Abstract
Ferrous calcium silicate slags, whose principal components are “FeO x ”-CaO-SiO2, are widely used in copper smelting and converting operations. In the current study, high-temperature equilibration and rapid quenching techniques were used to study the phase equilibria of the ferrous calcium silicate slags. The compositions of phases in the slags were measured accurately using electron probe X-ray microanalysis (EPMA). The phase equilibria of the system have been characterized at oxygen partial pressures between 10−5 atm and 10−7 atm at selected temperatures between 1473 K and 1623 K (1200 °C and 1350 °C). The effects of oxygen partial pressure and temperature on the compositions of phases in the slags are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most primary copper sulfide smelting, copper converting, and direct-to-copper smelting processes operate with silicate-based slags.[1,2] Despite the widespread use of silicate-based slags in copper-making processes, as well as in other nonferrous smelting, certain aspects of the chemistries and phase equilibria of the systems remain uncertain. Information on the chemistries and phase equilibria of the systems is needed for improvements of the current operations and the development of new processes.
The principal components present in these silicate-based slags are “FeO x ”-CaO-SiO2. A review of the literature shows that several studies are available on phase equilibria of “FeO”-CaO-SiO2 system that have been carried out at conditions relevant to copper production. Shigaki et al.[3] investigated the phase diagram of the “FeO”-CaO-SiO2 system at 1573 K (1300 °C) and an oxygen partial pressure of 10−7 atm. The equilibration of 1 g of sample in Pt foil crucible was carried out at the target temperature for 3 hours. After the equilibration, the sample was quenched into water and the electron probe X-ray microanalysis (EPMA) technique was used to analyze the composition of phases in the sample.
Kimura et al.[4] studied the effect of oxygen partial pressure (between 10−2.68 and 10−7.75 atm) on the liquidus of the “FeO”-CaO-SiO2 system at 1573 K (1300 °C). A platinum crucible was used to contain 8 g of sample. The equilibrated samples were cooled under an argon gas flow. The fully liquid slag in the sample was separated from the solid phases and was analyzed by using a bulk wet chemical analysis technique.
Henao et al.[5] investigated phase equilibria of the “FeO”-CaO-SiO2 system at oxygen partial pressures between 10−3.4 atm and 10−9 atm at a fixed temperature of 1573 K (1300 °C). Henao et al.[5] also investigated the system at a constant oxygen partial pressure of 10−6 atm at temperatures between 1623 K and 1673 K (1350 °C and 1400 °C). Equilibrations of samples were carried out in Pt foil crucibles for 24 hours. An argon gas stream was used to cool the equilibrated samples. The compositions of liquid and solid phases formed in the sample were determined using EPMA.
Nikolic et al.[6] carried out phase equilibria studies on the “FeO”-CaO-SiO2 system at 1473 K and 1573 K (1200 °C and 1300 °C) at an oxygen partial pressure of 10−6 atm, and at 1523 K (1250 °C) at an oxygen partial pressure of 10−5 atm. Equilibration of the samples in this study was carried out in Pt foil envelope for 24 hours followed by quenching of the samples into water. The compositions of the liquid and solid phases were determined by using EPMA.
A critical assessment of the experimental techniques commonly used in the high temperature equilibration of ferrous calcium silicate slag was carried out by Nikolic et al.[7] to identify possible sources of the differences in liquidus measurements reported by previous studies. It was shown that with careful attention to factors, such as, oxygen partial pressure, temperature, technique of measurement, sample preparation and sample quenching method, accurate measurements of phase equilibria of these slag systems can be obtained.[7] The approach has been used successfully in the investigation of various complex ferrous calcium silicate slags, for example, “FeO”-CaO-SiO2-“Cu2O”,[8] “FeO”-CaO-SiO2-Al2O3-MgO,[9] and “FeO”-CaO-SiO2-“Cu2O”-Al2O3-MgO.[10]
In the current study, the equilibration/quenching/EPMA techniques have been applied to determine accurately the phase equilibria of ferrous calcium silicate slags at intermediate oxygen partial pressures. Following the study by the previous authors,[6,7] the oxygen partial pressure range has been extended from 10−5 atm to 10−7 atm between temperatures of 1473 K and 1623 K (1200 °C and 1350 °C). The conditions selected are of industrial interest and fill gaps in fundamental information required to characterize and describe copper slag chemistries.
Experimental Techniques
Preparation of Oxide Mixtures and Containment Crucible
The high purity oxides and metals used as starting materials for the experiments were CaO powder (calcined at 1173 K (900 °C) from 99.0 wt pct pure CaCO3 powder), SiO2 (99.99 wt pct pure 1 to 3 mm fused lump that had been ground with an agate mortar and pestle), Fe2O3 powder (99.99 wt pct pure), and Fe powder (99.9 wt pct pure). Mixtures of various compositions were prepared by weighing the high-purity powders and mixing them thoroughly using an agate mortar and pestle. The initial compositions of the mixtures were selected such that at equilibrium there would be liquid phase in equilibrium with one or more solid phases (Figure 1). Each mixture was then compacted with pressure of 40 MPa to produce a pellet weighing less than 0.2 g.
To avoid contamination of the slag by crucible material, 10 mm × 12 mm envelopes made from 0.025-mm-thick platinum foil were used as sample containers. It was found that under the conditions investigated, only a small amount of iron was dissolved in the platinum. Although this changes the bulk composition of the mixture slightly (point b, in Figure 1), the compositions of phases at equilibrium (compositions of liquid phase (point a) and solid phase (point c) in Figure 1) do not change.
High-Temperature Equilibration Technique
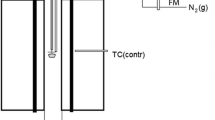
Equilibration experiments were carried out using a vertical tube furnace with design shown in Figure 2. The specimen was introduced from the bottom of the vertical tube furnace and suspended by a sample holder made of Pt wire. The 30-mm inside diameter recrystallized alumina reaction tube was preconditioned at the target temperature and oxygen partial pressure for more than 5 minutes, and the specimen was then raised into the uniform temperature hot zone of the furnace; premelting of the sample was carried out by increasing the temperature 25 K above the target temperature. After 30 minutes of premelting, the temperature of the furnace was decreased back to the target temperature and the sample was equilibrated inside the furnace for 24 hours. After the equilibration, the base of the furnace was immersed in iced water, the bottom end of the furnace was released, and the specimen was rapidly quenched by dropping it directly into the iced water. The quenched sample was dried on a hot plate, crushed into smaller pieces, and mounted in epoxy resin. It was then polished using conventional metallographic polishing techniques and carbon coated for subsequent electron probe EPMA examination.
Control of Temperature and Oxygen Partial Pressure
To monitor the actual temperature surrounding the sample, a working thermocouple was placed in a recrystallized alumina thermocouple sheath immediately adjacent to the sample. The working thermocouple was calibrated against a standard thermocouple (supplied by the National Measurement Institute of Australia, NSW, Australia). The temperature of the experiment was controlled continuously within ±1 K of the target temperature. It is estimated that the overall absolute temperature accuracy of the experiment is ±3 K.
The oxygen partial pressure inside the closed-system furnace was controlled by introducing a stream of gas having a specific CO/CO2 ratio. In this study, equilibrations were carried out at oxygen partial pressures between 10−5 atm and 10−7 atm. These oxygen partial pressures require low CO/CO2 ratios, thus premixed 5 pct CO diluted in high purity Argon gas (Beta standards with ±0.02 pct uncertainty; supplied by BOC, QLD, Australia) and high purity CO2 (99.995 pct pure; supplied by Coregas, NSW, Australia) were used to achieve the target CO/CO2 ratios. The proportion of each gas was controlled using U-tube pressure differential type flowmeter. The total flow-rate of the gas inside the reaction tube was between 400 and 900 mL/min, and the fluctuation of the gas flow rate was found to be less than 1 pct of the total flow rate.
An oxygen probe made of Y2O3-stabilized ZrO2 solid electrolyte cell (SIRO2, DS-type oxygen probe; supplied by Australian Oxytrol Systems, Victoria, Australia) was used to confirm the oxygen partial pressure of the experiment. This was done by directing the output gas from the equilibration furnace into a separate vertical tube furnace equipped with the oxygen probe. The equilibration furnace and the oxygen probe furnace were set at exactly the same temperature. By this arrangement, direct monitoring of the oxygen partial pressure during the equilibration was possible. Results of DS-type oxygen probe measurements using air as a reference for selected experiments, given in Table I, are within the accuracy of the DS-type oxygen probe, i.e., ±0.1 Log \( {\text{P}}_{{{\text{O}}_{2} }} \) units.[11]
Analysis Technique and Representation of Systems
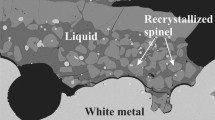
Backscattered scanning electron microscope (SEM) micrographs of typical quenched liquid slags in equilibrium with various solid phases are shown in Figure 3. At least five solid phases are found in the slags at the conditions investigated in the current study. The solid phases that have been identified are spinel, tridymite, dicalcium silicate, rankinite, and calcium metasilicate. The calcium metasilicate solid itself can be found in two different crystal structures, i.e., wollastonite and pseudowollastonite. Details on the identification technique of the two polymorphs will be delivered in the section about solid solutions in the system.
The rapid quenching technique retains the liquid slag successfully as a homogenous glassy phase. The compositions of various phases were measured using JEOL 8200L EPMA with wavelength dispersive detectors (JEOL is a trademark of Japan Electron Optics Ltd., Tokyo). Both 15-kV accelerating voltage and 15-nA probe current were selected for the microanalyzer operation. The Fe2O3 and CaSiO3 standards (from the Charles M. Taylor Co., Stanford, CA) were used for calibrations. The Duncumb–Philibert correction based on atomic number, absorption, and fluorescence (ZAF correction, supplied by JEOL) was applied. The accuracy of the EPMA measurements was reported to be within 1 wt pct.[12]
The EPMA analysis provides the concentrations of cations in the sample but does not distinguish between the different oxidation states of a cation. Both FeO and Fe2O3 are present in the ferrous calcium silicate slags. For the purpose of presentation, all iron oxide concentrations from the EPMA analysis were recalculated to FeO. The compositions were then normalized to 100 pct, thus projecting the compositions in the CaO-FeO-Fe2O3-SiO2 tetrahedron onto the FeO-CaO-SiO2 ternary plane as shown in Figure 4.
Compositional space in the Ca-Fe-Si-O system and projection onto the FeO-CaO-SiO2 plane.[6]
Assessment of Achievement of Equilibrium
Achievement of equilibrium was paid particular attention by adopting the following measures: (1) variation of equilibration time, (2) approach of equilibria from different directions, and (3) confirmation of homogeneity of phases by EPMA. Several dedicated sets of experiments were carried out for these purposes.
Table II summarizes the compositions of the liquid phase from the experiments. In the first set of experiments, the liquidus point at double saturation of spinel and tridymite at 1573 K (1300 °C) and \( {\text{P}}_{{{\text{O}}_{2} }} \) = 10−5 atm was approached from two directions, i.e., from a more reducing condition (mixture B81 made of Fe powder) and from a more oxidizing condition (mixture B82 made of Fe2O3 powder). It can be observed that both mixtures after 24 hours equilibration reached the same final liquid slag composition. The attainment of the same liquid slag composition from two different directions indicates that final equilibrium among gas, liquid, and solid phases was obtained at this condition.
Another series of experiments was undertaken to confirm the achievement of equilibrium between slag and gas at 1473 K (1200 °C) and \( {\text{P}}_{{{\text{O}}_{2} }} \) = 10−7 atm. Four different mixtures were used to approach the liquidus point at double saturation of spinel and tridymite. Three mixtures (B50, B56, and B57) approached equilibrium from the spinel primary phase field, and one mixture (B51) approached from the tridymite primary phase field. Mixtures B56 and B57 had the same proportions of Fe:CaO:SiO2; however, the B56 mixture was made using Fe powder, whereas the B57 mixture was made using Fe2O3 powder. Mixtures B50 and B51 used equimolar Fe and Fe2O3 powders. The four mixtures were equilibrated for 24 hours; the compositions of the resulting liquid slags are provided in Table II and are indicated as points B50’, B51’, B56’, and B57’ in Figure 5. In all these samples, a liquid phase with two crystals of primary phases, spinel and tridymite, were observed. It can be observed that mixtures B51 and B56, although approaching from two different primary phase fields, reached the same equilibrium point, i.e., B51’ and B56’.
In contrast, mixtures B50 and B57 had different final liquid slag compositions to those using mixtures B51 and B56. Mixture B50 with high iron concentration in the initial mixture, which was introduced in the form of Fe and Fe2O3 mixture, produced liquid slag with a higher iron oxide concentration (B50’). Mixture B57, which was prepared by using Fe2O3 powder, in contrast, produced liquid slag with low iron oxide concentration B57’. It seems that greater equilibration time is required for both of the mixtures to reach the final equilibrium point.
Experiments using mixtures B56 and B57 were then repeated for a total equilibration period of 72 hours. The final liquid compositions of both mixtures in Figure 5 are indicated as B56L’ and B57L’. In the case of mixture B56, no difference was found between the liquidus resulting from 24 hours equilibration (B56’) and 72 hours equilibration (B56L’). This finding implies that equilibrium between gas and slag for mixture B56 had been achieved in equilibration period of less than 24 hours, and the liquid slag compositions B56’ and B56L’ can be taken as the final equilibrium point. Meanwhile, for mixture B57, there was a movement of the liquidus toward the final equilibrium point, which indicates that even after 72 hours of equilibration, there was still reaction taking place in the system.
From this set of experiments, it can be observed that the final equilibrium point at 1573 K (1300 °C) at \( {\text{P}}_{{{\text{O}}_{2} }} \) = 10−5 atm can be achieved readily within 24 hours of equilibration time approaching from two different equilibration directions, i.e., using initial mixture containing Fe powder or using initial mixture containing Fe2O3 powder. In contrast, the attainment of equilibrium at 1473 K (1200 °C) at \( {\text{P}}_{{{\text{O}}_{2} }} \) = 10−7 atm is not straightforward. At this relatively low temperature, the final equilibrium condition in the tridymite primary phase field can be achieved easily within an equilibrium period of 24 hours starting with initial mixtures prepared by using Fe and Fe2O3 powders. The final equilibrium point in the spinel primary phase field at 1473 K (1200 °C) at \( {\text{P}}_{{{\text{O}}_{ 2} }} \) = 10−7 atm can only be achieved within 24 hours by having initial mixtures with compositions close to the final equilibrium points with iron introduced in the form of Fe powder. Introducing iron in the form of Fe2O3 powder in the spinel primary phase field at this condition, on the other hand, requires equilibration periods greater than 72 hours.
As a result of these experiments, the following equilibration experiments at oxygen partial pressures between 10−5 atm and 10−7 atm were carried out by using initial mixtures with compositions close to the target equilibration points with iron introduced in the form of Fe powder, or sometimes a Fe-Fe2O3 mixture with proportion representing stoichiometric FeO. In some cases, the equilibration time was extended more than 24 hours to ensure the achievement of equilibrium.
Results and Discussion
Liquidus in the “FeO”-CaO-SiO2 System at an Oxygen Partial Pressure of 10−5 atm and Selected Temperatures
The results of EPMA measurements for the “FeO”-CaO-SiO2 system at an oxygen partial pressure of 10−5 atm are summarized in Table III. The phase diagram of the “FeO”-CaO-SiO2 system at this condition is provided in Figure 6. The experimental liquid compositions reported by Nikolic et al.[6] at 1523 K (1250 °C) are indicated by closed circles, whereas the experimental liquid compositions from the current study at 1573 K (1300 °C) are indicated by open circles. The solid lines define the experimental liquidus of the primary phase fields, i.e., spinel (Fe3O4), tridymite (SiO2), and pseudowollastonite (CaSiO3). Additional scales of SiO2/Fe and CaO/SiO2 ratios, important parameters in fluxing, are included in the diagram for practical convenience.
Liquidus isotherms in the “FeO”-CaO-SiO2 system at 1523 K and 1573 K (1250 °C and 1300 °C) at an oxygen partial pressure of 10−5 atm (data from the present study indicated by open circle and data from Nikolic et al.[6] are indicated by closed circle)
At 1523 K (1250 °C) and an oxygen partial pressure of 10−5 atm, the fully liquid region is surrounded by three primary phase fields: spinel, tridymite, and pseudowollastonite. Increasing the temperature to 1573 K (1300 °C) increases the extent of the fully liquid area. The boundary between spinel and pseudowollastonite primary phase fields found at 1523 K (1250 °C) is not observed at 1573 K (1300 °C). At 1573 K (1300 °C), the spinel primary phase field shares a boundary with the dicalcium silicate primary phase field.
Liquidus in the “FeO”-CaO-SiO2 System at an Oxygen Partial Pressure of 10−6 atm and Selected Temperatures
Extensive work on the phase equilibria in the “FeO”-CaO-SiO2 system at an oxygen partial pressure of 10−6 atm between 1473 K and 1623 K (1200 °C and 1350 °C) has been carried out by Nikolic et al.,[6] at 1573 K (1300 °C) limited to CaO/SiO2 ratios lower than 1.2 and at 1623 K (1350 °C) limited only to the spinel primary phase field. In the current study, at an oxygen partial pressure of 10−6 atm, experimental measurements at 1573 K (1300 °C) have been extended to CaO/SiO2 ratios higher than 1.2 and at 1623 K (1350 °C) to include both tridymite and pseudowollastonite primary phase fields. At CaO/SiO2 ratios higher than 1.2, experimental difficulties arise with the presence of dicalcium silicate, which undergoes a polymorphic phase transformation during cooling, leading to the disintegration of the sample into small particles (the so-called “dusting” phenomenon).[13] The ability of EPMA to carry out compositional analysis on an area as small as 5 μm facilitates the examination of these small particles. Although “dusting” took place, a sufficiently large area for EPMA analysis can still be found to enable the composition of each phase to be measured accurately.[14]
Table IV shows the EPMA measurements obtained from the present experiments at an oxygen partial pressure of 10−6 atm. The phase diagram for the “FeO”-CaO-SiO2 system at this condition is provided in Figure 7. The dashed lines in the figure show the boundaries between the primary phase fields. The dash-dotted line gives the predicted wollastonite to pseudowollastonite transition. At 1573 K (1300 °C), the compositions of liquids at spinel and dicalcium silicate saturations, as well as at rankinite and dicalcium silicate saturations, have been determined successfully. The extent of the fully liquid area of the ferrous calcium silicate slag at 1573 K (1300 °C) at an oxygen partial pressure of 10−6 atm is characterized completely. At 1623 K (1350 °C), additional information on the positions of the liquidus in the tridymite and pseudowollastonite primary phase fields is now available.
Liquidus isotherms in the “FeO”-CaO-SiO2 system at 1473 K, 1523 K, 1573 K, and 1623 K (1200 °C, 1250 °C, 1300 °C, and 1350 °C) at an oxygen partial pressure of 10−6 atm (data from the current study indicated by open circle and data from Nikolic et al.[6] are indicated by closed circle)
Liquidus in the “FeO”-CaO-SiO2 System at an Oxygen Partial Pressures of 10−7 atm and Selected Temperatures
The compositions of the phases present in the “FeO”-CaO-SiO2 system at an oxygen partial pressure of 10−7 atm at temperatures of 1473 K and 1573 K (1200 °C and 1300 °C) for CaO/SiO2 ratios lower than 1.2 are summarized in Table V. At 1473 K (1200 °C), there is only a small range of compositions over which fully liquid slag is observed. The fully liquid region is surrounded by the spinel, tridymite, and wollastonite/pseudowollastonite primary phase fields. The fully liquid region expands with the increase of temperature from 1473 K to 1573 K (1200 °C to 1300 °C), principally because of the movement of the spinel liquidus. The movement of the tridymite liquidus is not as significant as that of spinel. For example, an increase of temperature in spinel primary phase field from 1473 K to 1573 K (1200 °C to 1300 °C) at fixed CaO/SiO2 ratio of 0.4 results in the movement of the liquidus position toward iron oxide corner by approximately 20 wt pct of “FeO.” In contrast, increasing the temperature in tridymite primary phase field from 1473 K to 1573 K (1200 °C to 1300 °C) at constant CaO/FeO ratio of 0.43 moves the liquidus position to the silica corner by less than 4 wt pct of SiO2. Finally, comparison of the fully liquid regions in Figures 6 through 8 at fixed temperature shows that the fully liquid region expands with decreasing oxygen partial pressure from 10−5 atm to 10−7 atm.
Solid Solutions in the “FeO”-CaO-SiO2 System at Oxygen Partial Pressures between 10−5 atm and 10−7 atm, at Selected Temperatures
The solid solutions in the “FeO”-CaO-SiO2 system at an oxygen partial pressure of 10−6 atm were reported by Nikolic et al.[6] The spinel solidus was found to contain relatively higher lime concentrations of up to 0.6 wt pct compared with silica, which had less than 0.45 wt pct of CaO. Tridymite was reported to dissolve appreciable amount of iron oxide compared to lime. Calcium metasilicate solid was found to dissolve an appreciable amount of iron oxide at 1473 K (1200 °C). At 1523 K and 1573 K (1250 °C and 1300 °C), the compositions of calcium metasilicate solid were close to stoichiometric CaSiO3. In general, it was found that the concentration of solute species in solids decreased with increasing temperature.
The following discussion focuses only on the results at oxygen partial pressures of 10−5 atm and 10−7 atm. Figures 9 through 11 summarize the experimentally determined solidus compositions of spinel, tridymite, and calcium metasilicate with tie-lines to the corresponding liquidus compositions. The figures provide information on the effects of oxygen partial pressure and temperature on the compositions of the solid phases.
The spinel phase seems to dissolve relatively higher concentrations of lime than silica. For example, at 1573 K (1300 °C) at an oxygen partial pressure of 10−5 atm, the maximum lime concentration in spinel is more than 1 wt pct, whereas the maximum silica concentration is only 0.3 wt pct (Figure 9(a)). This trend can also be observed at oxygen partial pressure of 10−7 atm for the spinel solidus at 1573 K (1300 °C) (Figure 9(b)). A comparison of the spinel solidus at 1573 K (1300 °C) at oxygen partial pressures of 10−5 atm and 10−7 atm indicates that there are no significant changes in the solubility ranges of silica and lime in solid spinel with the variation of oxygen potential. A comparison of the spinel solidus at fixed oxygen partial pressure (10−7 atm) at two different temperatures (1473 K and 1573 K [1200 °C and 1300 °C]) shows a significant effect of temperature on the spinel solidus. For example, at 0 wt pct CaO, it can be observed that the solubility of silica in the spinel increases with decreasing temperature.
The tridymite solidus is presented in Figure 10. In general, tridymite is found to dissolve an appreciable concentration of iron oxide and a limited amount of lime. The maximum concentration of iron oxide “FeO” in the tridymite solidus is approximately 1.1 wt pct at 1573 K (1300 °C) at an oxygen partial pressure of 10−5 atm (Figure 10(a)). The concentrations of solutes (lime and iron oxide) in tridymite change with temperature. It can be observed in Figure 10(b) that an increase in the temperature decreases the concentrations of solutes in the tridymite.
The compositions of the calcium metasilicate solid are presented in Figure 11. It is found that there is a decrease in the concentration of iron oxide in the calcium metasilicate solid with the increase of temperature. At 1473 K (1200 °C) at an oxygen partial pressure of 10−7 atm (Figure 11(b)), the calcium metasilicate solid can dissolve up to 4 wt pct of iron oxide. While at 1573 K (1300 °C) at oxygen partial pressures between 10−5 atm and 10−7 atm (Figures 11(a) and (b)), the calcium metasilicate is formed with composition approaching stoichiometric CaSiO3. The change in the ability of the calcium metasilicate solid to dissolve iron is an indication that there is a transformation of its crystal structure.
It was shown by Bowen et al.[15] that there are two polymorphs of calcium metasilicate, i.e., pseudowollastonite (α-CaSiO3) and wollastonite (β-CaSiO3). Pseudowollastonite is a pure CaSiO3 compound, while wollastonite is a CaSiO3 solid solution with appreciable FeSiO3 concentration. In the current study, the concentration of iron in the calcium metasilicate crystal has been used as a criterion to distinguish wollastonite from pseudowollastonite crystals. It is found that calcium metasilicate transforms from the wollastonite structure to the pseudowollastonite structure with the increase of temperature as can be observed in Figure 11(b).
Conclusions
The phase equilibria of the ferrous calcium silicate slags were characterized at oxygen partial pressures of 10−5 atm, 10−6 atm, and 10−7 atm at selected temperatures. The effects of oxygen partial pressure and temperature on the liquid and solid phases in the slags have been presented. It is found that the fully liquid region of the slags expands as a result of increasing the equilibration temperature or decreasing the oxygen partial pressure. The expansion of liquidus in the spinel primary phase field is more significant than those of tridymite and wollastonite/pseudowollastonite primary phase fields. In general, it is found that the concentrations of solutes in the solid primary phases decrease with increasing temperature.
References
W.G. Davenport, M. King, M. Schlesinger, and A.K. Biswas: Extractive Metallurgy of Copper, 4th ed., Elsevier Science Ltd, Oxford, UK, 2002.
J.P.T. Kapusta: JOM, 2004, vol. 56 (7), pp. 21–27.
I. Shigaki, M. Sawada, O. Tsuchiya, K. Yoshioka, and T. Takahashi: Tetsu-to-Hagane, 1984, vol. 70, no. 16, pp. 2208–15.
H. Kimura, S. Endo, K. Yajima, and F. Tsukihashi: ISIJ Int., 2004, vol. 44, no. 12, pp. 2040–45.
H.M. Henao, F. Kongoli, and K. Itagaki: Mater. Trans., 2005, vol. 46, no. 4, pp. 812–19.
S. Nikolic, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 179–88.
S. Nikolic, H. Henao, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2008, vol. 39B, no. 2, pp. 189–99.
S. Nikolic, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2008, vol. 39, pp. 200–09.
H. Henao, C. Nexhip, D.P. George-Kennedy, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 767–79.
H. Henao, C. Pizarro, C. Font, A. Moyano, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 1186–93.
R.A. Mendybaev, J.R. Becket, E. Stopler, and L. Grossman: Geochim. Cosmochim. Acta., 1998, vol. 62, pp. 3131–39.
E. Jak, P.C. Hayes, and H.G. Lee: Kor. IMM J., 1995, vol. 1, pp. 1–8.
S. Nikolic, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 210–17.
T. Hidayat, H.M. Henao, P.C. Hayes, and E. Jak: Proceedings of the 7th International Copper-Cobre Conf. 2010, GDMB, Hamburg, Germany, 2010, vol. 2, pp. 761–78.
N.L. Bowen, J.F. Schairer, and E. Posnjak: Am. J. Sci., 1933, vol. 26, pp. 193–284.
Acknowledgments
The authors would like to thank Australian Research Council Linkage program, Xstrata Technology, Xstrata Copper, BHP Billiton Olympic Dam Operation, Outotec Oyj, and Rio Tinto Kennecott Utah Copper, Corp., for their financial support. The authors also thank University of Queensland Research Scholarship (UQRS) and Endeavour International Postgraduate Research Scholarship (IPRS) for providing scholarships for T. Hidayat. The authors acknowledge Ms Belinda Chen, Ms Jie Yu, Dr Hector M. Henao, and Dr Baojun Zhao from PYROSEARCH at The University of Queensland for their valuable help and discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 3, 2011.
Rights and permissions
About this article
Cite this article
Hidayat, T., Hayes, P.C. & Jak, E. Experimental Study of Ferrous Calcium Silicate Slags: Phase Equilibria at \( {\text{P}}_{{{\text{O}}_{2} }} \) Between 10−5 atm and 10−7 atm. Metall Mater Trans B 43, 14–26 (2012). https://doi.org/10.1007/s11663-011-9569-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9569-3