Abstract
The main purpose of this study is to characterize and separate antimony from a stibnite concentrate through a low-temperature sulfur-fixing smelting process. This article reports on a study conducted on the optimization of process parameters, such as flux and zinc oxide weight percentage, in charging, smelting temperature, smelting duration on the antimony yield, resultant crude antimony grade, and sulfur-fixing rate. A maximum antimony recovery of 97.07 pct, crude antimony grade of 96.45 pct, and 98.61 pct sulfur-fixing rate are obtained when a charge (containing 63.20 wt pct of flux and 21.30 wt pct of stibnite, a flux composition of \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \) = 10/147, where W represents weight, and more than 10 pct of the stoichiometric requirement of zinc oxide dosage) is smelted at 1153 K (880 °C) for 120 minutes. This smelting operation is free from atmospheric pollution because zinc oxide is used as the sulfur-fixing agent. The solid residue is subjected to mineral dressing operation to obtain suspension, which is filtered ultimately to produce a cake, representing the solid particles of zinc sulfide. Based on the results of the chemical content analysis of as-resultant zinc sulfide, more than 90 pct zinc sulfide can be recovered, and the recovered zinc sulfide grade can reach 66.70 pct. This material can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is the largest producer of antimony in the world. Current estimates place the antimony output of China at more than 80 pct of the total global output. Antimony deposits are found in Guizhou, Guangxi, and in many other provinces throughout China; of these, Hunan has the richest deposits. In particular, Xikuangshan, near Lengshuijiang City, has been celebrated for a long time for the quality and quantity of its antimony.[1,2]
The commercial route for the extraction of antimony from its sulfide mineral stibnite at Xikuangshan is a pyrochemical process (1423 K to 1623 K [1150 °C to 1350 °C]), which involves roasting the concentrate in a blast furnace, volatilizing the resultant antimony trioxide, and reducing the trioxide with carbon to a metallic antimony in reverberatory furnaces.[1–3] However, smelting antimony under these high temperatures causes the following serious problems in the form of serious environmental pollution and large energy consumption:
(a) Serious environmental pollution. During roasting, considerable quantities of antimony sulfide and some associated low-boil-point metals, such as lead, arsenic, and cadmium, together with low concentrations of SO2, are emitted because of volatilization. At present, the smelting of nearly all nonferrous metals has overcome the problem of low concentrations of SO2 emission by adopting enhanced oxygen-rich smelting technologies such as the Ausmelt process, Noranda process, Mitsubishi process, etc. However, because of a comparatively small industry and because the antimony sulfide boil point is comparatively low (1353 K to 1363 K [1080 °C to 1090 °C]), it is difficult to apply the enhanced oxygen-rich smelting technology to antimony smelting. Therefore, large numbers of low-concentration SO2, together with some escaped heavy metals, result in serious environmental pollution in the areas surrounding the smelting plants brought about by the use of obsolete technology. In addition, antimony and its compounds are highly toxic. Although some improved methods have been developed,[4,5] traditional and improved methods for producing antimony are still carried out at high temperatures ranging from 1423 K to 1623 K (1150 °C to 1350 °C). At these temperatures, antimony sulfide, antimony, and its compounds possess a high volatility and, as a result, pass in considerable quantities into the gas phase, thereby polluting the environment with toxic antimony and low-concentration sulfurous gas. To date, not a single known method for antimony recovery can ensure adequate environmental control at the positions of the operator and in areas adjoining such antimony-recovering enterprises.[6–11] As a result, the antimony concentrations in the atmosphere around smelting plants in China exceed the permissible levels by as much as 0.5 mg m−3.
(b) Large energy consumption. To sustain this pyrochemical process (1423 K to 1623 K [1150 °C to 1350 °C]), large numbers of high-quality coal are consumed. Currently, more than three tons of standard coal is required to produce one ton of antimony in Xikuangshan. Furthermore, the current pyrochemical process requires a high-grade concentrate and is not suitable for the treatment of low-grade stibnite. This is because the process results in the formation of a stable antimony matte of impure elements, thereby rendering subsequent processing difficult and more energy consuming.
Therefore, the most promising process for antimony smelting is the low-temperature process. A proposed process exists for recovering antimony under low temperature consisting of the following steps: feeding flux, stibnite-containing feed, and a sulfur-fixing agent, such as zinc oxide and powdery coal, into a reaction with a temperature of less than 1173 K (900 °C). As a result, crude antimony metal, zinc sulfide, gangue, and a melt, mainly containing sodium carbonate withdrawn from the process, are obtained. After the resultant liquid crude antimony is discharged, the melt is discharged from the reaction zone and is subjected to a filtering operation while it is melting to obtain a slurry containing a mixture of solid particles of zinc sulfide, gangue, and a molten salt (To reuse the flux again, the discharged molten salt was subjected to a filtering operation while it was melting state. The filtrate [essentially containing sodium carbonate] can be fed into the reaction zone to reuse as the reaction flux again). The solid residue then is subjected to mineral dressing to obtain a suspension, which is filtered ultimately to produce a cake, representing solid particles of zinc sulfide that can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide.
Experimental
Materials
Stibnite was supplied by Xikuangshan ShanXing Antimony Co. Ltd. (Lengshuijiang, China). Its chemical composition and species analysis are given in Tables I and II. Zinc oxide and standard coal were supplied by GuangXi Hua-Xi Co. Ltd. (Lengshuijiang, China). The chemical composition of adopted coal in this research is shown in Table III. Sodium carbonate and caustic soda were of commercial grade and procured from local suppliers.
Procedure
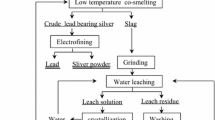
In a typical procedure, sodium carbonate, caustic soda, stibnite, and a sulfur-fixing agent, such as zinc oxide, are fed for 120 minutes into a reaction with a temperature of 1153 K (880 °C). As a result, crude antimony metal, zinc sulfide, a melt mainly containing sodium carbonate, and gangue (withdrawn from the process) are obtained. After liquid antimony is discharged from the bottom of a rotary kiln, the melt is discharged from the reaction zone and subjected to leaching while heating to obtain a slurry containing a mixture of solid particles of zinc sulfide, gangue, and a molten solution (essentially containing sodium carbonate, which can be fed into the reaction zone again as the inert reaction media). The solid residue is subjected to mineral dressing to obtain a suspension, which is filtered ultimately to produce a cake, representing solid particles of zinc sulfide that can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide. Figure 1 shows a flow diagram of the sequential steps in the extraction of antimony from stibnite using a low-temperature smelting process.
Main Principles of the Process
In the work, the molten salt of sodium carbonate and sodium hydroxide were used as the reaction media. The sodium slag was kept intact throughout the smelting process. The following pyrochemical reactions took place between stibnite, zinc oxide, and coal in this “inert” reaction media:
The antimony oxides contained in the stibnite were reduced by coal into metallic antimony expressed as follows:
The elements of gangue, such as SiO2, Al2O3, CaCO3, MgCO3, FeO, and CuS, were kept intact under a reaction temperature between 1053 K and 1253 K (780 °C and 980 °C). These materials form the slag, together with the resulting ZnS.
The sulfur-fixing rate is calculated, according to Eq. [3], which is expressed as follows:
In Eq. [3], W ss represents the total sulfur weight of the resultant slag, and W sc denotes the total sulfur weight of the added stibnite concentrate.
A single factor experiment was carried out using 200 g stibnite per time, whereas a confirmation experiment was carried out using 1000 g stibnite per time.
Results and Discussion
The extraction of antimony from stibnite was studied by smelting it in the presence of zinc oxide, sodium carbonate, and a small amount of sodium hydroxide. The addition of sodium hydroxide was meant to decrease the melting point and increase the fluidness of the flux. The smelting of antimony in the presence of zinc oxide and coal resulted in the reduction of antimony and associated lead into metallic antimony and lead, in accordance with Eqs. [1] and [2].
The sulfur present in the concentrate was captured by zinc oxide and converted into zinc sulfide because zinc sulfide can be roasted using enhanced oxygen-rich smelting technology to regenerate into zinc oxide and produce H2SO4, resulting in free from low-concentration-sulfur atmospheric pollution.
Low-Temperature Smelting Process
Smelting temperature
The results of the influence of the smelting temperature on the recovery of antimony and on the resultant crude antimony grade are presented in Table IV. All experiments were carried out under a charging composition of 63.20 wt pct flux and 21.30 wt pct of stibnite with smelting for 120 minutes. The flux composition, which is the ratio of weight of caustic soda to that of soda \( \left( {W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} } \right), \) is 10/147; zinc oxide is 10 pct in excess of the stoichiometric requirement.
Experimental results indicate that antimony recoveries improved from 89.69 pct to 98.56 pct, as the smelting temperature increased from 1053 K to 1153 K (780 °C to 880 °C) and as the antimony grade increased from 95.69 pct to 97.41 pct. These results are from the fact that the charge containing 63.20 wt pct flux and zinc oxide is 10 pct in excess of the stoichiometric requirement. Accordingly, the antimony in slag decreased from 3.54 pct to 0.95 pct, and the sulfur-fixing rate increased from 95.18 pct to 99.51 pct. Continued increase in the temperature of smelting is unnecessary. Therefore, results on the influence of smelting temperature indicate that 1153 K (880 °C) of smelting is adequate for the extraction of antimony and fixing sulfur.
Flux composition
The results of the influence of the flux composition on the recovery of antimony and on the resultant crude antimony grade are presented in Table V. All experiments were carried out under the following conditions: 63.20 wt pct flux and 21.30 wt pct of stibnite, 10 pct excess of zinc oxide over the stoichiometric requirement, and smelting temperature at 1153 K (880 °C) for 120 minutes. Studies on the effect of flux composition, \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \), decreased from 60/80 to 0/160. The experiment results indicate that the antimony yield increased from 76.69 pct to 96.08 pct as the weight of caustic soda to the weight of soda \( \left( {W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} } \right) \) decreased from 60/80 to10/147; in addition, the resulting crude antimony grade decreased from 97.84 pct to 95.39 pct. Accordingly, the antimony in slag decreased from 6.46 pct to 1.45 pct. Thus, a maximum antimony recovery of 96.08 pct and a crude antimony grade of 95.39 pct were obtained when \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \) was recorded at 10/147.
Zinc oxide dosage
The results of the influence of the dosage of zinc oxide in the charge composition on the recovery of antimony and on the resultant crude antimony grade are presented in Table VI. All experiments were carried out under a temperature of 1153 K (880 °C) for 120 minutes, with 63.20 wt pct flux, 21.30 wt pct of stibnite, and a flux composition \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \)= 10/147.
The results of the influence of zinc oxide dosage on the recovery of antimony and on the resulting crude antimony grade are presented in Table VI. Studies on the effect of the zinc oxide dosage indicate that the antimony recovery rate increased from 95.46 pct to 98.86 pct, when the zinc oxide dosage increased from 0 to 1.3 times that of the stoichiometric requirement. Moreover, the resulting crude antimony grade almost remained constant at around 96 pct to 97 pct. The sulfur-fixing rate increasing sharply from 1.28 pct to 99.77 pct indicates that the sulfur present in the stibnite concentrate is captured by zinc oxide and forms a stable zinc sulfide according to Eqs. [1] and [2]. Results on the influence of dosage of zinc oxide indicate that zinc oxide is 10 pct in excess of the stoichiometric requirement and is adequate for the extraction of antimony and fixing sulfur.
Flux dosage
All experiments were carried out under the following conditions: a temperature of 1153 K (880 °C), smelting duration of 120 minutes, 21.30 wt pct of stibnite, flux composition \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \)= 10/147, and more than 10 pct of the stoichiometric requirement of the zinc oxide dosage. The quantity of the total flux dosage increased from 40.30 wt pct to 78.40 wt pct. The results of the influence of flux dosage on the recovery of antimony and on the resultant crude antimony grade are presented in Table VII.
Experimental results indicate that antimony recoveries improved from 89.21 pct to 97.80 pct as the dosage of total flux increased from 40.30 wt pct to 63.20 wt pct and as the antimony grade increased from 81.17 pct to 97.24 pct. Accordingly, antimony in the slag decreased from 4.56 pct to 0.90 pct and the sulfur-fixing rate remained almost constant at around 99 pct. Continued increase in the dosage of flux was unnecessary. On the contrary, a higher dosage of flux causes the fluidity of molten salt to increase, resulting in more leakage from the rotary kiln. Therefore, results from the influence of flux dosage indicate that 63.20 wt pct of flux is adequate for the extraction antimony.
Smelting duration
Table VIII shows the extraction antimony from stibnite concentrates by low-temperature smelting in the presence of zinc oxide and under different smelting durations at a smelting temperature of 1153 K (880 °C), 63.20 wt pct of flux, and the flux composition \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \)= 10/147, with more than 10 pct of the stoichiometric requirement of zinc oxide dosage. Based on the results, the antimony recovery rate and the crude antimony grade increased with an increase in smelting duration from 30 minutes to 120 minutes. However, when the duration >120 minutes, no additional increase was apparent in the recovery rate and product grade. At the same time, the sulfur-fixing rate did not change significantly when the smelting duration reached 120 minutes. When the duration extended to more than 120 minutes, no considerable change occurred, whereas energy and the resultant antimony volatilization increased. Therefore, in this study, 120 minutes was selected as the optimized smelting duration.
Confirmations experiments
Based on the experiments, the optimum conditions for the low-temperature smelting process extraction of antimony from stibnite concentrates, conducted by smelting in the presence of zinc oxide, are as follows: a temperature of 1153 K (880 °C), 120 minutes smelting duration, 63.20 wt pct of flux and 21.30 wt pct of stibnite, flux composition \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \)= 10/147, and more than 10 pct of the stoichiometric requirement of zinc oxide dosage. These conditions then were applied in a confirmation experiment to extract antimony from stibnite. Table IX gives the results of the confirmation experiments. In total, 97.07 pct antimony was separated from the stibnite and 96.45 pct crude antimony was obtained. Sulfur contained in stibnite was nearly fixed (98.61 pct) in the slag in the form of zinc sulfide.
Zinc Sulfide Flotation
The resulting slag was mixed after being cooled and ground to a size of 200 mesh. The ground slag powder subsequently was subjected to mineral dressing, according to the procedure described here. A laboratory flotation machine, supplied with an automatic froth removal system, was used in the flotation tests. The sample for the laboratory flotation tests was collected at the industrial grinding circuit feed point and added to the tank of the flotation machine. Tap water then was added to complete the required volume. Reagents for zinc sulfide flotation (Na2CO3, dosed to raise the pulp pH to 9.5, dispersant, potassium isopropyl xanthate 70 g/t, and frother) were conditioned at 1600 rpm. The rotor speed then was reduced to 1200 rpm, the air flow rate was adjusted to 9 NL/min, and the flotation proceeded for 20 minutes. Flotation parameters and flotation results are shown in Table X. The flotation experiment results show that more than 90 pct zinc sulfide was recovered and that the recovered zinc sulfide grade reached 66.70 pct. The recovered zinc sulfide can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide.
Discussion
In a low-temperature smelting process, a maximum antimony recovery of 97.07 pct and crude antimony grade of 96.45 pct were obtained when a charge (containing 63.20 wt pct of flux and 21.30 wt pct of stibnite, a flux composition \( W_{\text{NaOH}} /W_{{{\text{Na}}_{ 2} {\text{CO}}_{3} }} \)= 10/147, and more than 10 pct of the stoichiometric requirement of zinc oxide dosage) was smelted at 1153 K (880 °C) for 120 minutes. This smelting operation is free from atmospheric pollution because of its use of zinc oxide as a sulfur-fixing agent (sulfur-fixing rate >98 pct). In comparison, the current antimony smelting process, called the “Xikuangshan process,” which involves roasting the concentrate in a blast furnace, volatilizing the resulting antimony trioxide, and reducing the trioxide with carbon to metallic antimony in reverberatory furnaces, always results in serious environmental pollution and higher energy consumption. The comparisons between the low-temperature smelting process and the “Xikuangshan process” are listed in Table XI.
Conclusions
In the presence of zinc oxide, a low-temperature smelting process has been developed for the extraction of antimony and for fixing sulfur from stibnite concentrate. Under optimized conditions, a high antimony recovery rate (>97 pct), a high crude antimony grade (>96 pct), and a high sulfur-fixing rate (>98 pct) have been achieved, resulting in a process that does not generate atmospheric pollution. The recovered zinc sulfide can be sold as zinc sulfide concentrate or roasted to regenerate into zinc oxide.
References
Z. Yi-feng and Z. Wen-tian: Miner. Process. Extr. Metall., 1984, vol. 3, pp. 687-98.
S. Gui-hua: Trans. Inst. Min. Metall., Sect. C, 1984, vol. 93, pp. 186-92.
T. Lager and K.S.E. Forssberg: Miner. Eng., 1989, vol. 4, pp. 543-56.
T. Mo-tang and J. Gui-zhong: Chin. J. Nonferr. Metal, 2007, vol. 3, pp. 34-36.
Ch. Yong-ming, H. Chao, T. Mo-tang, Y. Wei-yi, T. Chao-bo, and P. Guan-hua: Chin. J. Nonferr. Metal, 2005, vol. 15, pp. 1311–16.
P. Baláă, J. Briančin, V. Šepelák, T. Havlik, and M. Škrobian: Hydrometallurgy, 1992, vol. 31, pp. 201-12.
S. Ubaldini, F. Vegliò, P. Fornari, and C. Abbruzzese: Hydrometallurgy, 2000, vol. 57, pp. 187-99.
W. Jikun and L. Ting: Nonferr. Metal, 2000, vol. 52, pp. 44-8.
L. Lei: YunNan Metall., 2002, vol. 31, pp. 23-25.
P. Baláž and M. Achimovičová: Hydrometallurgy, 2006, vol. 84, pp. 60-68.
Y. Jian-guang, Y. Sheng-hai, and T. Chao-bo: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 523–34.
Acknowledgments
This project was supported by the Non-Ferrious Metals Science Foundation of HNG-CSU. Project (50804056) was supported by the National Nature Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 28, 2010.
Rights and permissions
About this article
Cite this article
Yang, JG., Tang, CB., Chen, YM. et al. Separation of Antimony from a Stibnite Concentrate Through a Low-Temperature Smelting Process to Eliminate SO2 Emission. Metall Mater Trans B 42, 30–36 (2011). https://doi.org/10.1007/s11663-010-9453-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9453-6