Abstract
The thermodynamic relation between silicon and aluminum in liquid iron was studied by measuring the effect of silicon on the solubility product of AlN in liquid Fe-Si-Al-N alloys containing silicon up to 1.5 mass pct in the temperature range from 1823 K to 1923 K (1550 °C to 1650 °C). The effects of aluminum and silicon on nitrogen solubility in liquid iron were separately determined in the same temperature range. The experimental results were thermodynamically analyzed using Wagner’s interaction parameter formalism to determine the first-order interaction parameters of silicon on nitrogen and aluminum in liquid iron as follows: \( e_{\text{N}}^{\text{Si}} = 0. 0 6 7 3, \;e_{\text{Al}}^{\text{Si}} = 0.009 \) (1823 K to 1923 K (1550 °C to 1650 °C), Si ≤ 1.5 mass pct)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An accurate control of complex deoxidation by Si-Al in liquid steel is very important in view of high deoxidation efficiency and inclusion controls in many grades of steels. Despite its importance, the reported data on the interaction parameter between silicon and aluminum in liquid iron are very limited. Chipman and Floridis[1] determined the first-order interaction parameter between aluminum and silicon in the Fe-Al-Si-C system as \( \varepsilon_{\text{Al}}^{\text{Si}} = 6.9 \) at 1873 K (1600 °C) using a silver bath isoactivity method for the four alloys containing silicon in the range of X Si = 0.13 to 0.38. They estimated the activity coefficient of Al, \( \gamma_{\text{Al}} \) in Fe-Al melts at 1873 K (1600 °C) using the extrapolated activity data of the solid binary Ag-Al system[2] based on the phase diagram[3] and the assumption that log\( \gamma_{\text{Al}} \) is inversely proportional to absolute temperature. Later, Wilder and Elliott[4] determined the activity of aluminum in liquid binary Ag-Al alloys at higher temperatures up to 1223 K (950 °C). They reexamined Chipman and Floridis’ data and recalculated the \( \varepsilon_{\text{Al}}^{\text{Si}} \) value as 7.0 at 1873 K (1600 °C). The value of \( \varepsilon_{\text{Al}}^{\text{Si}} \) can be converted to the \( e_{\text{Al}}^{\text{Si}} \) value as 0.056 at 1873 K (1600 °C) using the Lupis’ conversion relationship.[5] This \( e_{\text{Al}}^{\text{Si}} \) value is the only data compiled as the recommended value by the Japan Society for the Promotion of Science.[6] There is no information on the temperature effect for this important parameter value.
The interaction parameter, by its definition, is the effect of one solute on the activity coefficient of another solute in the dilute solution. Aluminum and silicon contents in liquid steel during the Al-Si codeoxidation process are normally less than 1 mass pct. In some special grades such as transformation-induced plasticity steels, their contents are as high as 2 mass pct.[7–9] In the authors’ recent study,[10] the effect of aluminum content on nitrogen solubility in liquid iron and the solubility product of aluminum and nitrogen in liquid iron saturated with pure solid AlN were measured as a function of temperature.
In the current study, the thermodynamic interaction between silicon and aluminum in liquid iron was determined by measuring the effect of silicon on the solubility product of AlN in Fe-Si melts in the temperature range from 1823 K to 1923 K (1550 °C to 1650 °C). The effect of silicon on nitrogen solubility was separately determined. The current results were thermodynamically analyzed to determine the interaction parameters of silicon on nitrogen and aluminum in Fe-Si-N and Fe-Al-Si melts, respectively.
Experimental
Experimental Procedures
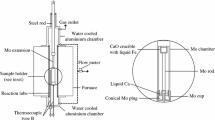
The metal–gas and the metal–nitride–gas equilibration experiments were carried out to determine the effect of silicon on the nitrogen solubility and the AlN solubility product in liquid iron, respectively. Detailed descriptions of the experimental apparatus and procedure are available in the authors’ recent studies on Fe-Al-N, Fe-Cr-N, and Fe-Cr-Al-N systems.[10–12]
Five hundred grams of high-purity electrolytic iron contained in an Al2O3 crucible (outer diameter [OD]: 56 mm, inner diameter [ID]: 50 mm, height [H]: 96 mm) was melted in the temperature range of 1823 K to 1923 K (1550 °C to 1650 °C) by a 15 kW/30 kHz high-frequency induction furnace. After melting the iron, the melt temperature was directly measured by a Pt/Pt-13 mass pct Rh thermocouple sheathed with an 8-mm OD alumina tube immersed in the melt, and the temperature was accurately controlled within 2 K during each experiment by a proportional–integral–derivative controller of the induction furnace. After the melt temperature was reached at a desired value, Ar-10 pct H2 gas was blown onto the melt surface at a high flow rate of ~5000 mL/min for 2 hours to deoxidize the iron melt. The oxygen content in the melt decreased to a value less than 20 mass ppm. Then, the gas was switched to a mixture of Ar-10 pct H2 and N2 gases to have nitrogen partial pressures of 0.3 and 1 atm. The flow rate of the gas mixture was controlled by a mass flow controller in the range of 1000 to 2000 mL/min depending on the nitrogen partial pressures in the gas. Strong agitation of the melt by an induction furnace resulted in a fast attainment of equilibrium nitrogen solubility in liquid iron under a nitrogen partial pressure within 1 hour.
Fe-Si-N System
In the case of silicon addition experiments to measure the nitrogen solubility in Fe-Si melts, pellets of silicon (99.99 pct purity) were added to liquid iron through an 18 mm OD quartz tube after confirming the equilibrium nitrogen solubility in pure liquid iron under a nitrogen partial pressure by the sampling and analysis. After each silicon addition, a new nitrogen solubility equilibrium was attained within 30 minutes. This was confirmed by sampling and in situ analysis after the silicon addition. Silicon additions were repeated up to 2 mass pct Si in liquid iron.
Fe-Al-Si-N System
In the case of the metal–nitride–gas equilibration experiments, an aluminum (99.9 pct purity) addition and sampling were repeated until a stable AlN layer was formed on the surface of the iron melt. The formation of AlN in the iron melt could be confirmed by a sharp decrease in the nitrogen content checked by an analysis of the metal samples during the experiment. After the saturation of AlN in liquid iron, silicon (99.99 pct purity) was added up to 1.5 mass pct Si in liquid iron. After each silicon addition, the new AlN solubility equilibrium was attained within 30 minutes. This result was confirmed by sampling and in situ analysis for nitrogen at 15-minute intervals after each silicon addition.
Chemical Analysis
The metal samples were carefully cut for the chemical analysis. In the authors’ previous studies,[10–12] the detailed procedure for chemical analysis is available. Four specimens of each metal sample were prepared for the analysis of nitrogen and oxygen. The nitrogen and oxygen contents in the metal sample were measured by the inert gas fusion-infrared absorptiometry technique (LECO TC-600 apparatus; LECO Corporation, St. Joseph, MI) with an accuracy of ±2 mass ppm. For the analysis of Al and Si, the metal sample (0.2 g) was dissolved in 20 mL HCl(1 + 1) in a glass beaker of 50 mL capacity heated in a water bath for 2 hours and analyzed by the inductively coupled plasma-AES (SPECTRO ARCOS apparatus, manufactured by Spectro Analytical Instruments, Kleve, Germany) using appropriate standard solutions containing the same amount of Fe (2000 mass ppm) as the sample solutions. The analytical limit for silicon and aluminum in the metal sample was 5 ± 1 mass ppm.
After each experiment, the melt remaining in the alumina crucible was quenched rapidly by blowing helium gas onto the melt surface. The quenched metal samples were cross sectioned and examined by an optical microscope and the scanning electron microscopy (SEM)-energy-dispersive X-ray spectroscopy (EDS) for the presence of AlN inclusions. The center part of metal sample was virtually clean without any noticeable inclusions. However, a few AlN inclusions was observed near the melt surface as shown in Figure 1.
Results and Discussion
Effect of Silicon on Nitrogen Solubility in Liquid Iron
The experimental results of nitrogen solubility measurements with silicon additions in liquid iron under different nitrogen partial pressures and temperatures are summarized in Table I. Figure 2 shows the nitrogen solubility of liquid iron containing silicon up to 2 mass pct under nitrogen partial pressures of 0.3 and 1 atm at 1823 K, 1873 K, and 1923 K (1550 °C, 1600 °C, and 1650 °C). Silicon decreases the nitrogen solubility in Fe-Si melt linearly.
The dissolution of nitrogen in liquid iron alloys can be written as[13]
where \( {\text{K}}_{ 1} \) is the equilibrium constant for Reaction [1] and \( f_{\text{N}} \) is the activity coefficient of nitrogen in the 1 mass pct standard state in liquid iron.
Using Wagner’s formalism,[14] the equilibrium constant of the preceding reaction can be rewritten as the following relation using interaction parameters:
where \( e_{\text{N}}^{i} \) and \( r_{\text{N}}^{i} \) are the first- and the second-order interaction parameters of elements on nitrogen in liquid Fe-Si-N alloys, respectively. As mentioned, the oxygen content was very low in the melt and the effect of oxygen on nitrogen was assumed to be negligible. The first- and the second-order self-interaction parameters of N, \( e_{\text{N}}^{\text{N}} \) and \( r_{\text{N}}^{\text{N}} \), respectively, are known to be zero at nitrogen contents up to 0.045 mass pct in liquid iron at 1879 K (1606 °C).[15]
The activity coefficient of silicon on nitrogen \( f_{\text{N}}^{\text{Si}} \) can be then defined as
Figure 3 shows the values of \( \log f_{\text{N}}^{\text{Si}} \) plotted vs silicon concentration in mass pct in liquid iron using the relation expressed by Eq. [4]. The data determined at different nitrogen partial pressures show an excellent linear relationship. No temperature dependence was determined in the temperature range from 1823 K to 1923 K (1550 °C to 1650 °C). The first-order interaction parameter \( e_{\text{N}}^{\text{Si}} \) can be determined as 0.0673 by a linear regression analysis of the data. Several groups including Pehlke and Elliott,[15] Maekawa and Nakagawa,[16] and Schenck et al.[17] measured the nitrogen solubility in Fe-Si melts at 1 atm nitrogen pressure using the Sieverts method[15] and the sampling method.[16,17] Table II summarizes the results, and the \( e_{\text{N}}^{\text{Si}} \) value determined in the current study is in good agreement with the value determined by Schenck et al.[17]
Effect of Silicon on Solubility Product of AlN in Liquid Iron
The experimental results of nitrogen solubility measurement with aluminum and silicon additions in liquid iron are also summarized in Table I. Figure 4 shows the variation of equilibrium nitrogen solubility in Fe-Al-N melts with aluminum additions under a nitrogen partial pressure of 1 atm at 1923 K (1650 °C). The nitrogen solubility decreases slightly as the aluminum content increases in liquid iron when the melt is not saturated with AlN as shown using open symbols in the figure. When the aluminum content exceeds the critical value, the nitrogen solubility decreases significantly because of the formation of AlN in the melt as shown as solid symbols in the figure. The solid line in Figure 4 is the equilibrium solubility product of aluminum and nitrogen for AlN formation in liquid iron at 1923 K (1650 °C) determined in the authors’ previous study on AlN formation in Fe-Al-N melts.[10]
Figure 5 shows the effect of silicon additions on the solubility of aluminum and nitrogen for AlN saturation in liquid iron under a nitrogen partial pressure of 1 atm at 1923 K (1650 °C). As the silicon content increases, the nitrogen solubility decreases slightly while the aluminum solubility is nearly constant. Figure 6 shows the effect of silicon additions on the solubility product of aluminum and nitrogen, \( \log [{\text{pct Al][pct N}}] \), for AlN saturation at different temperatures. Silicon decreases the solubility product of AlN in liquid iron primarily because of the effect of silicon on nitrogen solubility.
As shown in Figure 1, the inclusions formed in the melt during the metal–nitride–gas equilibration experiments were identified as pure aluminum nitride. In the authors’ recent study,[12] the X-ray diffraction (XRD) analysis on the filtrated residue after the acid dissolution of the metallic portion of quenched samples confirmed that the aluminum nitride formed in Fe-Al-N melts was a pure stoichiometric AlN.
The reaction equilibrium for the dissolution of pure solid AlN in liquid Fe-Al-Si-N alloys can be written as[10]
where \( {\text{K}}_{5} \) is the equilibrium constant for Reaction [5], and \( h_{\text{Al}} \) and \( h_{\text{N}} \) are the Henrian activities of aluminum and nitrogen relative to the 1 mass pct standard state in liquid iron, and \( f_{\text{Al}} \) and \( f_{\text{N}} \) are the activity coefficients of aluminum and nitrogen, respectively. Under the current experimental conditions, the activity of AlN is unity.
To determine the thermodynamic relation between silicon and aluminum from the AlN solubility product data in Fe-Si-Al-N melts, the equilibrium constant \( {\text{K}}_{ 5} \) can be rewritten as the following relation using Wagner’s formalism[14]:
where \( e_{\text{Al}}^{\text{i}} ,\;e_{\text{N}}^{\text{i}} ,\,r_{\text{Al}}^{\text{i}} ,\;{\text{and}}\;r_{\text{N}}^{\text{i}} \) are the first- and second-order interaction parameters of elements on aluminum and nitrogen in liquid Fe-Si-Al-N alloys, respectively.
The value of \(e_{\text{N}}^{\text{Si}} \) was determined as 0.0673 in the current study for Fe-Si-N melts containing silicon up to 2 mass pct in the temperature range from 1823 K to 1923 K (1550 °C to 1650 °C). In the authors’ recent work,[10] the thermodynamics of Fe-Al-N was studied as a function of temperature. The values of \( \log {\text{K}}_{5} \) and the interaction parameters used in Eq. [8] are summarized in Table III. The value of \( r_{\text{Al}}^{\text{Al}} \) was assumed to be zero in the current study.
Then, Eq. [8] can then be rearranged as
where \( f_{\text{Al}}^{\text{Si}} \) is the interaction coefficient of silicon on aluminum in liquid iron. As mentioned, the oxygen content in the melt was very low and the effect of oxygen on aluminum and nitrogen was assumed to be negligible.
Therefore, the values of \( e_{\text{Al}}^{\text{Si}} \) and \( r_{\text{Al}}^{\text{Si}} \) in Eq. [9] can be determined from the solubility product of AlN as a function of the silicon content in the Fe-Al-Si-N melts. Figure 7 shows the values of \( \log f_{\text{Al}}^{\text{Si}} \) plotted vs the percent silicon in Fe-Si-Al melts using the relation expressed by Eq. [9]. The \( \log f_{\text{Al}}^{\text{Si}} \) value increases linearly with silicon content up to 1.5 mass pct, but the effect is not significant. Also, the temperature effect is negligible as shown in the figure. Therefore, the values of \( e_{\text{Al}}^{\text{Si}} \) and \( r_{\text{Al}}^{\text{Si}} \) can be determined as 0.009 and 0, respectively, in the temperature range from 1823 K to 1923 K (1550 °C to 1650 °C) by a linear regression analysis of the data. The \( e_{\text{Al}}^{\text{Si}} \) value determined in the current study using the metal–nitride–gas equilibration technique in Fe-Si-Al-N melts was about six times lower than the value of 0.056 at 1873 K (1600 °C) reported by Wilder and Elliott.[4]
Wilder and Elliott[4] calculated the interaction parameter of silicon on aluminum in liquid iron from the Chipman and Floridis’ aluminum distribution data[1] between liquid Fe-Al-Si-C alloys and Ag-Al-Si melts at 1873 K (1600 °C) using the extrapolated Al activity data in liquid binary Ag-Al alloys at 1223 K (950 °C). However, the relatively high silicon content in Ag-Al-Si alloys (X Si < 0.0782) equilibrated with Fe-Al-Si-C alloys at 1873 K (1600 °C) in Chipman and Floridis’ work[1] does not warrant the accuracy of values for \( \log \gamma_{\text{Al}} \) in more dilute liquid alloy systems at 1873 K (1600 °C).
Conclusions
Using the metal–gas and the metal–nitride–gas equilibration techniques, thermodynamic relations among silicon, nitrogen, and aluminum in liquid iron were determined in the temperature range of 1823 K to 1923 K (1550 °C to 1650 °C). The main findings of this study can be summarized as follows:
-
1.
Silicon decreases the nitrogen solubility in Fe-Si-N melts, and the first- and the second-order interaction parameters of silicon on nitrogen were determined as
$$e_{\text{N}}^{\text{Si}}=0.0673,\;r_{\text{N}}^{\text{Si}}=0\quad(1823\;\text{K\;to}\;1923\;\text{K}\;[1550\;^{\circ}\text{C\;to}\;1650\;^{\circ}\text{C}],\text{Si}\leq 2\;{\text{mass\;pct}})$$ -
2.
The first- and the second-order interaction parameters between silicon and aluminum in liquid Fe-Si-Al alloys containing up to 1.5mass pct Si were determined as
$$e_{\text{Al}}^{\text{Si}}=0.009,\;r_{\text{Al}}^{\text{Si}}=0\quad(1823\,\text{K\;to\;}1923\,\text{K}\;[1550\;^{\circ}\text{C\;to\;}1650\;^{\circ}\text{C}],\text{Si} \leq 1.5\,{\text{mass\;pct}})$$
References
J. Chipman and T.P. Floridis: Acta Metall., 1955, vol. 3, pp. 456–59.
M. Hillert, B.L. Averbach, and M. Cohen: Acta Metall., 1956, vol. 4, pp. 31–36.
M. Hansen and K. Anderko: Constitution of Binary Alloys, McGraw-Hill Book Co., New York, NY, 1958.
T.C. Wilder and J.F. Elliott: J. Electrochem. Soc., 1960, vol. 107, pp. 628–35.
C.H.P. Lupis and J.F. Elliott: Acta Metall., 1960, vol. 14, pp. 529–38.
The 19th Committee in Steelmaking: Thermodynamic Data For Steelmaking, The Japan Society for Promotion of Science, Tohoku University Press, Sendai, Japan, 2010, p. 76.
J.K. Jung, O.Y. Lee, Y.K. Park, D.E. Kim, K.G. Jin, S.K. Kim, and K.H. Song: J. Kor. Inst. Metall. Mater., 2008, vol. 46, pp. 627–33.
C.Y. Choi, D.Y. Lee, I.B. Kim, Y.D. Kim, and Y.D. Park: Kor. J. Metall. Mater., 2011, vol. 49, pp. 967–76.
S.K. Lee, J.S. Kim, J.W. Choi, N.H. Kang, and K.M. Cho: Metall. Mater. Int., 2011, vol. 17, pp. 251–57.
W.Y. Kim, J.G. Kang, C.H. Park, J.B. Lee, and J.J. Pak: ISIJ Int., 2007, vol. 47, pp. 945–54.
W.Y. Kim, C.O. Lee, C.W. Yun, and J.J. Pak: ISIJ Int., 2009, vol. 49, pp. 1668–72.
J.O. Jo, M.S. Jung, J.H. Park, C.O. Lee, and J.J. Pak: ISIJ Int., 2011, vol. 51, pp. 208–13.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, p. 81.
C. Wagner: Thermodynamics of Alloys, Addison-Wesley Press, Cambridge, MA, 1952, pp. 47, 51.
R.D. Pehlke and J.F. Elliott: Trans. TMS-AIME, 1960, vol. 218, pp. 1088–101.
S. Maekawa and Y. Nakagawa: Tetsu-to-Hagané, 1960, vol. 46, no. 7, pp. 10–15.
H. Schenck, M. Frohberg, and H. Graf: Arch. Eisenhuttenwes., 1958, vol. 29, pp. 673–77.
Acknowledgment
This study was supported by the R&D Center for Valuable Recycling (Global-Top Environmental Technology Development Program) funded by the Ministry of Environment (Project No.: 11-C22-ID).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 7, 2011.
Rights and permissions
About this article
Cite this article
Kim, DH., Jung, MS., Nam, H. et al. Thermodynamic Relation between Silicon and Aluminum in Liquid Iron. Metall Mater Trans B 43, 1106–1112 (2012). https://doi.org/10.1007/s11663-012-9677-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9677-8