Abstract
The carbon solubility in the CaO-SiO2-3MgO-CaF2 slag system at 1773 K (1500 °C) was investigated under CO/Ar and CO/N2 gases. Higher extended basicity [(CaO + MgO)/SiO2) increased the carbon solubility in the slag as the activity of free oxygen ions (\( \varvec{a}_{{{\mathbf{O}}^{2 - } }} \)] promoted the reaction of the free carbide mechanism. Higher CaF2 also resulted in higher carbon dissolution into the slag as fluorine ions interact with the bridged oxygen (O0) in the melt structure to increase the activity of the free oxygen ions in the melt. Structural information obtained from the Fourier transformed infra-red (FT-IR) and Raman revealed a depolymerization of the network structure as the simpler structural units of NBO/Si = 4 increased and the Si-O-Si bending vibrations decreased with higher basicity and CaF2 content. This correlated well with higher free oxygen ions (O2−) in the slag system and subsequently higher carbon dissolution. A correlation of the theoretical optical basicity (Λth) with the logarithm of the carbon content in slag showed a relatively similar trend and an increase of carbon was observed with higher optical basicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The dominant carbon dissolution mechanisms in slags have been well-known to be the incorporated carbide or free carbide mechanisms depending on the composition and in particular the extended basicity (basic oxides/acidic oxides).[1–6] And when sufficient nitrogen is present in some slag systems, cyanide (–CN) has also been known to form with the incorporated and free carbide to significantly increase the carbon and nitrogen content of the slag compared to nitrogen-free reacting systems, as verified by the present authors in the CaO-10SiO2-Al2O3-CaF2 slag system.[1,4] The relative contents of slag constituents are provided in wt pct in the present paper.
In this study, the carbon solubility in the CaO-SiO2-MgO-CaF2 slag system has been investigated. The effect of the extended basicity [(CaO + MgO)/SiO2] and CaF2 content on the carbon solubility has been provided. In addition, the carbon solubility of the slag was correlated to the melt structure using Fourier transformed infra-red (FT-IR) and Raman spectroscopy of as-quenched slags from 1773 K (1500 °C).
Reagent grade chemicals (Junsei; Tokyo, Japan) of CaCO3 (99.5 pct), SiO2 (99.5 pct), MgO (98 pct), and CaF2 (99 pct) were used. A total of 4 g was pre-melted in a Pt-10Rh crucible for 5 hours at 1773 K (1500 °C) with Ar gas flowing at 4.2 cm3/s. The post-experimental chemical compositions were analyzed using X-ray fluorescence spectroscopy (XRF; S4 Explorer; Bruker AXS GmbH, Karlsruhe, Germany), as provided in Table I. There are slight changes in the composition of the slags and the post-experimental compositions were used in subsequent results. In past work by Kor and Richardson,[7] it was inferred that the CaF2 and H2O could react to form CaO and HF, which could affect not only the partial pressure of H2O, but also the activity of O2− in the slag as CaO is increased. Changes in the activity of O2− can have a significant effect on the carbon solubility.[4,6] However, in the present work, ultra high purity grade gases (99.9999 pct) were passed through columns of drierite and soda lime to remove impurities such as H2O and CO2 that may be present in the gases and there is likely negligible amount of moisture to react with the CaF2 in the slag. Thus, the very slight change in the weighed and post-experimental chemical analysis of the slag, if any, is likely due to the formation of SiF4 according to earlier work by Mitchell[8] expressed in Eq. [1].
The CaF2 and SiO2 loss decreases with increasing CaO and seems to agree with the findings of Mitchell and Kumar et al.[9] This change in the composition can increase the oxygen activity resulting in higher carbon solubility. However, as the CaF2 content decreases, lower carbon solubility can occur[4] and thus there could be a mutual competing effect of CaF2 and CaO following volatilization through Reaction [1]. It should be noted that reference samples containing reagent grade chemicals of CaF2 at concentrations of 3, 5, 8, 10, 12, and 15 wt pct was used to obtain a calibration curve for specifically CaF2 since compositional changes in CaF2 could occur in the CaO-SiO2-CaF2-3MgO system and accurate determination of the fluorine content is of importance. Furthermore, preliminary experiments showed the small changes in the composition for the present slag system occurred during the 5 hours pre-melting stages and compositional changes through volatilization was not significant during the 24 hours high temperature chemical equilibrium experiments. This compositional change during the pre-melting stages and the kinetics is a topic of further research. All results in the present study use the post-experimental chemical compositions and accounts for minute changes in the slag composition and its effect on the carbon solubility, if any.
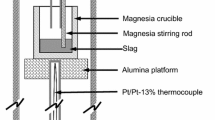
Details of the experimental apparatus have been discussed elsewhere,[4] but the slag sample was placed in a carbon crucible (OD: 1.5 cm, ID: 1.1 cm, H: 7.2 cm) within a Müllite reaction tube under a mixture of CO with Ar or N2 gases (6:4) of 4.2 cm3/s. A MoSi2 (Kanthal; Hallstahammar, Sweden) vertical resistance furnace was set at a target temperature of 1773 K (1500 °C). The oxygen partial pressure was maintained using the C/CO equilibrium reaction resulting in an oxygen partial pressure of approximately 10−16 atm, which is above the oxygen partial pressure for the decomposition of the Müllite reaction tube. Preliminary work with slags showed the equilibrium reaction time for the slag/carbon to be approximately more than 18 hours; thus, the equilibration time was taken to be 24 hours.[4] The sample was removed from the hot zone of the furnace after the reaction was completed and quickly quenched with Ar gas. Carbon was measured by a CS-300 analyzer (LECO; St. Joseph, MI).
FT-IR (Spectrum-100; PerkinElmer, Waltham, MA) and Raman (PD-RSM300; Photon Design, Tokyo, Japan) spectroscopy on as-quenched slag samples were used to ascertain structural information of the slags. A sample of 2 mg was mixed with 200 mg of KBr, which was then pressed into 10 mm diameter and 1-mm-thick disk pellets for FT-IR analysis.[10,11] The characteristic structural units of the silicate based slags verified with the FT-IR and Raman are typically concentrated within the wavenumber between 1400 and 400 cm−1, where the various symmetric stretching and bending vibrations of the Si-O bonds constitute the greater part of the slag structure.
From the Raman spectroscopy (PD-RSM300; Photon Design; Tokyo, Japan), the obtained spectra can be deconvoluted and the area integrated to ascertain quantitative information on the amounts of the individual silicate structural units within the melt.
Figure 1 shows the carbon solubility in the CaO-SiO2-3MgO-9CaF2 slag at 1773 K (1500 °C) as a function of the extended basicity [(CaO + MgO)/SiO2 ratio] under a CO and Ar or N2 gas mixture. The carbon solubility in the slag seems to slightly increase with higher basicity under both atmospheric conditions. Although the amount of carbon is much less, this trend is similar to the results obtained from past work done by the authors in an alumina rich CaO-10SiO2-Al2O3-10CaF2 slag at 1773 K (1500 °C) shown by the open circles, which is expected for a (CaO + MgO)/SiO2 above unity.[4] If the free carbide ion dominates in the compositional range of the present study, the carbide ions increase with increasing activity of free oxygen ions according to Reaction [2].
The carbon solubility in the CaO-10SiO2-Al2O3-10CaF2 slag system is also provided and shows comparable trends with the CaO/(SiO2 + Al2O3) ratio. From the comparison of the absolute values of the aluminate-rich and silicate-rich slag systems, it can be inferred that the aluminate-rich slags are more effective in dissolving carbon. Furthermore, unlike the carbon solubility of the CaO-10SiO2-Al2O3-10CaF2 system, where significant cyanide (−CN) formed under a CO/N2 atmosphere, the present CaO-SiO2-3MgO-9CaF2 system under identical conditions did not show dramatic differences in the carbon content with CO/Ar and CO/N2 atmospheres. Only a slight increase in the carbon content was verified with the CO/N2 combination and thus suggests only minimal formation of –CN compounds. The carbon content of the present study showed comparable results to Berryman and Sommerville.[2] The increasing trend was similar to their results above a CaO/SiO2 of 0.9, where the free carbide mechanism begins to dominate. In the present work, the oxygen partial pressure is less than 10−16 atm, where the carbide formation is dominant and the carbonate formation mechanism is not expected.[12]
The effect of CaF2 on the carbon solubility in the CaO-SiO2-3MgO-CaF2 slag at 1773 K (1500 °C) and fixed extended basicity of 1.2 is shown in Figure 2. Higher CaF2 content seems to increase the carbon solubility of the slag in both CO/Ar and CO/N2 atmospheres. CaF2 has been known to be a network modifier, which can interact with the bridged oxygen (O0) in the silicate melts to release free oxygen ions (O2−). Thus with the addition of CaF2, the activity of free oxygen ions would likely increase and similar to higher basicity push Reaction [1] forward to increase the carbon solubility in the slag. The carbon content in the melt at 1773 K (1500 °C) for CO/N2 was found to be higher than the CO/Ar condition. This difference in the carbon content is to be the aforementioned additional formation of cyanide compounds within the slag as was observed by Park and Sohn.[4]
The carbon solubility in the present slag system at 1773 K (1500 °C) was found to be directly affected by the compositional variations of the extended basicity and CaF2 additions. These modifications in the composition and carbon solubility can also be correlated to the slag structure. Using FT-IR and Raman spectroscopy of as-quenched slags from 1773 K (1500 °C), the changes in the basicity and CaF2 can influence the various structural units that comprise the molten slag. Figure 3(a) describes the Si-O tetrahedral stretching vibrations as a function of the basicity in the CaO-SiO2-3MgO-9CaF2 slag system. The depth of the NBO/Si = 4 trough convoluted within the symmetric [SiO4]-tetrahedral stretching vibrations located approximately at 850 cm−1 generally increases with higher basicity and the right peak of the NBO/Si = 4 shifts toward lower wavenumber.[13,14] NBO/Si is the number of non-bridged oxygen per tetrahedral cation and a higher value corresponds to a less complex and simple structural unit, which can be identified with depolymerization of the melt structure. As the peak shifts toward lower wavenumbers, the center of gravity within the symmetric [SiO4]4−-tetrahedral stretching bands moves toward lower wavenumbers. This suggests that the simple structural units of the NBO/Si = 4 are becoming more pronounced and a depolymerization of the slag is occurring with higher basicity, which is expected as the free oxygen ions provided at higher basicity can interact with the bridged oxygen to form non-bridged oxygens as anticipated by Reaction [3].[15–17]
In addition, the Si-O-Si bending and its transmittance trough located near 700 cm−1 shifts toward lower wavenumbers and becomes less pronounced with higher basicity. Considering the Si-O-Si bending vibrations are characteristic of complex structural units, higher basicity seems to decrease the intensity of the Si-O-Si bending and suggest a depolymerization of the network to simpler structural units. Figure 3(b) shows the effect of CaF2 on the slag structure. With higher CaF2 content, the depth of the NBO/Si = 4 trough becomes deeper and more pronounced indicating a depolymerization to simpler structures corresponding to NBO/Si = 4. Thus, with higher basicity the slag structure becomes more depolymerized with the supply of excess free oxygen ions and subsequently increases the activity of free oxygen ions to increase the carbon solubility by pushing Reaction [1] forward. The Si-O-Si bending also becomes less pronounced further supporting the depolymerization of the structure and subsequent affinity for the free carbide mechanism to be dominant as the bridged oxygen ions decrease.
A more quantitative analysis can be performed using Raman spectroscopy of the as-quenched slags. Table II provides the fraction of structural units obtained from the integration of the deconvoluted Raman spectra comprising the slag as a function of basicity. It was apparent that the fraction of structural units including NBO/Si = 1 and NBO/Si = 3 decreased, while the NBO/Si = 2 and NBO/Si = 4 increased with higher basicity. This illustrates the network modification of the slag structure with higher basicity as the complex structures of NBO/Si = 1 become depolymerized and increase the fraction of less complex NBO/Si = 2 and NBO/Si = 4 structural units within the melt. This corresponds well with the results of the FT-IR and the carbon solubility plots with higher basicity.
Similar to the FT-IR, Figure 4 shows the depolymerization of the structure with higher CaF2 content in the slag. As expected, higher CaF2 sequentially decreases the complex structural units of NBO/Si = 1 and NBO/Si = 3, while the less complex structural units of NBO/Si = 2 and NBO/Si = 4 increase. From the FT-IR and Raman results, the complex silicate structural units decrease with higher basicity and CaF2 content subsequently lowering the available bridged oxygen ions and increasing the free oxygen ion activity to facilitate the dominance of the free carbide mechanism and increase the carbon dissolution into the melt.
The compositional variations in the present study include changes in both the apparent basicity defined as the mass ratio of CaO/SiO2 and CaF2. To comprehend both effects into a single variable, the theoretical optical basicity first proposed by Duffy and Ingram[16] has been used in Figure 5. The details of the theoretical optical basicity (Λth) have been described in detail elsewhere, but the calculation can be obtained from Eq. [4].[18]
where the theoretical optical basicity of the compound is calculated from the addition of each individual pure component i optical basicity (Λ i ) of a j component comprised system. In the CaO-SiO2-3MgO-CaF2 system considered in the present study, there are four components and the theoretical optical basicity of the pure constituents CaO, SiO2, MgO, and CaF2 estimated from the average electron density according to Nakamura et al.[18] was 1.00, 0.47, 0.92, and 0.67, respectively. x i is the mole fraction of component i and n i is the number of anions with respect to unit cation in the compound. Figure 5 shows a relatively similar trend of the logarithm of carbon content as a function of the theoretical optical basicity, which correlates well with the depolymerization of the melt structure and subsequent increase in the carbon content with both increasing CaO/SiO2 and CaF2 content in the slag. The overall trend in carbon with optical basicity seems to agree well with work done by others.[19,20]
The effect of the extended basicity and CaF2 on the carbon solubility in the CaO-SiO2-3MgO-CaF2 slag system at 1773 K (1500 °C) was investigated under CO/Ar and CO/N2. In the present study, the carbon solubility increased with increasing activity of free oxygen ions or increasing basicity. The carbon seems to dissolute as a carbide ion. Higher CaF2 also resulted in higher carbon dissolution into the slag as the network modifying fluorine ions interacts with the bridged oxygen in the melt structure to increase the activity of the free oxygen ions in the melt subsequently driving the forward reaction of the free carbide mechanism. Slightly higher carbon content was observed for the CO/N2 atmosphere compared to CO/Ar, which may be the formation of the cyanide compounds within the melt. Structural information obtained from the FT-IR and Raman revealed a depolymerization of the network structure as the simpler structural units of NBO/Si = 4 increased with higher basicity and CaF2 content. The Si-O-Si bending vibration also decreased with higher basicity and CaF2 to support the depolymerization of the melt and decreasing the bridged oxygen ions and increasing the activity of free oxygen ions to facilitate the free carbide mechanism. A correlation of the theoretical optical basicity with the logarithm of the carbon content in slag showed a similar trend and an increase of carbon was observed with higher optical basicity.
References
K. Schwerdtfeger and H.G Schubert: Metall. Trans. B., 1977, vol. 8B, pp. 535-540.
R.A. Berryman and I.D. Sommerville: Metall. Trans. B., 1992, vol. 23B, pp. 223-227.
I. Sohn, D.J. Min, and J.H. Park: Steel Res., 1999, vol. 70, pp. 215-220.
J.-Y. Park and I. Sohn: Metall. Mater. Trans. B., 2013, vol. 44B, pp. 123-132.
M. Kuwata and H. Suito: Metall. Mater. Trans. B., 1996, vol. 28B, pp. 57-64.
J.H. Park, D.J. Min, and H.S. Song: ISIJ Int., 2002, vol. 42, pp. 127-131.
G. J. W. Kor and F. D. Richardson: Trans. of the Metall. Society of AIME, 1969, vol. 245, pp. 319-327.
A. Mitchell: Trans. Faraday Society, 1967, vol. 63, pp. 1408-1417.
D. Kumar, R. G. Ward and D. J. Williams: Disc. Faraday Society, 1961, vol. 32, pp. 147-155.
G.-H. Kim, C.-S., Kim, and I. Sohn: ISIJ Int., 2013, vol. 53, pp. 170–76.
J.-Y. Park, S.-J. Park, W.-S. Chang, and I. Sohn: J. Am. Cer. Soc., 2012, vol. 95, pp. 1756-1763.
M. Kuwata and H. Suito: MMTB, 1996, vol. 27B, pp. 57-64.
B.O. Mysen, D. Virgo, and C. M. Scarfe: Am. Mineral., 1980, vol. 65, pp. 690-710.
P. McMillan: Am. Mineral., 1984, vol. 69, pp. 645-659.
H.W. Nesbitt, G.M. Bancroft, G.S. Henderson, R.Ho, K.N. Dalby, Y. Huang, and Z. Yan: J. Non-Cryst. Solids, 2011, vol. 357, pp. 170-180.
J. A. Duffy and M.D. Ingram: Phys. Chem. Glasses, 1975, vol. 16, pp. 119-123.
C. J. B. Fincham and F. D. Richardson: Proc. Roy. Soc., 1954, vol. A223, pp. 40-62.
T. Nakamura, T. Yokoyama, and J.M. Toguri: ISIJ Int., 1993, vol. 33, pp. 204-209.
J.H. Park and D.J. Min: Metall. Mater. Trans. B., 1999, vol. 30B, pp. 1045-1052.
J.H. Park, G.H. Park and Y.E. Lee: ISIJ Int., 2010, vol. 50, pp. 1078-1083.
This study was partially supported by the BK21PLUS Project at the Division of the Eco-Humantronics Information Materials, the National Science Foundation of Korea Project No. 2012-8-0486 and the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093823).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 27, 2013.
Rights and permissions
About this article
Cite this article
Park, JY., Jung, SM. & Sohn, I. Carbon Solubility in the CaO-SiO2-3MgO-CaF2 Slag System. Metall Mater Trans B 45, 329–333 (2014). https://doi.org/10.1007/s11663-014-0028-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0028-9