Abstract
Ilmenite produced from the Panxi area in China has high impurities such as Ca and Mg. High-grade titanium (Ti) slag can be obtained by the electric arc furnace process, a traditional method of treating ilmenite. Thus, Ti slag prepared from the Panxi ilmenite contains high CaO and MgO, exceeding 5 pct of the slag content. This high CaO and MgO content confers considerable difficulty in producing titania (TiO2) white using fluidizing chlorination. In this study, a new process named vacuum separation was found to produce high-grade TiO2 materials. The effects of separation temperature and time on the TiO2 grade were studied. The high-grade TiO2 slag, which has 93 pct TiO2, <0.1 pct MgO, <1.2 pct SiO2, and <0.5 pct CaO, can be produced at 1823 K (1550 °C) in 45 minutes through the proposed method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ilmenite and rutile are the main raw materials for producing titanium pigment and metallic titanium. The ilmenite reserve in China has about 2 × 108 tons in the form of titania (TiO2), accounting for approximately 30 pct of the world’s ilmenite reserves. Most of these reserves are located in the Panxi area, China. This area has become an important titanium production base in China.[1] However, most of these reserve materials are paragenetic and titanium ores containing minerals such as high Ca and Mg. These characteristics of ilmenite have conferred considerable complexity and difficulty in using smelting technology.[2,3] In 2006, an electric arc furnace (EAF, 25.5 MW) was constructed to produce TiO2-rich slag for an ilmenite smelting plant in Panzhihua. The TiO2 grade in the produced slag was merely about 80 pct, and more impurities remained in the slag. Subsequent processing was required before chlorination was adopted to manufacture TiO2 and titanium sponge.[4,5] Calcium and magnesium oxides can be removed by the hydrochloric acid method, but it has disadvantages such as serious corrosion of equipment and large amount of waste acid and by-products.[6] These problems also limit the efficiency of titanium resource utilization and the development of the titanium industry in Panzhihua. Thus, a new technology that produces high-quality titanium-rich material must be established.
In this paper, the vacuum separation method, which is a new process for producing high-quality titanium-rich material, is proposed. In the vacuum atmosphere, reactions that result in increased gas volume during production can be promoted and reactions such as metal gasification, evaporation, and metal compound decomposition can be accelerated.[7,8] This vacuum separation technology can be used to remove the high vapor pressure of substances, such as MgO, SiO2, and ZnO,[9–16] during the reduction process. In the proposed process, most Mg and Si can be removed from the slag, and the TiO2 slag can reach a grade as high as 90 pct. By contrast, given that the ilmenite produced from Panzhihua contains very high amounts of CaO, MgO, and SiO2, a slag with 75 to 80 pct TiO2 can be produced through the proposed EAF process.
Experimental
The chemical compositions of ilmenite and coke, which were supplied by Panzhihua Iron & Steel (Group) Co., China, are shown in Tables I and II.
Before the experiment, 100 pct ilmenite, 12 pct coal powder, and 2‰ resin were mixed based on the ratio of mass. The mixed raw materials were placed into a mold with a diameter of 30 mm and then pressed under a pressure of 20 MPa to form the cylindrical pellets. The pellets were approximately 10 mm in height and 100 g in mass. The pellets were dried at 473 K (200 °C) for 4 hours to remove the water. The pre-reduction process was conducted in a vertical tube electric resistance furnace. The pre-reduction temperature and holding time were 1653 K (1380 °C) and 30 minutes, respectively, and argon gas was blown into the furnace during the reduction and cooling steps to avoid re-oxidation of the metal.
After pre-reduction, the samples were placed into a graphite crucible with a graphite lid, which was then loaded in the vacuum vertical tube furnace with a graphite heater to separate the slag and the metal. During the separation process, pressure in the furnace was maintained at 100 Pa, temperatures were fixed at 1723 K, 1773 K, and 1823 K (1450 °C, 1500 °C, and 1550 °C), with holding times at 15, 30, and 45 minutes, respectively. After the separation, the samples were analyzed using X-ray diffraction and chemical analysis.
Results and Discussion
Effect of Temperature in the Vacuum Smelting Process on Separation Behavior
Figure 1 shows the effect of temperature on the content of Ti2O3 and TiO2 in the high-grade TiO2 slag after smelting. The grade of the titanium-rich material increased as temperature increased. The grade achieved about 88 pct when the temperature was 1823 K (1550 °C). The TiO2 content decreased when the temperature increased from 1723 K to 1823 K (1450 °C to 1500 °C), and it slightly changed to approximately 68 pct when the temperature exceeded 1773 K (1500 °C). The Ti2O3 content increased as the temperature increased, which indicated that TiO2 was gradually reduced along with Ti2O3 as the temperature increased. The increase in temperature can promote the reduction of TiO2 in the vacuum smelting process.
Figure 2 shows the effect of temperature on MgO, SiO2, CaO, FeO, and MFe content of the high-grade TiO2 slag after smelting. The MgO content decreased as the temperature increased. The SiO2 content decreased as the temperature increased from 1723 K to 1773 K (1450 °C to 1500 °C), and it slightly changed to approximately 2 pct when the temperature exceeded 1773 K (1500 °C). The CaO content increased when the temperature increased. These results indicated that MgO, SiO2, and CaO were gradually reduced and then became weathered as the temperature increased during the vacuum smelting process.
Figure 2 shows that FeO and MFe content decreased as the temperature increased. The increase in the FeO amount as the temperature increased was more obvious in the initial reduction stage than the later stage. The MFe content decreased as the temperature increased from 1773 K to 1823 K (1500 °C to 1550 °C), and it changed slightly when the temperature was below 1773 K (1500 °C). This result indicated that FeO was gradually reduced along with MFe as the temperature increased. This phenomenon can improve the separation process between the slag and the MFe by increasing the temperature during vacuum smelting.
Effect of Holding Time in the Vacuum Smelting Process on Separation Behavior
The relationship of holding time and content of titanium oxides (Ti2O3 and TiO2) in the slag is shown in Figures 3 and 4. The grade of the titanium-rich material increased as the holding time increased. The grade was approximately 90 pct when the holding time was 45 minutes. The Ti2O3 content first increased and then remained relatively constant at about 65 pct when the holding time increased from 15 to 30 minutes, whereas the TiO2 content first decreased and then remained roughly constant at about 25 pct at 1500 °C. The Ti2O3 content increased and TiO2 content decreased slowly as the holding time increased from 15 to 30 minutes; however, the change in the Ti2O3 and TiO2 content was rapid when the holding time increased from 30 to 45 minutes at 1823 K (1550 °C). This result indicated that the TiO2 and Ti2O3 contents were gradually reduced over the holding time. Furthermore, the TiO2 content was easily reduced to a low-valence titanium in the vacuum smelting process.
The relationship of holding time and content of MFe and FeO is shown in Figure 5, which indicates that MFe and FeO content decreased as the holding time increased. The MFe and FeO content increased slowly as the holding time increased from 15 to 30 minutes; however, this transformation occurred rapidly when the holding time increased from 30 to 45 minutes at 1823 K (1550 °C). This result indicated that the FeO and MFe contents were gradually reduced as the holding time increased. The iron metallization ratio of the pellets was 85 pct after pre-reduction. However, MFe was less than 1.5 pct when the holding time and the temperature were 45 minutes and 1823 K (1550 °C), respectively. Thus, the vacuum separation method can improve the separation process between the slag and the MFe by increasing the holding time.
At 1773 K and 1823 K (1500 °C and 1550 °C), the graph of the relationship between the MgO, SiO2, and CaO contents and the holding time is shown in Figure 6. The MgO and SiO2 content decreased as the holding time increased. When the holding time was 45 minutes, the content of MgO was less than 0.1 pct, which decreased to about 96 pct after vacuum smelting. The contents of SiO2 were 1.83 and 1.17 pct, which were reduced by about 63 and 74 pct, respectively, after vacuum smelting. At 1773 K (1500 °C), the CaO content increased as the holding time increased, then increased as the holding time increased from 15 to 30 minutes, and then decreased rapidly when the holding time increased from 30 to 45 minutes. The CaO content was finally reduced by approximately 64 pct. This finding indicated that MgO, SiO2, and CaO contents were gradually reduced and then weathered as the holding time increased in the vacuum smelting process.
The mass of the metal phases cannot be easily obtained based on the experiment because the metal phases of gaseous Fe, Ca, Mg, and SiO could not be collected from the vacuum vertical tube furnace. The samples were loaded into the corundum crucible with a graphite cover. This graphite cover can be oxidized by the oxygen left in the furnace; therefore, obtaining an accurate mass of the sample left in the corundum crucible and the cover is difficult. However, the gaseous Fe and other metals were found on the surface of the cover, which are shown in Figure 7.
Phase Transformation of the Slag in the Smelting Process
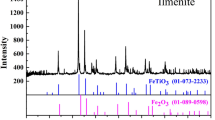
In the vacuum smelting experiment, the phases of the samples were analyzed by XRD. The XRD patterns are shown in Figures 8 and 9 at different holding times. The on-site slag had only two main phases, namely, TiO2 and brookite. However, several new peaks appeared in the XRD pattern of the sample after the vacuum smelting experiment. The main phases were TiO2 and Ti2O3 after vacuum smelt separation at a temperature of 1773 K (1500 °C). The intensity of TiO2 and Ti2O3 significantly decreased as the holding time increased. A number of diffraction peaks of the low-cost titanium compounds (TiO) were observed when the temperature was 1773 K (1500 °C) and the holding time was 45 minutes. The XRD pattern of the sample obtained at 1823 K (1550 °C) and a holding time of 45 minutes shows that the TiO2 phase disappeared completely, whereas the mixture phases of TiC, TiO, and Ti2O3 still existed. However, a TiC phase was generated when the holding time was 30 minutes. Thus, TiO2 was gradually reduced to the low-valence titanium compounds as the holding time and the temperature increased.
Theoretical Calculation and Comparisons
Ilmenite pellets are loose and porous after pre-reduction. CO gas can easily spread out if the vacuum is 100 pa. Therefore, the following gas–solid and solid–solid reactions may occur in the system:
FactSage software was used to calculate the Gibbs energy of the above reactions at a system pressure of 100 Pa and 1 atm; the results are shown in Figures 10 and 11. It can be seen from Figure 10 that the Gibbs free energy of the gas–solid reaction is still positive, indicating that a reaction cannot occur. By contrast, the Gibbs free energy of the solid–solid reactions is negative, indicating that a reaction can occur. The reaction order from easy to difficult is FeO, SiO2 MgO, and CaO. Thus, calculations using the Panzhihua ilmenite vacuum carbothermal reduction theory show that the reaction mechanism is a direct reduction solid–solid reaction of carbon particles. The results of this study are consistent with those of others.[17] Figure 11 shows that the Gibbs free energy of the first and fourth reactions is negative, indicating that reactions can occur. The fourth reaction requires high temperatures to occur. The other reactions are still positive, indicating that the reaction cannot occur. The results of this study are consistent with those of others.[18–20] Figure 10 also shows that the vacuum smelting process effectively removes impurities, such as Fe, Mg, and Si, and successfully enriches titanium slag.
The masses of different oxides and the mass percentages of various oxides in the slag phase vs temperature are shown in Figure 12(a) and (b). FactSage software was used to perform the required calculations. The calculation conditions were carbon content and pressure at 12 pct and 100 Pa, respectively. When the temperature exceeds 1573 K (1300 °C), the slag phase would form. The quality of the SiO2 in the slag decreases rapidly with increasing temperature, whereas the quality of Ti2O3 and TiO2 increases with increasing temperature. The quality of Al2O3 and CaO increases from 1573 K to 1623 K (1300 °C to 1350 °C) and then remains roughly constant. The dissolved quantity of Al2O3 and CaO increases with increasing temperature, and cannot be reduced to metal gas under 100 pa pressure when the temperature ranges from 1573 K to 1623 K (1300 °C to 1350 °C). The quality of MgO and SiO2 increased with increasing temperature. However, MgO, SiO2, and CaO percentage compositions initially decrease with increasing temperature and then remain roughly constant at about 0.7, 0.2, and 2.4 pct, respectively, at temperatures exceeding 1923 K (1650 °C). Ti2O3 and TiO2 contents increase with increasing temperature when the temperature is below 1923 K (1650 °C) and then remain roughly constant. There is a 16 pct liquid phase when the temperature is 1623 K (1350 °C), a 40 pct liquid phase when the temperature is 1823 K (1550 °C), an 80 pct liquid phase when the temperature is 1923 K (1650 °C), and a 100 pct liquid phase when the temperature is about 1973 K (1700 °C). After theoretical calculations, the slag oxides TiO2 and Ti2O3 contents reach 94 pct. These theoretical calculations were consistent with the experimental results.
Conclusions
High-grade TiO2 slag was prepared using ilmenite from Panzhihua by means of the vacuum smelting method. The conclusions are summarized as follows:
-
1.
Ti2O3 content initially increased and TiO2 content initially decreased and then remained roughly constant with increasing holding time. The grade of the titanium-rich material increased with increasing holding time. A TiO2 grade of 91 pct was achieved at a temperature of 1823 K (1550 °C) and a holding time of 45 minutes.
-
2.
MgO, CaO, and SiO2 contents decreased with increasing holding time and temperature. Given a holding time of 45 minutes, and temperatures of 1773 K and 1823 K (1500 °C and 1550 °C), MgO content was less than 0.1 pct at both temperatures, SiO2 content was 1.83 and 1.17 pct, respectively, and CaO content was 2.83 and 0.44 pct, respectively.
-
3.
FeO amount and the metallization of iron content decreased with increasing holding time and temperature. At a holding time of 45 minutes, FeO content was less than 4 pct no matter the temperature.
-
4.
The main phases of the TiO2 and Ti2O3 samples weakened with increasing holding time after vacuum smelt separation at 1773 K (1500 °C). The main phases became TiC, Ti2O3, and TiO. The diffraction peak TiO2 was reduced to become another diffraction peak of the low-valence titanium compound when the holding time was 45 minutes. The TiC phase was generated when the holding time was 30 minutes at 1823 K (1550 °C).
-
5.
After theoretical calculation, TiO2 and Ti2O3 contents reached 94 pct. These theoretical calculations were consistent with the experimental results.
References
Y.M. Wang: Mining and Metallurgical Engineering, 2011, vol.31 (5) pp.66-68.
H. Wang: Titanium Ind. Progress, 1999, vol. 4, 6.
W. Mo, G.Z. Deng, and F. Luo: Titanium Metallurgy, Metallurgical Industry Press, Beijing, 1998.
H.J. Ma and K. Wang: Rare Met., 1981, vol. 3, pp. 21-26.
G.Z. Deng and W.H. Yu: Iron Steel Vanadium Titanium, 2003, vol.24 (2),pp.34-38.
G.Z. Qiu and Y.F. Guo: Multipurp. Util. Miner. Resour., 1998 vol. 5, pp. 29–33.
Y.N. Dai and B. Yang: Nonferrous Metal Vacuum Metallurgy, Metallurgy Industry Press, Beijing, 2009, p. 158.
Y.N. Dai and Z. Zhao: Vacuum Metallurgy, Metallurgy Industry Press, Beijing, 2002.
Q. Luo, T. Qu and D.C. Liu: J. Centr. South Univ. Sci. Technol., 2012, vol. 11, pp. 4190–98.
Q. Luo, D.C. Liu and T. Qu: J. Centr. South Univ. Sci. Technol., 2012, vol. 08, pp. 2900–908.
T. Yang, T. Qu, B. Yang, Y.N. Dai and B.Q. Xu: Metallurgical and Materials Transactions B, 2012, vol.43(3), pp.657–61.
Y.B. Feng, B. Yang and Y.N. Dai: Chin. J. Nonferr. Met., 2011, vol. 07, pp. 1748–755.
J. Safarian, and M. Tangstad: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1427–445.
L.Z. Xiong, Q.Y. Chen and L. Zhuo: Chin. J. Process Eng., 2010, vol. 01, pp. 133-137.
H.X. Liu, Y.N. Dai and Y.F. Li: Vacuum, 2009, vol. 05, pp. 82-86.
L.X. Kong, B. Yang, Y.F. Li, B.Q. Xu, D.C. Liu and G.B. Jia: Metallurgical and Materials Transactions B, 2012, vol.43 (6), pp. 1649-1656.
Q. Luo, T. Qu and D.C. Liu, South University: Science and Technology, 2012 vol. 11, pp. 4190.
Z.H. Li, Y.N. Dai and H.S. Xue: Nonferrous metals, 2005, vol.57 (1), pp. 56-59.
M. Wang, J. Tan and Q.Y. Chen: Nonferrous Metals ExtractiveMetallurgy, 2012, vol. 05, pp. 1–4.
Q.C. Yu, B. Yang and H.W. Ma: Chin. J. Vac. Sci. Technol., 2009, vol. 29, 68.
Acknowledgments
The authors are especially grateful for the financial support from the National Basic Research Program of China (973 Program-2013CB632603). The chemical compositions of all the samples were analyzed by Panzhihua Iron & Steel Research Institute, Pan Gang Group.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 19, 2013.
Rights and permissions
About this article
Cite this article
Zhang, K., Lv, X., Huang, R. et al. Preparation of High-Grade Titania Slag from Ilmenite-Bearing High Ca and Mg by Vacuum Smelting Method. Metall Mater Trans B 45, 923–928 (2014). https://doi.org/10.1007/s11663-013-9982-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9982-x