J.H. Park and D.J. Min: ISIJ Int., 2007, vol. 47, pp. 1368-69.
J.H. Park, H. Kim, and D.J. Min: Metall. Mater. Trans. B, 2008, vol. 39 B, pp. 150-53.
Y. Miyabayashi, M. Nakamoto, T. Tanaka and T. Yamamoto: ISIJ Int., 2009, vol. 49, pp. 343-48.
H. Kim, W.H. Kim, J.H. Park, and D.J. Min: Steel Res. Int., 2010, vol. 81, pp. 17-24.
H.S. Park, H. Kim, and I. Sohn: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 324-30.
L. Wu, J. Gran, and D. Sichen: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 928-31.
J.H. Park: ISIJ Int., 2012, vol. 52, pp. 1627-36.
J.H. Park: ISIJ Int., 2012, vol. 52, pp. 2303-04.
J.H. Park: J. Non-Cryst. Solids, 2012, vol. 358, pp. 3096-3102.
O. Takeda, T. Okawara, and Y. Sato: ISIJ Int., 2012, vol. 52, pp. 1544-49.
K.Y. Ko and J.H. Park: ISIJ Int., 2013, vol. 53, pp. 958-65.
T.S. Kim and J.H. Park: ISIJ Int., 2014, vol. 54, pp. 2031-38.
J.H. Park: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 938-47.
J.H. Park and D.J. Min: Steel Res. Int., 2004, vol. 75, pp. 807-11.
J.H. Heo, Y. Chung, and J.H. Park: Metall. Mater. Trans. B, 2015, vol. 46B, in press.
A. Navrotsky: Physics and Chemistry of Earth Materials, Cambridge University Press, New York, NY, 1994.
C.A. Francis and P.H. Ribbe: Am. Mineral., 1980, vol. 65, pp. 1263-69.
Q. Williams, P. McMillan, and T.F. Cooney: Phys. Chem. Minerals, 1989, vol. 16, pp. 352-59.
T.F. Cooney and S.K. Sharma: J. Non-Cryst. Solids, 1990, vol. 122, pp. 10-32.
H. Toraya and S. Yamazaki: Acta Crystallogr. B, 2002, vol. 58B, pp. 613-21.
B.O. Mysen and P. Richet: Silicate Glasses and Melts: Properties and Structure, Elsevier, Amsterdam, Netherlands, 2005.
D. Virgo, B.O. Mysen, and I. Kushiro: Science, 1980, vol. 208, pp. 1371-73.
B.O. Mysen: Am. Mineral., 1980, vol. 65, pp. 690-710.
B.O. Mysen: Eur. J. Mineral., 2003, vol. 15, pp. 781-802.
B.O. Mysen: Earth Sci. Rev., 1990, vol. 27, pp. 281-365.
J. Etchepare: Study by Raman spectroscopy of crystalline and glassy diopside, in Amorphous Materials, eds. R.W. Douglas and E. Ellis, Wiley–Interscience, New York, NY, 1972.
T. Furukawa, K.E. Fox, and W.B. White: J. Chem. Phys., 1981, vol. 75, pp. 3226-37.
S.A. Brawer and W.B. White: J. Non-Cryst. Solids, 1977, vol. 23, pp. 261-78.
S.A. Brawer and W.B. White: J. Chem. Phys., 1975, vol. 63, pp. 2421-32.
P. McMillan: Am. Mineral., 1984, vol. 69, pp. 622-44.
Y. Iguchi, S. Kashio, T. Goto, Y. Nishina and T. Fuwa: Can. Metall. Quart., 1981, vol. 20, pp. 51-56.
C.M. Schramm, B.H.W.S. DeJong, and V.F. Parziale: J. Am. Chem. Soc., 1984, vol. 106, pp. 4396-4402.
J.F. Stebbins, J.B. Murdoch, E. Schneider, I.S.E. Carmichael, and A. Pines: Nature, 1985, vol. 314, pp. 250-52.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 25, 2014.
Rights and permissions
About this article
Cite this article
Park, J.H., Ko, K.Y. & Kim, T.S. Influence of CaF2 on the Viscosity and Structure of Manganese Ferroalloys Smelting Slags. Metall Mater Trans B 46, 741–748 (2015). https://doi.org/10.1007/s11663-014-0269-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0269-7