Abstract
Density measurements of a low-silica CaO-SiO2-Al2O3 system were carried out using the Archimedes principle. A Pt 30 pct Rh bob and wire arrangement was used for this purpose. The results obtained were in good agreement with those obtained from the model developed in the current group as well as with other results reported earlier. The density for the CaO-SiO2 and the CaO-Al2O3 binary slag systems also was estimated from the ternary values. The extrapolation of density values for high-silica systems also showed good agreement with previous works. An estimation for the density value of CaO was made from the current experimental data. The density decrease at high temperatures was interpreted based on the silicate structure. As the mole percent of SiO2 was below the 33 pct required for the orthosilicate composition, discrete \( {\text{SiO}}_{4}^{4 - } \) tetrahedral units in the silicate melt would exist along with O2– ions. The change in melt expansivity may be attributed to the ionic expansions in the order of
Structural changes in the ternary slag also could be correlated to a drastic change in the value of enthalpy of mixing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Density is the most fundamental thermophysical property that is directly amenable to the structure correlations of molten slags. Molar volume, which is the reciprocal of density, is a thermochemical property and has direct reflections of Gibbs energies of the slag system. A better understanding of these properties leads researchers to give conclusive evidence in regard to the types and packing of ions present in molten slags. Density is required to estimate other fundamental properties, like surface tension, viscosity, and thermal conductivity from thermal diffusivity so that a relatively complete picture in regard to the behavior of high-temperature molten oxides could be made. It is also a widely used thermodynamic quantity for calculating other critical dimensionless parameters (viz. Reynolds, Prandtl, Nusselts, and Grashoffs numbers), which are used in the heat- and mass-transfer calculations.
The Division of Materials Process Science, KTH (Stockholm, Sweden) is involved in extensive research work during the last decade to develop a model that could provide a better approximation for the densities of molten oxide systems.[1] This was only possible by using mutually compatible thermochemical and thermophysical data in the same model and establishing a suitable relation between the two.[2] The key to the model developed was that the cation–anion attraction forces should be reflected both in the relative integral molar enthalpies of mixing and in the molar volume of the silicate melts.[3] A direct relationship could be made between the two as they are correlated directly to the bonding between the nearest and next nearest neighbor ions in the silicate system.
Density measurements of slag systems at high temperatures have been carried out by various methods, some of the most prominent being the Maximum bubble pressure method,[4] Pycnometric method,[5] Levitation method,[6] Sessile drop method,[7,8] and Archimedean method.[9] The CaO-SiO2-Al2O3 ternary is the most extensively studied silicate system with several researchers carrying out density measurements at various compositions.[10–13] However, few researchers have made measurements in the low-silica region.[4,14] Some actually have tried to find an explanation for the change in silicate structure with an increase in temperature and have succeeded to some extent.[4]
The aim of the current work is to determine the density of low-silica CaO-SiO2-Al2O3 slags and its variation with respect to temperature. A comparison is made between the corresponding values obtained from the density model developed by the division and with the work of Zielinski and Sikora.[14] An attempt is made to find the corresponding densities of the binary systems and to provide an approximate estimation of the density of pure CaO from the experimental values obtained. Furthermore, based on enthalpy and expansivity variations, some approximations to the silicate structure variation with a change in temperature are suggested. Attempts are made in the current laboratory to design suitable experiments to determine the diffusion coefficient of sulfur in the CaO-SiO2-Al2O3 ternary slag system. The results of the current density measurements are required for these diffusion studies.
Experimental
In the current work, the Archimedean method has been employed, as it allows maintaining and controlling the oxygen potential of the melt easier as compared with the maximum bubble pressure method and minimizes the observation errors while using the sessile drop technique. However, this method has some inherent errors such as the effect of surface tension of the molten slag exerted on the shank/wire of the bob. This error could be reduced by reducing the diameter of the suspending wire/shank, which at high temperatures is a limitation. Another method to decrease this error is to increase the mass of the bob to an extent that the effect of the surface tension on the bob could be neglected or by using a double bob. Material selection is of crucial importance for high-temperature measurements, especially in slag systems. In this experiment, Platinum 30 pct Rhodium bob and wire were used, as this alloy is inert to chemical attack by the slag and functions without any mechanical breakdown at the experimental temperatures. The measurement temperature range was 1683 K (1410 °C) to 1733 K (1460 °C).
The CaO-Al2O3-SiO2 slag was prepared from pure raw materials supplied by Alfa Aesar (Karlsruhe, Germany), shown in Table I. The raw materials were dried in a muffle furnace maintained at 1273 K (1000 °C) for 24 hours before mixing thoroughly. The slag compositions were selected from Slag Atlas,[15] and their melting points were verified by ThermoCalc (ThermoCalc Software AB, Stockholm, Sweden) so that the slags were in the homogeneous liquid phase region above 1673 K (1400 °C).
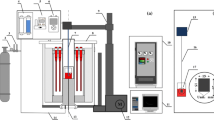
The experimental setup is shown in Figure 1. The furnace used was acquired from Thermal Technology, GMBH (1000-3060-FP20; Santa Rosa, CA) with graphite heating elements and is capable of attaining temperatures higher than 2500 K (2227 °C). The furnace is equipped with a Eurotherm 818 digital controller and a B-Type thermocouple to maintain constant temperatures within ±2 K (±2 °C). The reaction tube is made of recrystallized alumina with radiation shield arrangements on either end to maintain an even temperature zone corresponding to the crucible height. The furnace has provisions to be operated under vacuum, inert gas, or gas mixtures.
Armco iron crucibles were used for the measurement; hence, the maximum temperature for the density measurement was limited. A Pt 30 pct Rh bob with a gross weight of 178.8 g, suspended by a wire of the same material, was used for the density measurement using the Archimedes principle. The radiation shield arrangement helped to prevent the Pt 30 pct Rh wire to be exposed directly to high temperatures, hence, reducing its tendency to wear out. Additional cooling coils were provided at the top and bottom of the furnace. Kern ALJ 220-5DNM (Kern & Sohn GmbH, Balingen, Germany) type weighing balance with a reproducibility of 0.15 mg was used to monitor the weight of the bob by suspending it from the hook provided below the balance. The balance was connected to a laptop through an RS 232 cable, and the weight measurements were recorded with the help of Balance Connection SCE-3.0 Version 3.2 software. It was ensured that the Pt 30 pct Rh wire did not touch the sides of the alumina reaction tube so that accurate measurements could be made. A cooling plate was provided under the balance to ensure that the temperature of the balance was within workable limits to prevent any error/damage to the system. The gas-cleaning system used for purifying the argon gas, with initial purity >99.99 pct, from moisture, oxygen, and carbon dioxide was mentioned elsewhere.[16] The purified argon gas was made to pass through a ZrO2-CaO oxygen probe, with air as the reference electrode, which was used to monitor continuously the partial pressure of oxygen before passing through the furnace. The probe initially was calibrated with O2 gas, prior to the experiment. A \( {\text{P}}_{{{\text{O}}_{ 2} }} \) level of the order of 10−15 to 10−17 Pa was easily attainable and was recorded using a data logger.
Experimental Procedure
After the heat treatment of the individual slag components, the materials were mixed thoroughly and then loaded in an iron crucible. To prevent any iron oxide from entering the system, the empty crucible initially was heat treated at 1423 K (1150 °C) in a purified argon atmosphere maintained in the furnace. After the heat treatment, the crucible was observed to have metallic shine without any trace of oxide formation on the surface. Approximately 120 g slag was loaded into the crucible. This amount was sufficient to provide enough molten slag height to enable a known volume of the bob to be inserted completely. An argon flow rate of 300 ml/min was maintained using a Bronkorst EL (Bronkhorst High-Tech B.V., Ruurlo, The Netherlands) flow meter and controller throughout the experiment. The argon gas was purified by using a gas-cleaning system to maintain the partial oxygen pressure below 10−7 Pa and the settings were monitored continuously using a data logger. The slag mixture was heated to 1683 K (1410 °C) and was maintained at this temperature for 90 minutes. After the slag was stabilized at this temperature, the Pt 30 pct Rh wire holding the bob was connected to one lead of a multimeter (with an input impedance of 10 MΩ) via a Pt wire, whereas the other lead was connected to one arm of the B-Type thermocouple wire in contact with the bottom of the crucible holding the slag as shown in Figure 1. The B-type thermocouple was pushed so that it touched the bottom of the crucible. Later, during the measurement of the molten slag height, the bob was lowered slowly so that when its lower tip touched the molten slag, the molten slag along with the bob and the thermocouple formed a closed circuit, and the multimeter showed a reading that indicated that the bob actually had touched the molten surface, thus estimating its height from the bottom of the crucible. The electrical resistance values thus obtained were in the range of 1 to 2 Ω at 1723 K (1450 °C). Once the molten slag height was estimated, the bob was lowered so that it was immersed completely in the molten slag. The difference in the weight of the bob in argon and inside the slag gave the weight loss as a result of the buoyant force applied by the molten slag bath. The volume of the slag being displaced by the bob would be equal to the volume of the bob immersed multiplied by a factor containing the volumetric expansion ratio for Pt 30 pct Rh[17] at any particular temperature T (K).
Density at any temperature is expressed as follows:
The weight of the bob was 178.8 g. Based on the values for the surface tension and the wetting angle for CaO-SiO2-Al2O3 slags and Pt substrate obtained by previous research, the surface tension could be approximated to 500 mN/m.[18,19] This estimation results in a buoyancy force of the slag on the bob, 2πrσCosθ, of approximately 0.15 g. Hence, the effect of surface tension could be neglected, as the weight of the bob is high in comparison to the buoyant force applied by the slag on the bob. The slag densities were measured for two different temperatures (viz. 1683 K (1410 °C) and 1733 K (1460 °C)). Selected experiments were repeated under heating and cooling modes, and the results were reproducible.
Results
The density measurements were obtained at 1683 K (1410 °C) and 1733 K (1460 °C). The aim was to compare the densities obtained from the experiment with the model developed earlier in the group by Persson et al.[1,3] as well as the experimental values at similar compositions reported earlier by Zieliński and Sikora[14] using the maximum bubble pressure method. The various slag compositions investigated in the current work and the results obtained are presented in Table II. It could be observed that as the silica content of the slags studied increased, the density decreased significantly. A similar trend has been verified in the measurements with binary FeO-SiO2 slags studied by Lee and Gaskel,[4] CaF2-SiO2 melts by Yakobashvili,[20] ternary CaO-Al2O3-SiO2 slags studied by Bochorishvili and Yakobashvili[13] and Zieliński and Sikora,[14] and in quaternary Al2O3-CaO-MgO-SiO2 slags studied by Winterhager et al.[11] The experimental error was calculated in accordance with the probable measurement errors occurring (i.e., the error from the measurement of the mass of the bob and from the measurement of the height of the molten slag using the multimeter setup).
The error occurring as a result of the mass measurement using the balance would depend on the inherent error provided by the instrument and by the number of readings that possibly could be taken at a particular temperature. In each case, the mass measurement error was in line with the calibration error (±0.025 g) of the instrument. The number of readings taken at 1683 K (1410 °C) and 1733 K (1460 °C) at 5 pct silica addition was lower compared with the remaining slag compositions, which resulted in a larger error bar for this composition. The error as a result of the estimation of the molten slag level using the bob–thermocouple arrangement was of the order of 1 mm. This was taken on an assumption that the minimum error possible during height estimation was of the same order of magnitude. This error in measurement would result in a volumetric error of 0.028 cm3.
Figure 2 shows the comparison of the experimental results with those predicted using the model developed by Persson et al.[3] It is to be noted that the model values have an error inherent in them, as it is difficult to incorporate fixed density values for pure CaO, Al2O3, and SiO2. Persson et al.[3] have mentioned that there were difficulties in retrieving reliable experimental data required for precise model calculations. The authors also mention that, for systems containing CaO as a component, a marked deviation from experimental data was to be expected, owing to the unreliable molar volume value of CaO in a super-cooled state, which induced uncertainties in the measurements of densities for binary systems like CaO-SiO2, and Al2O3-CaO within ±10 pct and ±5% error limits, respectively. Taking this into consideration, the agreement between the current experimental data and those predicted by the model can be considered reasonable.
Comparison between density values of the current work with the model proposed by Persson et al.[1] at 1683 K (1410 °C) and the results of Zieliński and Sikora[14] at 1723 K (1450 °C). Experimental error bar values are shown for the current work, indicating good agreement with both model and experimental results
The experimental values obtained at 1733 K (1460 °C) were compared with those obtained with similar compositions carried out by Zielinski and Sikora[14] using the maximum bubble pressure method. Good agreement between the values can be observed in Figure 2.
Discussion
Prediction of Binary Densities
The model developed by Persson et al.[1] actually estimates the molar volumes/densities of ternary slag melts from the corresponding binary slag systems. Conversely, it could be possible to find the densities of the binary slags from the experimental values. As the experiment was carried out at a fixed weight percentage of calcium oxide, the predictions of the densities of binary slag sub systems (viz. CaO-SiO2 and CaO-Al2O3) were facilitated. The density values for the CaO-Al2O3-SiO2 slag was plotted with varying SiO2 content for a given temperature as shown in Figure 3. A linear fit was made in the graph obtained as an approximation that showed the variation of the density as a function of silica content. The molar volume of CaO and FeO were calculated by Hara et al.[9] from the extrapolation of binary systems. Similar methods were used in the current work to find the densities of the binary CaO-SiO2 and CaO-Al2O3 slag systems by extrapolating from the ternary to the silica-rich as well as silica-deficient zones, respectively. The values obtained were compared with density values from the model mentioned as well as other references.[14,21]
Table III shows the predicted binary slag density values using the linear fit equation. The model values were higher compared with the results from the linear fit model; the reason for this deviation could be because of uncertainties in the model predictions based on uncertainties in the available literature data related to activity measurements and to the estimation of molar volume of super-cooled CaO. Another cause for the deviation would be the degree of linear fit as a result of fewer data points.
The comparison of the predicted density of CaO-Al2O3 slag at 1683 K (1410 °C) was in good agreement with the values of Zielinski and Sikora.[14] The reference value used for CaO-SiO2 corresponds to a composition of 50 wt pct/50 wt pct of both components taken at 1773 K (1500 °C).
Comparison with High-Silica Systems
From the equation derived by the linear fitting of the density data points as explained in the previous section, it is possible to estimate approximately the densities of high-silica compositions at 1733 K (1460 °C). Table IV shows the density estimates and their comparisons with established research data. Good agreement was observed with the density values obtained at the similar temperatures with high-silica contents.
Prediction of Density Value for CaO from Experimental Data
In an attempt to refine the molar volume values obtained from the density model by Persson et al.[1,3] for binary as well as for higher order systems containing CaO, the density variation of pure super-cooled CaO as a function of temperature was derived from the experimental data. The densities for the individual pure oxides were used for calculating the molar volumes of higher order systems. Currently, established density data for pure super-cooled liquid Al2O3 as a function of temperature was investigated by Mitin and Nagibin[22] and was expressed as follows:
Similarly, the density value for pure super-cooled liquid SiO2 was established from the classic works of Bacon et al.[23] and was equated as follows:
Using these values for pure oxides and back-calculating from the model, the density value for pure super-cooled liquid CaO could be calculated for the experimental temperatures from the following equation:
From Eq. [1], the density of pure super-cooled liquid CaO could be calculated for different temperatures. The density value for room temperature was required to steer the density value. The density of pure super-cooled liquid CaO as a function of temperature could be evaluated as follows:
Using the new density values of the pure oxides, the densities of the ternary slag systems was calculated. The results are tabulated in Table V.
It is shown that the density values obtained from the model developed by Persson et al.[3] after the incorporation of the estimated density value for pure CaO exhibited a better agreement with the experimental data.
Structural Interpretations
Explanations for the decrease in density with an increase in temperature can be traced to structural factors. For ternary silicate melts, if the binary silicates are of equal silica mole fraction and if the cations are of similar size and valency, then mixing could be considered ideal.[24,25] However, in the current situation, the cations are of different valence states and size; hence, nonideal mixing would result. In the current slag compositions studies, the mole percent of SiO2 was well below the 33 pct required for the orthosilicate composition. This would correspond to the existence of discrete \( {\text{SiO}}_{4}^{4 - } \) tetrahedral units along with O2– in the silicate melt and thus would rule out the possibility of the impact of silicate polymerization on the volumetric expansion at high temperatures. Hence, it is felt that the major focus should be given to the ionic bonding for the explanation of the decrease in melt densities with an increase in temperature.
It is widely understood that any ion with an ion-oxygen parameter, (defined by the columbic force between the cation and oxygen anion) in the range of 0.7 to 1.7 could behave either as a network former or modifier depending on the melt environment.[26] The melt system would consist of Ca2+, \( {\text{AlO}}_{4}^{ - 5} \), \( {\text{SiO}}_{4}^{4 - } \), O2–, and O– ions. The high CaO and Al2O3 contents in the slag suggest that the predominant bonding would be between the two. Some CaO would behave as a network modifier and hence create more nonbridging oxygen in the melt. As the ion-oxygen parameter also represents the coulombic interaction between the oxygen ion and cation, and with Al3+ being on the higher side (1.66) along with Si4+ (2.45) compared with Ca2+ (0.7), the predominant ionic expansion should be that contributed by the Ca-O bonding.
Figure 4 shows the volumetric expansion coefficient plotted against the molar fraction of alumina content in the slag. The CaO-SiO2 and CaO-Al2O3 binaries are at the left and right extremes, respectively. It could be observed that the melt expansivity decreases with an increase in alumina content. The region corresponding to the current work is that between 0.25 and 0.3 mole fraction of Al2O3. In this region, as there is very little variation in the CaO content (0.53 to 0.64), it could be concluded that there was a minimum base expansivity in the system as a result the Ca-O bonding, which is likely to be the major reason for the density decrease. Furthermore, as the mole fraction of CaO was greater than that of Al2O3, the melt expansivity would be expected to be predominantly because of the ionic expansion of the Ca-O bonds.
The decrease in the melt expansivity toward the CaO-Al2O3 binary suggests that a stable structure with respect to Ca-Al-O bonds seems to be taking place that could lead to microsegregation. In other words, the drooping melt expansivity curve could indicate that the inherent ionic bond expansivity is being counteracted by the thermal relaxation caused by microsgeregation. This was explained earlier by Lee and Gaskel[4] in FeO-SiO2 and CaO-SiO2 melt systems. As the mole fraction of CaO was greater than that of Al2O3, the melt expansivity predominantly could be a result of the ionic expansion of the Ca-O bonds.
Lee and Gaskel[4] have mentioned that the inherent expansivities of the bond types and the degree of polymerization are two major factors influencing the expansivity of a binary silicate melt; this observation could be true for ternary melts as well. As Ca2+ is the predominant cation in the melt, the change in the volume, which resulted in the decrease in density of the melt, could be attributed to the bond expansivities in the following order:
The change in slag structure could be identified by evaluating the enthalpies of mixing for the different slag compositions. In an earlier work, Seetharaman and Staffansson[27] showed that, for Ag-Sn metallic system, a minimum enthalpy of mixing indicated a corresponding increase in volume contraction. According to Kubaschewski and Alcock, the negative enthalpies might be the result of better packing of the atoms based on the chemical bond theory. A change in structure causes a drastic difference in the enthalpy of mixing, as there is reordering of bonds to optimize the total energy of the system.[28] The enthalpies of mixing values were calculated for the current slag composition using Eq. [1] and taking the λ parameter as –0.06. Similarly H m values for other compositions studies by Bochorishvili and Yakobashvili[13] and Zielinski and Sikora[14] also were evaluated. These values are plotted against the mass percentage of SiO2, as shown in Figure 5. The drastic changes in the enthalpy values can be correlated to the phase change, as shown in Figure 6 .[15] In the current work, the decrease in the values of H m could be correlated from the ternary phase diagram as the phase change from 3CaO-Al2O3 to 2CaO-SiO2. Similarly, the changes in the enthalpy of mixing values for the density values quoted by Bochorishvili and Yakobashvili[13] and Zielinski and Sikora[14] could be attributed to the phase changes from 3CaO·Al2O3 - 2CaO·SiO2 – 2CaO·Al2O3·SiO2 and 12CaO·7Al2O3 - CaO·Al2O3 - 2CaO·Al2O3·SiO2 respectively.
Conclusions
Measurements of densities of low-silica CaO-SiO2-Al2O3 systems were made using Archimedes principle, and the results obtained were in good agreement with those obtained from the model developed earlier in the present group by Persson et al.[3] as well as with the work of Zielinski and Sikora.[14] A suitable value for the densities of CaO-Al2O3 and CaO-SiO2 binaries was obtained by extrapolating the ternary densities. Higher silica systems also were assessed, and the experimental values showed good agreement. An attempt to give an estimation for the density value for pure CaO was made, the substitution of which into the model developed by Persson et al.[3] resulted in a better agreement with the experimental results. As the current slag composition had mole percent of SiO2 well below the 33 pct required for the orthosilicate composition, discrete \( {\text{SiO}}_{4}^{4 - } \) tetrahedral units would exist along with free O2– ions. Thus, the impact of silicate polymerization for volumetric expansion at high temperatures would be negligible. Hence, it was concluded that the change in expansivity was a result of the ionic expansion, which was contributed predominantly by the Ca2+-O2− and Ca2+-O– bonds present in the melt. Structural changes to the slag were identified by plotting the variation of the enthalpy of mixing with the weight percentage SiO2. This was verified by plotting the corresponding points on the CaO-Al2O3-SiO2 ternary phase diagram.[15]
Abbreviations
- T :
-
Temperature, K
- W argon :
-
Weight of the bob in argon atmosphere, g
- W molten slag :
-
Weight of the bob in molten slag, g
- V 0 :
-
Volume of the slag being displaced, cm3
- L T /L273:
-
Ratio in the change in length of a Pt 30 pct Rh wire at any temperature T (K) to the same at 273 K
- ρ expt :
-
Experimental density value, g/cm3
- R:
-
Gas constant, J/K mol
- X i :
-
Molar fractions of the individual oxides
- M i :
-
Molecular weights of the oxides, mol
- V mi :
-
Molar volumes of the oxides given as the ratio of the molecular weight by the density of the oxide, cm3/mol
- H m :
-
Relative integral molar enthalpy of mixing derived by solving the Gibbs–Helmoltz equation for the relative integral molar Gibbs energy of mixing. The enthalpy thus derived is a function of the ionic fractions of cations and anions in their respective grouping and the interaction variables derived for various binary oxide systems and temperature, J/mol
- λ :
-
Constant and is evaluated for binary systems using experimental density data in literature
References
M. Persson, J. Zhang, and S. Seetharaman: Steel Res. Int., 2007, vol. 78, no. 4, pp. 290-98.
Royal Institute of Technology: Thermoslag, Division of Materials Process Science, Royal Institute of Technology, Stockholm, Sweden, 1996.
M. Persson, T. Matsushita, J. Zhang, and S. Seetharaman: Steel Res. Int., 2007, vol. 78, no. 2, pp. 102-08.
Y.E. Lee and D.R. Gaskell: Metall. Trans., 1974, vol. 5, pp. 853-74.
Y. Sato, T. Nishizuka, K. Hara, T. Yamamura, and Y. Waseeda: Int. J. Thermophys., 2000, vol. 21, no. 6, pp. 1463-71.
T. Matsushita, T. Ishikawa, P.F. Paradis, K. Mukai, and S. Seetharaman: ISIJ Int., 2006, vol. 46, no. 4, pp. 606-10.
A.I Sergienko: Avt. Svarka, 1965, vol. 18, no. 6, pp. 26-31.
T. Takayanagi, M. Kato, and S. Minowa: J. Jap. Foundry Soc., 1976, vol. 48, no. 12, pp. 779-83.
S. Hara, K. Irie, D.R. Gaskel, and K. Ogino: Trans. JIM, 1988, vol. 29, pp. 977-89.
E.V. Krinochkin, K.T. Kurochin, P.V. Umrikhin, F.K. Poverkh, and Y. Rasp: Ed. V.N. Eremenko, Naukova Dumka, Kiev, Ukraine, 1971, pp. 179–83.
H. Winterhager, L. Greiner, and R. Kamnel: Forschungsberichte des Landes Nordrhein-Westfalen, no. 1630, Westdeutscher Verlag, Germany, 1966.
L. R. Barett and A.G. Thomas: J. Glass Technol., 1959, vol. 43, p. 179.
A.I. Bochorishvili and S.B Yakobashvili: Svar. Proiz., 1968, vol. 10, pp. 13-15.
M. Zieliński and B. Sikora: Pr. Inst. Metall. Zelaza im St. Staszica, 1977, vol. 29, nos. 3-4, pp. 229-32.
Verein Deutscher Eisenhűttenleute: Slag Atlas, Verlag Stahleisen m.b.h, Dusseldorf, Germany, 1981, p. 57.
A. Jakobsson, N.N. Vishwanathan, S. Du, and S. Seetharaman: Metall. Mater. Trans. B, 2000, vol. 31B, p. 974.
B. Barter and A.S. Darling: Platinum Metals Rev., 1960, vol. 4, pp. 138-40.
Verein Deutscher Eisenhűttenleute: Slag Atlas, Verlag Stahleisen m.b.h, Dusseldorf, Germany, 1981, pp. 239.
G. Parry and O. Ostrovski: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 681-89.
S.B. Yakobashvili: Auto Weld, 1970, vol. 23, no. 6, pp. 20.
V.I. Sokolov, S.I. Popel, and O.A. Esin: Černaja Metallugija, 1970, vol. 13, no. 2, pp. 10-15.
B.S. Mitin and Yu. A. Nagibin: Russ. J. Phys. Chem., 1970, vol. 44, no. 5, pp. 741-42.
J.F. Bacon, A.A. Hasapis, and J.W. Wholley: J. Phys. Chem. Glasses, 1960, vol. 1, pp. 90-98.
F.D. Richardson: Trans. Faraday Soc. 1956, vol. 52, p. 1312.
C.J.B. Fincham and F.D. Richardson: Proc. Roy. Soc., 1954, vol. 223A, p. 40.
Y. Waseda and J.M. Touguri: The Structure and Properties of Oxide Melts, World Scientific, Hackensack, NJ, 1998, pp. 4.
S. Seetharaman and L.I. Staffansson: Chemica Scripta, 1976, vol. 10, pp. 61-66.
P. Kubaschewski and C.B. Alcock: Thermochimie, Colloq. Internat. Centre National Recher. Scientif., No. 201, Marseille, Paris, 1972, France, p. 211.
Acknowledgments
The authors would like to thank the Swedish Research Council (Project No: H 6971) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 17, 2009.
Rights and permissions
About this article
Cite this article
Muhmood, L., Seetharaman, S. Density Measurements of Low Silica CaO-SiO2-Al2O3 Slags. Metall Mater Trans B 41, 833–840 (2010). https://doi.org/10.1007/s11663-010-9385-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9385-1









