Abstract
KF-NaF-AlF3-based electrolyte is a promising low-temperature electrolyte for aluminum reduction. Alumina solubility in molten KF-NaF-AlF3-based electrolyte was determined as a function of the melt composition and temperature by measuring the weight loss of a rotating corundum disk and by using a LECO RO500 oxygen analyzer (LECO Corporation, St. Joseph, MI). The investigated temperature range is 1023 K to 1073 K (750 °C to 800 °C), and the total cryolite molar ratio (CRt = ([KF] + [NaF])/[AlF3]) is 1.3 to 1.5; the content of NaF ranges from 0 mol pct to 50 mol pct. The effect of temperature, CaF2, and LiF on alumina solubility is discussed as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum has been produced by the Hall-Heroult process for more than 100 years. Carbon anode and liquid aluminum cathode are used in this process to decompose alumina dissolved in molten NaF-AlF3-based cryolite, in which the cryolite ratio (the mole ratio of NaF to AlF3) ranges from 2.0 to 2.7. However, the electrolytic process is operated at temperature of 1183 K to 1233 K (910 °C to 960 °C), which is a high-energy-consumption process. Lowering the operating temperature of the aluminum reduction cell can reduce the energy consumption efficiently; therefore, producing aluminum at lower temperature [below 1123 K (850 °C)] is a popular research field studied by many laboratories.[1–5] One key problem is to find a solvent for alumina with a lower liquidus temperature and suitable physicochemical properties.
The KF-AlF3-based electrolyte has a much lower liquidus temperature[6] and a much wider range of low-temperature liquid composition than that of the NaF-AlF3 system.[7,8] Furthermore, the alumina solubility in KF-AlF3-based electrolyte is higher than that in NaF-AlF3-based electrolyte. Thus, the KF-AlF3-based electrolyte is considered a promising electrolyte used in aluminum electrolysis. However, the KF-AlF3 system will be changed into KF-NaF-AlF3 system inevitably because of the accumulation of sodium (which is brought in by alumina in the electrolysis process) in the electrolyte. As a whole, the KF-NaF-AlF3 system is the most possible low-temperature electrolyte for aluminum electrolysis. It can promote the development of inert anode and help to realize the industrialization of low-temperature aluminum electrolysis as well.
The published physical-chemical data of KF-NaF-AlF3 system electrolyte is deficient because it is a new electrolyte system. Belyaev et al.,[9] Barton et al.,[10] and Danielik and Gabcova[11] reported a partial rough-phase diagram of KF-NaF-AlF3 system, but the detailed data cannot be obtained in the phase diagram. Apisarov et al.[12] measured the liquidus temperature and conductivity at [KF]/([KF] + [NaF]) molar ratio ranging from 0 to 1 and at the fixed values of ([KF] + [NaF])/[AlF3] molar ratio (CRt) equal to 1.3, 1.5, and 1.7. In our previous work,[13] the liquidus of the KF-NaF-AlF3-based electrolyte has been measured at the CRt is 1.3, 1.41, and 1.5 with the content of NaF ranging from 0 mol pct to 50 mol pct.
Little information is available on alumina solubility in KF-NaF-AlF3-based electrolyte. Robert et al.[14] and Yang et al.[15] reported the alumina solubility in KF-AlF3-based electrolyte, and their results are listed in Figure 1. Yang et al.[15] reported the alumina solubility in KF-NaF-AlF3-based electrolyte only with NaF ranging from 0 to 8 mol pct at 973 K (700 °C). Apisarov et al.[16] measured alumina solubility in KF-NaF-AlF3-based electrolyte at the CRt = 1.3 and 1.5 at 973 K, 1023 K, and 1073 K (700 °C, 750 °C, and 800 °C) [most of the measurements were done at 1073 K (800 °C)], and regression equations were given to calculate alumina solubility in the KF-NaF-AlF3 system in CRt range from 1.3 to 3.0 depending on the concentration of the components and temperature.
Experimental
Chemical
KF, NaF, CaF2, and LiF are analytical reagents from Aladdin Reagent (Shanghai, China), and the purity is higher than 99.7 pct. Corundum disks contain a minimum of 99.6 pct Al2O3. AlF3 is prepared from AlF3·3H2O (analytical reagents, Aladdin Reagent) as follows: First, aluminum fluoride trihydrate is air-blast dried at 393 K to 473 K (120 °C to 200 °C) for at least 8 hours, then it is mixed with ammonium hydrogen fluoride (analytical reagents, Aladdin Reagent) and baked at 623 K to 873 K (350 °C to 600 °C) for approximately 6 hours in an air-tight furnace. The exhaust gas is absorbed by a sodium hydrate solution and a hydrochloric acid solution. The typical content of impurities in aluminum fluoride is listed in Table I; the purity of the aluminum fluoride prepared with the preceding method was greater than 99.5 pct. Before the experiment, all reagents are dried in a vacuum oven with P2O5 at 423 K (150 °C) for at least 4 hours.
Experimental Setup and Procedure
The experimental setup and procedure for measuring alumina solubility is similar to that in Reference 15.
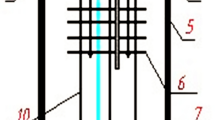
The experimental setup is shown in Figure 2. A thermal radiation-proof assembly is hung with a brass lid to ensure the homogeneity of temperature. The brass lid is tightened with a brass O-ring holder to press a fluorocarbon rubber O ring between them to seal the one-end closed corundum furnace tube. The brass lid is kept cool with cooling water circulated through a chiller at 293 K (20 °C). All the rods and pipes through the brass lid are fixed air tight. The setup is heated up with argon purging the furnace tube. The temperature of bath is measured by a calibrated Pt-Pt/Rh thermocouple, whose accuracy is within ±0.2 K (°C) at 1073 K (800 °C); the thermocouple is protected by a SiC sheath (Hexoloy SE; Saint-Gobain Ceramics Structural Ceramics, Niagara Falls, NY).
Experimental setup for the determination on alumina solubility 1—stainless steel rod; 2—corundum pipe (Ar gas inlet); 3—copper pipe (cooling water inlet); 4—O ring; 5—thermal radiation-proof assembly; 6—a small graphite cap; 7—bath; 8—corrundum disk; 9—graphite crucible; 10—thermocouple; 11—a big graphite lid; 12—corundum furnace tube; 13—fastening bolt; and 14—brass lid
The measuring procedure is as follows: Weigh and mix the electrolyte (the total mass is 600 g) in a glove box under nitrogen atmosphere, and then transfer to a high-purity graphite crucible (inner diameter is 95 mm) with a graphite lid. The graphite crucible held by 2 stainless steel rods is moved quickly into a corundum furnace tube (inner diameter is 130 mm). A high-purity corundum disk fixed with a stainless steel rod by screw caps is hung above the electrolyte while it is heating up. After the electrolyte is molten, a sample is taken to determine the initial alumina content by a LECO RO500 oxygen analyzer (LECO Corporation, St. Joseph, MI). After the initial sampling, the corundum disk is put into the bath and rotated by an agitator at the rotating speed of 260 rpm. The samples for alumina analysis are withdrawn with a graphite ladle[15] through a hole in the graphite lid. In most cases, only two samples are taken in one experiment. After sampling, the hole is covered with a small graphite cap to reduce volatilization of the bath. After the last sampling, the corundum disk and the thermocouple are removed out of the bath. When the furnace cooled down below 373 K (100 °C), the graphite crucible is taken out, and the adherent electrolyte is removed from the corundum disk in a hot AlCl3 solution.
Two methods are used to determine the alumina solubility. One is measuring the weight loss of a rotating corundum disc; the other is measured directly by the LECO RO500 oxygen analyzer. As for the former method, the saturated alumina concentration is calculated by Eq. [1]:
where W sat AO is the saturated alumina concentration, W 0 AO is the initial alumina concentration measured by LECO RO500 oxygen analyzer, it usually ranges from 0.1 to 0.3 wt pct. W 0 d is the initial weight of the corundum disk, W 1 d is the final weight of the disk, W s is the weight of the initial sample, and 600 is the initial weight of the bath; all the weight units are presented in grams.
The LECO method is as follows: First, the sample is pulverized in a glove box under high-purity nitrogen atmosphere. An approximately 0.04-g sample is weighted with a balance (Sartorius BSA-124S; Sartorius AG, Goettingen, Germany). The weighted sample is sealed in a tin capsule (LECO501-059), and then the tin capsule is put in a nickel basket (LECO502-344). When the LECO RO500 oxygen analyzer is ready, the nickel basket was put into the sample loader, and the analysis was started. The alumina concentration is calculated from the oxygen content of the sample. The measuring parameter of oxygen analyzer is listed in Table II. The RO500 oxygen analyzer is calibrated by an iron powder standard (LECO502-399), which contains 1.09 wt pct oxygen.
Results and Discussion
To determine the equilibrium time for corundum disk being dissolved in the melt at 1023 K (750 °C), a serial of samples were taken at various contact times during experiment. The rotating speed rate of the corundum disk is 260 rpm. The melt is composed of KF-NaF-AlF3 with the CRt = 1.3, and it contained 20 mol pct NaF. The samples were analyzed by a LECO RO500 oxygen analyzer. As shown in Figure 3, the concentration of alumina reached a steady value after 4 hours. Based on the results shown in Figure 3 and other similar tests, 5 to 6 hours are chosen as the rotating time of the corundum disk in the melt to ensure the saturation of the melt with alumina.
In most cases, the results from two methods show good consistency (see Table III). But the repeatability of the weight loss method is better than LECO method. The uncertainty of former method (by measuring the weight loss of a rotating corundum disk) is less than ±0.1 wt pct, and the uncertainty of LECO method is less than ±0.2 wt pct. The possible explanations is that the sample used for analyzing alumina concentration by LECO method is also less (the mass is usually 0.03 to 0.05 g) to obtain a steady result. Furthermore, the instrumental error of LECO RO500, the segregation of melt during sampling, and water absorption during analysis process might lead to a deviation of results as well. Therefore, the results from the weight loss method were taken as final.
Alumina solubility in CR = 1.3 and 1.5 KF-AlF3 melts at the temperature range of 973 K to 1073 K (700 °C to 800 °C) was measured, and the obtained results are compared with the reported data. Among four obtained results, three results are in good agreement with that reported by Yang et al.,[15] but some deviation was found between our results and the results reported by Apisarov et al.[16] (Table IV).
The alumina solubility in a KF-NaF-AlF3-based electrolyte was measured at the ([KF] + [NaF])/[AlF3] molar ratio (CRt), and it was 1.3 and 1.41 at 1023 K (750 °C). The content of NaF ranged from 0 to 50 mol pct with the interval of 5 mol pct. The experimental results are shown in Figure 4.
As shown in Figure 4, as a whole, the saturated alumina concentration tends to decrease as the NaF content increases. This could be explained as follows. According to the report by Robert et al.,[14] and Gilbert et al.,[17] AlF −4 and AlF 2−5 are the dominant anions in MF-AlF3 (M = Na, K) melt with 1 ≤ CR ≤ 3, and the formation of oxide complex in MF-AlF3 (M = Na, K) melts mainly follow reactions [2] through [4]:
Because the potassium cation radius is larger than that of the sodium cation, which results in an increase on the electric potential of the M+–A− interactions in the sequence K+ < Na+, the interactions between K+ and the A− (including AlF6 3−, AlF5 2−, and AlF4 −) are weaker than the interactions between Na+ and those anions. So, when NaF is added to the melt, the equilibriums in reactions [2] through [4] will shift to the left and result in the reduction in alumina solubility. The alumina solubility also tends to increase as CRt increases, possibly because as the concentration of AlF5 2− increases as CRt increases, it will shift the equilibriums in reactions [2] through [4] to the right.
Alumina solubility decreases rapidly with the addition of NaF when the content of NaF ranges from 0 to 20 mol pct. When CRt is 1.3 and the content of NaF ranges from 20 to 30 mol pct, the alumina solubility changes little. When the content of NaF is higher than 30 mol pct, the alumina solubility exhibits considerable reduction. When the content of NaF reaches 50 mol pct, the saturated alumina concentration is 2.5 wt pct’ it is not high enough for aluminum electrolysis. When CRt is 1.41, the alumina solubility changes little with the content of NaF ranging from 20 to 35 mol pct, and the alumina solubility exhibits considerable reduction as the content of NaF is higher than 40 mol pct. The explanation to those variation trends of alumina solubility need to be studied even more.
The alumina solubility was measured at 1023 K, 1048 K, and 1073 K (750 °C, 775 °C, and 800 °C) to study the influence of temperature on alumina solubility. The effect of CaF2 and temperature on the alumina solubility in the melts with CRt = 1.3 and 1.35, as well as NaF = 30 mol pct, was also investigated. The results are shown in Figure 5.
As shown in Figure 5, without the addition of CaF2, the alumina solubility in melts almost exhibits a linear relationship with the temperature. The addition of CaF2 will reduce alumina solubility, but the effect will weaken when the temperature increases. When the content of CaF2 reaches 5 wt pct, CaF2 has great effect on alumina solubility in the melts with CRt = 1.3, 1.35, and NaF = 30 mol pct. The saturated alumina concentration is lower than 1 wt pct at 1023 K (750 °C) in the melts with CRt = 1.3 or 1.35, and NaF = 30 mol pct. It is notable that when the temperature reaches 1073 K (800 °C), the addition of CaF2 has a lesser effect on alumina solubility.
The possible reason for the effect of CaF2 on alumina solubility could be as follows: According to the report by Gilbert et al.,[17] the dissolution of CaF2 in MF-AlF3 (M = Na, K) melt may follow Eq. [5]:
Here, n varies from 2 to 0. When CaF2 is dissolved according to this model, it associates with AlF5 2−, forming a mixed complex, and F− will be released. This will shift the equilibriums in reactions [2] through [4] to the left and will result in the reduction in alumina solubility in melt. Furthermore, X-ray diffraction (XRD) analysis (Figure 6) of the solid samples show the presence of KCaAl2F9 and NaCaAlF6 in KF-NaF-CaF2-AlF3-based electrolyte with CRt = 1.3, NaF = 30 mol pct and CaF2 = 5 wt pct, which indicate that KCaAl2F9 and/or NaCaAlF6 might exist in the melt above 1023 K (750 °C). It may lead to the great increase of activation energy with calcium fluoride addition and result in the significant reduction in alumina solubility.
Table V lists the alumina solubility in a KF-NaF-CaF2-LiF-AlF3-based electrolyte at 1023 K (750 °C). The results listed in Table IV indicate that the alumina solubility tends to decrease with the addition of CaF2 and LiF. The explanation on the influence of CaF2 on alumina solubility was given previously. As for the LiF, the interaction between Li+ and AlF 2−5 is stronger than that of K+ and Na+, and it will shift the equilibriums in reactions [2] through [4] to the left and reduce the Al-O-F complex formation. Thus, the alumina solubility is lower. In addition, an XRD analysis (Figure 7) of the solid samples show the presence of Na3Li3(AlF6)2 in KF-NaF-LiF-AlF3-based electrolyte with CRt = 1.3, NaF = 30 mol pct, and LiF = 3 wt pct. It may also lead to the reduction in alumina solubility. The results listed in Table V also indicate that the LiF has less influence on alumina solubility than CaF2 at the same weight percent.
The results listed in Table V indicate also that the addition of a CaF2 in KF-NaF-lF3-based electrolyte with CRt = 1.41 has a lesser influence on alumina solubility when compared with CRt = 1.3. When both LiF and CaF2 are added in the melts, the alumina solubility will decrease significantly, especially when the CRt is 1.3. This condition should be avoided in aluminum reduction. The reason for that combination effect is not clear yet.
Conclusions
Alumina solubility in KF-NaF-AlF3-based electrolyte with CRt = 1.3 and 1.4 decreases as the NaF content and CRt increase. The solubility of alumina does not change much at 1023 K (750 °C) when the content of NaF ranges from 20 mol pct to 30 mol pct. The alumina solubility decreases with the addition of CaF2 and LiF. The addition of CaF2 has great influence on alumina solubility in melt with CRt = 1.3, 1.35, and NaF = 30 mol pct when the content of CaF2 reaches 5 wt pct. When both LiF and CaF2 are added in the melts, the alumina solubility will decrease significantly.
References
W.C. Sleppy and C.N. Cochran: Light Metals, TMS, Warrendale, PA, 1979, pp. 385-89.
T.R. Beck: Light Metals, TMS, Warrendale, PA, 1994, pp. 417-23.
C.W. Brown: Light Metals, Warrendale, PA, 2000, pp. 391–96.
J. Thonstad and A. Solheim: Aluminium, 1986, vol. 62, no. 12, pp. 938-41.
T.R. Beck: Light Metals, Warrendale, PA, 1994, pp. 417–23.
K. Grjotheim, C. Krohn, M. Malinovsky, K. Matia-sovsky, and J. Thonstad: Aluminum Electrolysis—Fundamentals of the Hall-Heroult Process, 2nd ed., Aluminium-Verlag, Düsseldorf, Germany, 1982.
B. Phillips, C.M. Warshaw, and I. Mockrin: Am. Ceram. Soc., 1966, vol. 49, no. 12, p. 633.
J. Thonstad, P. Fellner, and G.M. Haarberg: Aluminum Electrolysis—Fundamentals of the Hall-Heroult Process, 3rd ed., Aluminium-Verlag, Düsseldorf, Germany, 2001.
A.I. Belyaev, M.B. Rappaport, L.A. Firsanova: Electrometallurgiya Alyuminiya. Metallurgizdat, Metallurgizdat Indyustriya, Moscow, Russia, 1953.
C.J. Barton, L.M. Bratcher, and W.R. Grimes: Am. Ceram. Soc., 1969, p. 1153.
V. Danielik and J. Gabcova: J. Therm. Anal. Calorim., 2004, vol. 76, pp. 763–73.
A. Apisarov, A. Dedyukhin, E. Nikolaeva, P. Tin’ghaev, O. Tkacheva, A. Redkin, and Y. Zaikov: Light-Metals, Warrendale, PA, 2009, pp. 401–03.
H. Yan, W. Li, J. Yang, Yang, S. Chen, and S. Wang: XVIII International Symposium of ICSOBA, TRAVAUX, 2010, vol. 35 (39), pp. 607–11.
E. Robert, J.E. Olsen, V. Danek, E. Tixhon, T. Østvold, and B. Gilbert: J. Phys. Chem. B, 1997, vol. 101, pp. 9447-57.
J. Yang, D.G. Graczyk, C. Wunsch, and J.N. Hryn: Light Metals, Warrendale, PA, 2007, pp. 537–41.
A.P. Apisarov, A.E. Dedyukhin, A.A. Red’kin, O.Y. Tkacheva, Y.P. Zaikov: Russ. J. Electrochem., 2010, vol. 46, no. 6, pp. 633-39.
B. Gilbert, E. Robert, E. Tixhon, J.E. Olsen, and T. Østvold: Inorg. Chem., 1996, vol. 35, pp. 4198-4210.
Acknowledgment
Financial support from Chalco is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 13, 2011.
Rights and permissions
About this article
Cite this article
Yan, H., Yang, J., Li, W. et al. Alumina Solubility in KF-NaF-AlF3-Based Low-Temperature Electrolyte. Metall Mater Trans B 42, 1065–1070 (2011). https://doi.org/10.1007/s11663-011-9535-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9535-0