Abstract
Because of the growing demand of cleaner and defect-free steels, faster and more reliable offline steel cleanliness determination methods combined with online techniques are finding increasing usage to study steel refining and casting issues. Because a single-steel characterization technique is not sufficient to provide answers to all process problems, a combination of these techniques is often used to find a customized solution of the problem. This article reviews the past experiences on steel cleanliness examination to standardize the inclusion-characterization techniques and their applications for both experienced and novice researchers. The article also proposes a suite of techniques as a reference tool to facilitate clean steelmaking research at ArcelorMittal Group more effectively in the future. Examples of use of automated scanning electron microscopy and pulse discrimination analysis are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
ArcelorMittal, similar to other worldwide steel producers, is making every effort to make cleaner steels to meet customer demands while improving steelmaking and casting processes. One key area is to characterize and control the inclusions in the steel that affect its mechanical properties. After the merger between former Arcelor S.A. and Mittal Steel, researchers with expertise in the area of inclusion engineering teamed up to exchange the experiences on clean steelmaking, which are available separately in predecessor companies, and recommended to standardize the characterization techniques across the entire group. As a follow-up of these recommendations, a series of research was carried out to develop a suite of techniques to perform future clean steelmaking studies more efficiently. As reports that describe the characterization techniques in detail[1–4] were already available, efforts in the current exercise were directed to understand the applicability of these techniques by performing characterization on the steel grades in common production.
The article begins with a discussion about the inclusions that create problems in the steel grades of interest coupled with suggestions for appropriate sampling and techniques to carry out inclusion characterization. This is followed by a summary of the proposed tools for an inclusion analysis for use in research and plant studies to diagnose the root cause of the industrial problem. Thereafter, a round robin analysis conducted on selected steel samples is presented. The article finishes with a few examples of use of these techniques to analyze the steelmaking problems.
Figure 1 explains the methodology to conduct inclusion characterization as a part of the projects studying the issues that affect either the quality of the steel or its end use properties. To initiate steel characterization, the obvious questions to answer are as follows: What is the purpose of characterizing inclusions, and which methods can provide faster and reliable results? This process includes steel sampling followed by the sample analysis, and combining the inclusion analysis results with modeling and previous experience to propose a solution. Sometimes, the entire process has to be repeated after conducting plant trials to confirm the success of the proposed solution. The inclusion characterization process, in contrast, can be divided into a series of smaller steps that identify the dependency of a problem with steel grades and inclusions, as well as the dependency of sampling with techniques and problems. This relationship can be expressed in multiple tabular formats, and it forms part of the discussion in the subsequent sections.
Common Inclusions in Various Steel Grades and Their Link to Industrial Problems
As a first step, it is necessary to have some knowledge about the chemistry of inclusions and their nature (i.e., problematic or not) in the steel grade to be studied. This information can be obtained from the literature survey and the expertise of peer researchers. In addition, as new information from the characterization of steel grades becomes available, the previous experience can be updated. Table I lists the inclusions commonly observed in different steel grades. It should be noted that stainless steel grades are not discussed in this article. For advanced high strength steels (AHSS) grades, more research is necessary to complete the information in Table I. It is worth mentioning that not all inclusions behave the same because of the differences in their physicochemical properties at the steelmaking temperatures.
In Table I, the inclusions are classified in families based on their chemistry. The problematic inclusions in various steel grades are marked as “X,” whereas those inclusions that may or may not pose a problem in a particular grade are shown as “Y.” For example, the presence of pure MgO.Al2O3 phase or Mg-spinels is often considered questionable for their influence on the production and properties of various steel grades. Similarly, alumina inclusions are considered detrimental in many grades including Ti-stabilized ultralow carbon (TiSULC) steels, low-carbon Al-killed (LCAK) steels, tire cord steels, etc.,[2,3,5,11,27] but their effect on the castability of high-Al transformation induced plasticity (TRIP) steels is still under study. It is important to mention that AlN are major inclusions in the Al-TRIP steel grades.[21]
The inclusions that were considered detrimental for regular drawing and ironing (D&I) grades are no longer considered problematic for ultralow aluminum (ULA) D&I grades, except for mold slag containing particles. Mold slag, in fact, is not considered desirable in most steel grades as it causes surface and internal defects in the flat and long products. Nitride inclusions in the high-alloyed steels and sulfide inclusions, specifically MnS, in plate grade steels are also unwanted because of their effects on the process and properties of steel. Therefore, a first indication of type of inclusions that could potentially be present (and harmful) can be obtained from Table I for the steel grade(s) of interest.
Table II lists the same inclusions shown in Table I but link their effect(s) with commonly observed industrial issues. For example, the flat-rolled sheet steels are known for their clogging tendency and product rejections because of sliver defects. The main reasons for these defects are the entrapment of mold powder particles and the deoxidation/reoxidation inclusions in the first solidified shell. The inclusions associated with Ca or Ti aggravate the clogging and declogging phenomena[5,11] causing flow instabilities in mold and thus favoring the sliver defects appearance. Therefore, the characterization should be carried out knowing that these inclusions will be present in the TiSULC steel grade samples.
Likewise, silica, alumina, Mg-spinel, and Mn-spinel inclusions are of importance in long products and would be of interest to the researchers studying these grades for a cleanliness assessment. For AHSS grades, large Ca-aluminate inclusions were considered detrimental,[5] but more research is needed on these grades in the future to distinguish clearly the inclusions of interest with other exogenous particles. The reoxidation of steel, as a problem by itself or as the origin of certain inclusions, is considered as a part of the steelmaking process, and therefore, it is omitted from subsequent discussion. Whereas the problems shown in Table II are not the only problems in the flat and long steel products related to their end use properties, they are the frequently observed issues in the quality control of these steel grades. In summary, Table II explains the need to focus on the inclusions that were found associated with the defects experienced in the past.
Compiling the information presented in Tables I and II, a separate table (Table III) is created that associates the common industrial problems encountered in different steel grades produced in flat and long product segments. The data in this table are recorded based on both the internal expertise within ArcelorMittal and the published literature, except for AHSS steel grades.
Steel Sampling and Characterization Techniques for Different Problems and Steel Grades
A good sample is the key to a successful inclusion characterization. The samples can be collected either from the cast semis or from the liquid steel. Previous work[40,41] indicated the use of liquid steel samples to study inclusions. As chemical samples are collected during a variable production environment, the differences among sampling techniques, sampling location, sampler type, and immersion depth could lead to results that could not be repeatable and comparable among heats or plants. As most integrated steel plants are equipped with automatic sampling poles, their use minimizes the chances of variability of the location and depth of sample collection in a steel reactor such as the tundish or ladle, including variability resulting from operator-assisted sampling. Moreover, moisture and slag protection covers of samplers can contaminate the steel sample contributing to erroneous results. Recently, some research has been conducted to study the impact of the sampler type on the results of the steel cleanliness determination techniques including total oxygen[42] and electrolytic extraction.[43]
Table IV lists the type of samples recommended to study a particular problem. The most appropriate location to collect the sample is also listed for each case. The most widely used samples to study the process problems are from the final product such as the coil and the corresponding chemical samples obtained from the tundish. The LUS samples (massive samples collected from the tundish for an examination of the cooling phenomena that would happen in slabs thereby avoiding slab cross sectioning; these samples are no longer manufactured) and the pin samples were used previously to conduct total oxygen (OT) or optical emission spectroscopy pulse discrimination analysis (OES-PDA); however, recently, dual thickness chemical samples are found to conduct a good OT analysis. Silica is recommended over glass as the sampler tube material for pin samplers as a result of the contamination of inclusions by Na2O. Although the research is currently ongoing to use LSHR (liquid steel, hot-rolled) samples[44] to conduct inclusion characterization, the results from slab-milled samples can provide only partial indication and without statistical representativity (Table IV). Equally important are ladle-slag samples and refractory samples, if needed, to make thermodynamic calculations to support and compare the inclusion analysis results from the steel samples. It should be noted that tundish or mold slag sampling requires attention to safety and to avoid unintentional slag entrapment in liquid steel.
A number of techniques can be used to characterize the same sample or the same steel grade, as shown in Tables V and VI. A short description of these techniques is provided in the literature.[1,3–5,52] Sometimes, a technique can be used on a certain sample type but is usually not recommended, as outlined in Table V, because of the difficulties associated either with the sample or with the technique such that the resulting information may not be sufficient to make a conclusion. The measurement of OT is one such method. As known, the OT method was an industry standard to explain the qualifications of heats for many years in the past and was applied for all steel grades (Table VI). It is still a good indicator to measure the general steel cleanliness of the heats, but it lacks the detailed characteristics that define inclusions. Therefore, observations from just the OT data cannot be entirely conclusive. However, when the OT data are used in combination with the inclusion analysis results from SEM, it can be a useful tool in explaining complex issues.[7]
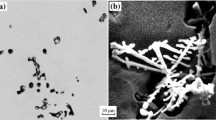
Recently, the inclusion analysis on steel samples is conducted using a combination of optical and electron microscopy (SEM) techniques. It is common to conduct an operator-assisted SEM examination prior to or after an automated SEM inclusion analysis, thereby acquiring information about the nature and composition of inclusions separately such that an automated SEM analysis does not mislead the overall analysis. Inclusion characterization using automated SEM can be conducted on the steel samples of any grade with certain minimum metallographic preparation. This process includes grinding followed by polishing of the samples using a 1 μm oil-based diamond suspension. Besides inclusion characterization on the chemical samples, a newer technique is to use OES-PDA that discriminates the insoluble elemental content from the soluble portion by the application of low-energy matter extraction from the steel matrix,[45,53] and it can be performed using industrial spectrometers that are also used to analyze steel chemistry. Lately portable OES is finding application in determination of defect chemistry on coil samples of TiSULC and D&I grades, as mentioned in Table VI.
The remelt button (RB) technique is the best to study inclusion morphology in three dimensions and its influence on the process parameters. The samples for the remelt buttons are good when obtained using thin pin or from the slab samples, as the chances of surface contamination or cavities are minimal. It is worth noting that because of the sample size in the remelt button technique (a few grams), it may not be suitable to study a plant issue that is related with a higher population of inclusions larger than 100 μm. The remelt button technique has been used extensively for castability and cleanliness studies in the past with using a cathodoluminescence (CLM) microscope,[46,47] but more research is required to use RB samples on D&I and free machining or tool steels.
MIDAS and other ultrasonic tests are designed mostly to work on the slab samples for inclusion determination and counting; however, it is said to be a tedious activity. The use of MIDAS for ultrasonic testing was conducted in the past[48]; however, because of inadequate experience on this technique in ArcelorMittal Group, no comments are made on this technique in Table V. A previously, and perhaps commonly, used electrolytic dissolution technique is a slow process and requires massive samples to account for inclusions of all sizes; hence, the massive liquid steel or slab samples are useful for this technique. Nevertheless, this technique is applicable to more steel grade families. According to the results from the IRSID-NSC collaboration on the steel cleanness assessment, the amount of steel in the LUS samples (less than 300 g) is too small to show the presence of larger inclusions in liquid steel. It was, therefore, recommended to use samples of several kilograms to obtain significant results when assessing the size of larger inclusions.[49]
The previously mentioned tests are mostly offline methods that are conducted after the completion of the production of a heat. However, in-line inspection methods to detect inclusions on a coil samples, including automatic surface inspection system, are gaining momentum besides other common methods such as the eddy current or lamb waves technique (mainly used for D&I grades). The advantage of these online methods is that extensive characterizations on a large section of steel surface can be made compared with the amount of steel that can be characterized using small samples, and thus, they are adapted particularly to evaluate macro inclusions.
The ultimate test of steel cleanliness is through mechanical tests on the product, such as the HIC test on plate samples, to ensure its lifelong application. Having the desired inclusions’ shape and size with their appropriate distribution in the product may not be helpful if the final product does not adhere to customer specifications. Therefore, the results of mechanical tests serve as a guideline for the characterization of appropriate samples of a heat to study the process problem. The list shown in Tables V and VI is not comprehensive, as there are methods such as laser techniques[50] for inclusion identification and online liquid steel inclusion measurement probes.[51] These methods are relatively new and are still in the process of development; therefore, they are not discussed in this study.
A Suite of Techniques for Cleanliness Characterization
As an outcome of several discussions after a literature survey, the authors agreed that the following techniques can be used for inclusion characterization on a routine basis.
Research Tools
To diagnose the plant problem as a research project, obtaining a complete chemical analysis of a steel sample including nitrogen and total oxygen is necessary. The inclusion analysis of the sample should be conducted using an automated SEM, and the results should be compared with thermodynamic estimations. CLM has been used extensively in the past at ArcelorMittal Global R&D to study the influence of the inclusion shapes on clogging,[7,47,54] and still a preferred method to study inclusion shapes as electrolytic etching of inclusions from a steel sample is a time-consuming activity. In addition to CLM, studies on the confocal laser microscope[55–57] have shown that it is a good tool to study inclusion shapes in situ and also is helpful in understanding their relative interaction in liquid steel.
In-Plant Use
The use of OES-PDA in plants is suggested as the primary investigative means for the evaluation of steel cleanliness on a regular basis, where possible. The slag composition after completion of the ladle treatment process (end of secondary metallurgy) can be used as an alternative technique. The actual slag composition and thermodynamic computations should be used in conjunction to understand the deviations in the process route from equilibrium. Although not all steel-producing plants may be equipped with spectrometers that could perform OES-PDA, the ladle slag compositions can serve as a good indicator if correlations with downstream process anomalies or product rejections are established.
In all studies, fast-cooling liquid steel samples are considered useful, and these samples should be collected using Ar-flushed sucking samplers. A killing agent in the samplers is not recommended. The slab samples, although recommended, are sometimes not useful because of the heterogeneous inclusion content and their location in the slab relative to the inclusion floatation zone in the caster, and therefore, they are helpful only to study macroinclusions in some specific studies. The massive samples collected from tundish are thus considered better to study the phenomena during cooling and solidification to represent the slab samples from the same heat.
Other techniques that can be used either as offline or on-site testing are listed in Table VII in order of preference. This table categorizes the techniques based on the characteristics of inclusions and is a summary of the information outlined in Tables I through VI. The tables listed in this article constitute a suite of techniques proposed by the authors as reference tools to pursue clean steel studies more effectively. This suite is also helpful in picking a technique quickly that is relevant to the steel grade and the process problem to be dealt. We recommend that a minimum of 30 heats from different cast sequences should be sampled and characterized to make statistical correlations of inclusion data with process variables.
Comparison of Results on Preferred Techniques Conducted Using Different Machines
From Table VII, it is clear that the automated SEM, PDA, and OT/RB are strong choices over other techniques as both research and plant tools for inclusion characterization. Therefore, it is worth investigating whether these techniques can produce repeatable, comparable, and reliable results. Although OT analysis can be made on standard samples to test the preceding criteria, standard samples are not available to conduct a round robin analysis for PDA and, more importantly, automated SEM analysis. This section, therefore, covers some issues related to the comparison of the results of these techniques on industrial samples.
Automated SEM Analysis
The results of inclusion characterization using two automated SEM machines from different suppliers (SEM A and SEM B) on two TiSULC heats are discussed in this article. The predefined quality event for sample selection was the castability of the heats—one that showed good castability (heat X) and the other that showed strong clogging (heat Y). Additionally, these two heats showed a clear distinction in terms of the average composition and the relative scatter of the inclusion chemistry. The tundish samples of these heats were reanalyzed using two different automated SEMs to compare the inclusion analysis results. A close (and similar) sample area was examined in this exercise.
From the examination of inclusion analysis data, it was found that heat X contained mostly Al-Mg-O inclusions and they were detected by both SEMs. In heat Y, the number density of the Al-Mg-O inclusions provided by either machine was close; however, their relative fraction was much higher in the inclusion analysis obtained from SEM B. This was because more Al2O3-TiO x -type inclusions were detected by SEM A.
Although a slight mismatch occurred in the number density of inclusions from the two SEMs, a better way to compare the inclusion analysis is to examine the average chemistry of all the inclusions that are selected and plotted on a particular ternary diagram. The composition of inclusions in heat X and heat Y are plotted on the ternary diagrams in Figures 2 and 3, respectively. The average composition of inclusions is indicated by the red points for the data from SEM A and the blue points for the data from SEM B. All the inclusions in Figures 2 and 3 are greater than 1 μm in size.
Comparison of inclusion chemistries in heat X (good castability) on two different ternary diagrams; all inclusions were >1 μm. The average chemistry of all inclusions is shown by a red point in (a) and (c) and a blue point in (b) and (d); (a) and (c) for heat X analyzed with SEM A and (b) and (d) for heat X analyzed with SEM B. (Color figure online)
Comparison of inclusion chemistries in heat Y (poor castability) on two different ternary diagrams; all inclusions were >1 μm. The average chemistry of all inclusions is shown by a red point in (a) and (c) and a blue point in (b) and (d); (a) and (c) for heat Y analyzed with SEM A and (b) and (d) for heat Y analyzed with SEM B. (Color figure online)
A comparison of the inclusion chemistry found in heat X analyzed using SEM A and SEM B, i.e., comparing Figures 2(a) with (b) and comparing Figures 2(c) with (d), it seems that the inclusion compositions are close from either analysis, and the average chemistry of inclusions is moderately comparable. Some differences are noted such as the detection of more inclusions containing Al2O3-TiO x from SEM A, as mentioned previously. Because of the presence of these inclusions, the average chemistry of inclusions computed and plotted on the MgO-TiO2-Al2O3 diagram reports a higher fraction of TiO2. A similar analysis can be made for heat Y by comparing the diagrams in Figure 3(a) with Figure 3(b).
The poor castability of heat Y cannot be explained alone by inspecting the MgO-TiO2-Al2O3 diagrams for heats X and Y shown in Figures 2(a) and (b) and Figures 3(a) and (b). Although heat X had spinel-TiO x -type inclusions, heat Y contained Al2O3-MgO inclusions with small fraction of TiO2, as shown in Figures 2(a) and (b) as well as Figures 3(a) and (b). These results do not help explain the difference in the castability behavior of heat X and heat Y. In contrast, CaO-Al2O3-MgO diagrams for these heats shown in Figures 2(c) and (d) as well as Figures 3(c) and (d) explain that many Ca-Mg aluminates are present in heat Y, whereas heat X contains a large population of spinels inclusions (MgO. Al2O3) and calcium-free Mg-aluminate inclusions. Although solid Ca aluminate inclusions were reported to accelerate nozzle clogging during casting operations,[13] the influence of spinel inclusions and spinel-TiO2 on castability is not clear because they do not agglomerate as much as alumina or solid calcium aluminate inclusions.[55,56] The presence of a higher fraction of solid Ca aluminate inclusions could be one reason why heat Y showed poor castability compared with heat X.
A comparison of inclusion analysis data from two SEMs in this study indicated that quantitative inclusion indices, e.g., number density, were dissimilar and variable. This finding is attributed to the differences in the analytical methods of two SEMs and the subsequent classification of energy-dispersive X-ray spectroscopy (EDX) data into inclusion categories. Moreover, the EDX chemistry of the individual inclusions analyzed by two machines shows a slight mismatch, particularly for inclusions smaller than 2 μm, and this difference is attributed to the specifics of each SEM. A more reliable way to compare the characterization results is to compare a general trend of the inclusion chemistries plotted on various ternary diagrams and to examine the corresponding average inclusion composition. It should be noted that the exact match between the average inclusion chemistry may not be achieved if some components of the ternary diagrams tend to be the elements that have overlapping EDX spectrum peaks or differences in sample polishing leading to poor spectrum quality or noise. However, for the two TiSULC heats studied in this article, the individual inclusion analysis results from either machine suggested that heat Y had Ca-Mg aluminate inclusions that affected its castability.
Pulse Discrimination Analysis
The TiSULC steel and Ca-treated medium carbon Al-killed (MCAK) steel grades were selected to conduct a round robin PDA analysis. Some selected tundish samples were analyzed using two spectrometers, C and D (from different suppliers). Spectrometer C has been used in the past to compare a PDA analysis with SEM and, therefore, serve as reference for all PDA work at ArcelorMittal Global R&D. The samples were analyzed first by the spectrometer D followed by their analysis on the spectrometer C for the round robin purpose. Before repeating the PDA technique using spectrometer C, the samples were milled to obtain a solid cross section free of contamination and spark marks.
The data from the PDA were used to compute the glob index (GI) using a computer algorithm. The details about the GI can be obtained from a previous publication[40]; it refers to the average intensity ratio of CaO/Al2O3 in oxide inclusions over certain threshold intensity values, which were detected by spectrometers in the low-energy spark mode. The GI values measured on the TiSULC and MCAK samples using the two spectrometers are plotted in Figure 4. As observed, the GI values follow a linear trend and are smaller from the measurements made using spectrometer D. As spectrometer C serves as a reference, the charts similar to Figure 4 are necessary for all spectrometers of interest using industrial samples such that the spectrometer in-test can provide GI or other PDA indices comparable with that provided by spectrometer C. It is worth noting that the GI can be used to diagnose steelmaking and casting issues. Recent developments on the determination of inclusions using PDA are promising,[53] and therefore, this tool shows a potential to become a daily monitoring quality-control technique in the coming years.
Total Oxygen Analysis
The OT was measured on the selected standard samples for the round robin study. Five standard samples in the range of 8 to 50 ppm were chosen and analyzed using a LECO combustion analyzer (LECO Corporation, St. Joseph Charter Township, MI). The samples were analyzed with three LECO machines viz. E, F, and G located at three different ArcelorMittal locations. The results of the OT analyses are shown in Figure 5. As shown, all the machines provide reproducible results within the marginal error of 1 ppm, which increases as the aim oxygen value in standard samples increases. Noticeably, the OT analyses from all three LECO measurements are slightly higher than the aim value of 29 ppm in one of the standard samples (sample 1092). It may be either because of the contamination within the sample or possibly because of an incorrect aim value. These results indicate that the OT analysis on standard samples is reasonably repeatable and comparable when measured with different machines. However, for industrial samples, variations of OT could exist within the same sample,[42] and therefore, the choice of the sampler and sample preparation techniques need consistency before the OT results can be compared and correlated to the process conditions of industrial heats.
Example Studies Using Automated SEM and OES-PDA
Although recent publications[6–9,16,34,38] report the use of automated SEM to study steelmaking process problems, this article presents some specific examples of the issues pertaining to the use of different ferroalloys in the steelmaking processes, and the application of an automated SEM and PDA to understand them better. The residuals in certain ferroalloys can produce inclusions in steel that can affect the castability of steel or produce surface defects in the finished steel products. One such issue occurred when the FeTi (~70 pct Ti) alloy was replaced with the one containing 33 pct Ti. The FeTi 70 pct is produced by melting Ti scrap whereas FeTi 33 pct is a product of the thermite process. The switching of FeTi 70 pct with FeTi 33 pct occurred as a result of the lower cost of the latter compared with the former alloy.
A detailed analysis of the sliver data on the finished coils of the TiSULC grades showed the relationship of the rejections with the source of FeTi, which is plotted in Figure 6. From a physical examination, it seemed that the slivers were inclusion-related defects. Consequently, a study was conducted in which the tundish samples were picked from the heats that were produced using the two different sources of FeTi. These samples were analyzed for inclusions using automated SEM A. The third heats in different cast sequences were chosen to eliminate the effects of tundish refractory contamination during the initial tundish filling and turbulence, steel reoxidation, and nozzle exchange.
Figure 7 plots the area fraction of Al2O3 and Al2O3-TiO x (AlTi) inclusions in the heats alloyed using FeTi 70 pct and FeTi 33 pct at two different plants P1 and P2. As seen, the area fraction of alumina inclusions is similar when either source of FeTi was used in both plants. Besides as higher alumina inclusion content was observed in the samples from plant P2, it did not correspond to a higher rejection index at plant P1 compared with plant P2 (Figure 6). However, a remarkable difference was observed in the area fraction of AlTi inclusions larger than 10 μm among the heats alloyed with FeTi 70 pct and FeTi 33 pct. This difference was higher for the AlTi inclusions in the heats produced in plant P1 than in plant P2. The relative change in the area fraction of larger AlTi inclusions between the plants matched the ratio of the effect of switching the type of FeTi on the slivers.
With extensive sampling of the FeTi 70 pct and FeTi 33 pct alloys and their SEM examination, it was found that the FeTi 33 pct used in plant P1 contained alumina particles as large as 20 μm in the alloy matrix, which were absent in the FeTi 70 pct used in the same plant and in both types of the FeTi alloys used at plant P2. Additionally, the total oxygen and Al measured in the FeTi 33 pct alloy used in plant P1 was approximately 1.2 pct and 3 pct, respectively, whereas in the FeTi 70 pct, there was 0.15 pct total O and 0.8 pct Al. Therefore, one reason for the higher sliver index at plant P1 was attributed to the compromised quality of FeTi 33 pct. This example corresponds to the findings of Dippenaar,[58] who suggested that the inclusions in ferroalloys can alter the outcome of the steel refining and cleanliness control practices significantly, and these should be considered in the selection and use of ferroalloys.
In another example, the inclusion cleanliness assessment was made on two different interstitial-free (IF) grades (standard IF and rephosphorized IF). The main difference between the two processes is that in rephosphorized IF heats, FeP alloys are added to trim the steel chemistry after steel deoxidation. In this case, the tundish samples were analyzed for inclusions using OES-PDA on the spectrometer C. The observations of this study are reported in Figure 8. The fraction of the Ca-containing inclusions (Al-Ca and Al-Ca-Mg) was found to be higher in the rephosphorized IF heats than in the standard IF heats. These results were confirmed later by a manual SEM analysis. The chemical analysis of the FeP revealed presence of Ca, suggesting that Ca from FeP reacted with alumina inclusions in steel to form solid Ca aluminates thus affecting the castability of the rephosphorized IF heats.
Another example where OES-PDA became useful was the quick detection of the difference in the inclusion population depending on the type of FeSi alloy used to trim the Si content of steel. A higher fraction of Ca-aluminate inclusions (Al-Ca) was observed in the heats made using FeSi N (Figure 9). In fact, whereas FeSi N contained about 0.35 pct Ca, FeSi M only had 0.03 pct Ca. In this case, the direct influence on the castability was established also (Figure 9).
The GI was also computed from the PDA data for the heats of interest. In the heats alloyed with FeSi M, the value of GI was close to zero, implying that the inclusions were almost pure alumina. In the heats treated using FeSi N, the calculated value of GI was approximately 1.9. This value corresponds to the domain of solid Ca-aluminates[59] and is dependent on the spectrometer. Thus GI values supported the theory that the heats with FeSi N had solid Ca-aluminates and affected the castability of the heats. The effect of residuals in FeSi and FeP on clogging tendency of heats, as found in this study, is in agreement with the previous work of Story.[8,38]
In the future, the questions to answer are as follows: How much inclusions (volume or amount), of what size, and which nature are acceptable, particularly in AHSS grades as the customer requirements become more stringent in coming years? Should more research be invested in developing online inclusion analyzers that can provide results in few minutes such that the problem can be corrected before the heat is sent for casting? How can thermodynamic models help in assessing the success of trials if implemented in an online process? The quest for the answers to these questions continues.
Summary and Conclusions
Faster and reliable offline methods combined with online techniques are necessary to evaluate completely the steelmaking and casting process issues. Appropriate sampling during plant trials followed by a sample analysis and previous experience with modeling helps in proposing a solution to a particular process problem. The choice of the sample and technique is dependent on the steel grade and the problem to be studied, and it can be identified using one of the tables reported in this article. Regardless, a complete steel chemical analysis including total oxygen, automated SEM, and OES-PDA, along with thermodynamic computations, is often used to the study steelmaking and casting issues at ArcelorMittal Global R&D. Round robin analyses of the steel samples with the previously mentioned techniques resulted in repeatable and comparable results. The results from the use of an automated SEM and a PDA showed that solid calcium aluminate inclusions are formed in liquid steel when ferroalloys containing higher amounts of Ca (as residual) are charged into a steel ladle that leads to poor castability of the heat.
Abbreviations
- A B:
-
automated SEM machines used in round robin study to compare inclusion indices
- C D:
-
spectrometers used in round robin study to measure the glob index (GI)
- E F G:
-
LECO combustion analyzers used in round robin study to measure total oxygen (OT)
- P1 P2:
-
plants that showed different sliver performance with use of FeTi 70 pct and FeTi 30 pct
- M N:
-
FeSi alloys studied for their impact on castability
- X Y:
-
two TiSULC heats with clear differences in inclusion composition and clogging behavior
References
C. Louis, P. Gauje, F. Ruby-Meyer, P. Osmont, P. Dunand, M. Nadif, M. Burty, and J.-M. Jolivet: “Inclusion Cleanliness Characterization Strategy,” ArcelorMittal Internal Report, no. PCMO/01/R/005, Metz, France, 2000, pp. 1–27.
L. Zhang and B.G. Thomas: Met. Trans. B, 2003, vol. 43, no. 3, pp. 271–91.
L.D. Way: Mater. Sci. Technol., 2001, vol. 17, pp. 1175–90.
H. Jacobi and K. Wunnenberg: IISI Study on Clean Steel: State of the Art and Process Technology in Clean Steelmaking, International Iron and Steel Institute, Brussels, Belgium, 2004, pp. 287–334.
P. Kaushik, H. Pielet, and H. Yin: Iron Steel Tech., 2009, vol. 6, no. 11, pp. 82–99.
P. Kaushik, H. Pielet, and H. Yin: Ironmaking Steelmaking, 2009, vol. 36, no. 8, pp. 561–71.
P. Kaushik, H. Yin, H. Pielet, and M. Lowry: Iron Steel Tech., 2011, vol. 8, no. 10, pp. 81–98.
S.R. Story, S.M. Smith, R.J. Fruehan, G.S. Casuccio, M.S. Potter, and T.L. Lersch: AISTech Conf. Proc., Nashville, TN, 2004, pp. 1147–56.
S.R. Story, G.E. Goldsmith, and G.E. Klepzig: Proc. of 7th Int. Conf. on Clean Steel, Balatonfured, Hungary, 2007.
L. Zhang, B. Thomas, K. Cai, J. Cui, and L. Zhu: ISSTech Conf. Proc., Indianapolis, IN, 2003, pp. 141–56.
M. Burty, L. Peeters, E. Perrin, S. Munzer, P. Colucci, D. Salvadori, F. Schadow, J.-M. Valcarcel, and J. Claes: 5th European Continuous Casting Conf. (ECCC) Proc., Nice, France, 2005, pp. 462–67.
I.-H. Jung, M.-K., Sun, and H.-G. Lee: Proc. of 4th International Congress of Science and Technology of Steelmaking (ICS), Gifu, Japan, 2008, pp. 420–23.
G.N. Faulring, J.W. Farrell, and D.C. Hilty: ISS EAF Conf Proc., Detroit, MI, 1979, pp. 219–29.
D. Janke, Z. Ma, P. Valentin, and A. Heinen: Proc. of Japan-Germany Seminar on the Fundamentals of Iron and Steelmaking, Tokyo, Japan, 1999, pp. 1–10.
M. Burty, L. Peeters, R. Fabien, and P. Osmont: Baoshang Annual Conf. Proc., Shanghai, China, 2006 (CD-ROM).
E.B. Pretorius, H.G. Oltmann, and T. Cash: AISTech Conf. Proc., Indianapolis, IN, 2009, pp. 1035–49.
S. Yang, L. Zhang, J. Li, L. Sun, K. Peaslee, and Y. Chen: AISTech Conf. Proc., Indianapolis, IN, 2011, pp. 591–606.
M. Thunman and D. Sichen: Steel Res. Int., 2008, vol. 79, no. 2, pp. 124–31.
J.H. Park and H. Todoroki: ISIJ Int., 2010, vol. 50, no. 10, pp. 1333–46.
H. Zheng, W. Chen, and Y. Hu: AISTech Conf. Proc., Nashville, TN, 2004, pp. 937–45.
H. Yin: Proc. AISTech, vol. 2, Charlotte, NC, 2005, pp. 89–97.
C. Garlick, P. Whitehouse, and M. Powell: ISS Ironmaking Conf. Proc., 2001, pp. 1089–1107.
T. Usui, K. Yamada, Y. Miyashita, H. Tanabe, M. Hanmyo, and K. Taguchi: Proc. of Scaninject II, part I, 1980, pp. 12:1–24.
A. Dash, P. Kaushik, and E. Mantel: Iron Steel Tech., 2012, vol. 9, no. 2, pp. 186–201.
C. Marique and K. Wunnenberg: IISI Study on Clean Steel: State of the Art and Process Technology in Clean Steelmaking, International Iron and Steel Institute, Brussels, Belgium, 2004, pp. 443–54.
A. Hamoen and W. Tiekink: ISS Steelmaking Conf. Proc., 1998, pp. 229–34.
L. Zhang, Y. Wang, D. Zhou, X. Hu, and K. Quian: Proc. of Materials Science and Technology, New Orleans, LA, 2004, pp. 703–14.
K. Steneholm, M. Andersson, M. Nzotta, and P. Jonsson: Steel Res. Int., 2007, vol. 78, no. 7, pp. 522–30.
J.-H. Qi, Z.-L. Xue, J. Wu, C.-G. Cheng, and Y.-M. Gao: J. Iron Steel Res., 2010, vol. 17, no. 7, pp. 63–67.
S. Ogibayashi, K. Yamaguchi, M. Hirai, H. Goto, H. Yamaguchi, and K. Tanaka: Proc. of the 6th International Iron and Steel Congress, Nagoya, Japan, 1990.
S. Liu, M. Liang, S. Niu, C. Li, L. Zhang, and X. Wang: AISTech Conf. Proc., Cleveland, OH, 2006, pp. 915–27.
S. Basu, S.K. Choudhary, and N.U. Girase: ISIJ Int., 2004, vol. 44, no. 10, pp. 1653–60.
R. Dekkers, N. Jokanovic, A. Rombout, B. Blanpain, and P. Wollants: Steel Res. Int., 2005, vol. 76, no. 7, pp. 475–80.
S.R. Story, N. Gupta, G. Casuccio, and M. Potter: AISTech Conf. Proc., vol. 2, Pittsburgh, PA, 2010, pp. 1–10.
H. Yamamura, Y. Ueshima, and T. Matsumiya: Proc. of SCANMET–II, Lulea, Sweden, 2004, pp. 365–75.
P. Kaushik, D. Kruse, and M. Ozgu: Rev. Metall., 2008, no. 2, pp. 92–101.
K. Wunnenberg and H.-J. Weddige: IISI Study on Clean Steel: State of the Art and Process Technology in Clean Steelmaking, International Iron and Steel Institute, Brussels, Belgium, 2004, pp. 455–62.
S.R. Story, G.E. Goldsmith, R.J. Fruehan, G.S. Casuccio, M.S. Potter, and D.M. Williams: AISTech Conf. Proc., Cleveland, OH, 2006, pp. 879–89.
S. Millman and K.-Y. Lee: IISI Study on Clean Steel: State of the Art and Process Technology in Clean Steelmaking, International Iron and Steel Institute, Brussels, Belgium, 2004, pp. 407–42.
F. Ruby-Meyer, J. Lehmann, R. Meilland, A. Nicelli, and E. Perrin: Proc. of Liquid Metal Processing Conference (LMPC), Nancy, France, 2003 (CD-ROM).
T. Hansen, G. Runnsjo, H. Hagglund, and P. Jonsson: 7th Clean Steel Conf. Proc, Balatonfured, Hungary, 2007 (CD-ROM).
O.T. Ericsson, A.V. Karasev, and P.G. Jonsson: Steel Res. Int., 2011, vol. 82, no. 3, pp. 222–29.
O.T. Ericsson, M. Lionet, A.V. Karasev, R. Inoue, and P.G. Jonsson: Ironmaking Steelmaking, 2012, vol. 39, no. 1, pp. 67–75.
T. Hansen, P. Jonsson, S.-E. Lundberg, and K. Torresvoll: Steel Res. Int., 2006, vol. 77, no. 3, pp. 177–85.
R. Meilland and L. Dosdat: Rev. Metall., 2002, no. 4, pp. 373–82.
H. Yin and H.T. Tsai: ISS Tech Conf. Proc., Indianapolis, IN, 2003, pp. 217–26.
P. Kaushik, H. Pielet, and H. Yin: Ironmaking Steelmaking, 2009, vol. 36, no. 8, pp. 572–82.
H. Yin and H.T. Tsai: AISTech Conf. Proc., Nashville, TN, 2004, pp. 1137–46.
M. Burty, C. Louis, P. Dunand, P. Osmont, M. Nadif, T. Isono, E. Takeuchi, and T. Toh: Rev. Metall., 2000, pp. 775–82.
A.V. Karasev, R. Inoue, and H. Suito: CAMP-ISIJ, 2008, vol. 21, p. 157.
R.P. Stone, C.C. Liu, and P.C. Glaws: 7th Clean Steel Conf. Proc., Balatonfured, Hungary, 2007 (CD-ROM).
H. Kondo: Tetsu-to-Hagané, 2004, vol. 90, no. 10, pp. 8–15.
J.-M. Bohlen, K. Li, J.-L., Dorier, and E. Halasz: 8th Int. Workshop on Progress in Analytical Chemistry and Materials Characterization in the Steel and Metal Industries, Luxemburg, 2011, pp. 101–17.
H.M. Pielet, H.T. Tsai, and R.T. Gass: ISSTech Conf. Proc., Indianapolis, IN, 2003, pp. 243–55.
M. Guo, B. Blanpain, and P. Wollants: CAMP-ISIJ, 2011, vol. 24, pp. 480–83.
Y. Kang, B. Sahebkar, P.R. Scheller, K. Morita, and D. Sichen: Metall. Mater. Trans. B, 2011, vol. 42, no. 3, pp. 522–34.
H. Yin, H. Shibata, T. Emi, and M. Suzuki: ISIJ Int., 1997, vol. 37, no. 10, pp. 946–55.
R.J. Dippenaar: Proc. of the Tenth International Ferroalloys Congress, Cape Town, South Africa, 2004, pp. 741–56.
F. Ruby-Meyer: “Influence of Process Parameter Modifications on Inclusion Cleanliness: Overview of Results Obtained by PDA-OES Assessment,” ArcelorMittal Internal Report, no. PE/04/R/041, Metz, France, 2004, pp. 1–17.
Acknowledgments
The authors acknowledge the help of P. Osmont, B. Barbi, J.-L. Gandar, S. Jacques, S. Schuldt, N. Jin, L. Keippel, D. Funk, K. Abretske, L. Laus, and J. Thacker of ArcelorMittal R&D for the analytical work performed on the steel samples for the round robin study. They express their appreciation to H. Pielet (project leader), H. Yin, D. Kruse, and M. Lowry for their valuable comments on this research. They also thank the technical staff of R.J. Lee Group, Pittsburgh, PA, for comments about comparative study on SEMs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript Submitted August 5, 2011.
Rights and permissions
About this article
Cite this article
Kaushik, P., Lehmann, J. & Nadif, M. State of the Art in Control of Inclusions, Their Characterization, and Future Requirements. Metall Mater Trans B 43, 710–725 (2012). https://doi.org/10.1007/s11663-012-9646-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9646-2