Abstract
Analysis has been undertaken on the kinetics of gaseous hydrogen reduction of solid nickel oxide by considering elementary steps commonly found in gas-solid reactions, i.e., gas-film mass transfer, gas pore diffusion, and chemical reaction. It is demonstrated that these reaction steps alone cannot account for the observed rates. The analysis has therefore been extended to include recently obtained microstructural information on the reduction product. The study provides strong evidence for direct links between the overall reduction kinetics and the changes to the reduction product microstructures generated at the Ni-NiO interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The industrial application of the gaseous hydrogen of solid NiO is found in a number of metallurgical processes. In the Caron process[1] carried out in the BHP Billiton Yabulu refinery (Townsville, QLD, Australia), the intermediate product of basic nickel carbonate (BNC) is decomposed to form NiO and, subsequently, the NiO is reduced at high temperature to obtain a high-grade nickel metal. In the Inco Goro process (Vale Inco, New Caledonia), NiO produced by the pyrohydrolysis of nickel chloride solutions is reduced by hydrogen.[2] The overall reaction of the reduction process can be expressed as
In these processes, the reduction rates and residual oxygen concentrations are important factors that determine the production rates and the value of the final Ni product, respectively.[3]
The kinetics of reduction of NiO by hydrogen has been studied over a wide range of temperatures, between 155 °C and 1000 °C, and reaction conditions. A review[4] of the literature on this topic has shown that there are disagreements between researchers on the magnitudes of the reduction rates, rate-limiting reaction mechanisms, and apparent temperature dependence. The review concluded that the values of the apparent activation energies could be divided into four categories, which, it was argued, corresponded to conditions from gas-phase mass transfer (17 to 22 kJ·mole−1) to chemical reaction (98 to 133 kJ·mole−1) limited processes.
Those studies specifically designed to eliminate the effects of gas-phase mass transfer from the bulk gas and porous diffusion have included (1) the direct observation of metal nuclei growth on the oxide,[5] (2) thermogravimetric studies of thin NiO plates[6] and small-sized spherical pellets,[2] and (3) metallographic studies of initial interface growth.[7] Even when the effects of gas-phase mass transfer have been eliminated, these measurements have shown that the kinetics of reduction exhibit a range of behaviors. In the temperature range 227 °C to 300 °C, the reaction rate appears to be independent of the hydrogen partial pressure; between 300 °C and 400 °C, the rates are of variable order with respect to the hydrogen partial pressure.[5] At temperatures above approximately 400 °C, the rate of nickel growth varies from \( p_{{{\text{H}}_{ 2} }}^{0.5} \)[5] to \( p_{{{\text{H}}_{ 2} }} . \)[7] At temperatures above approximately 700 °C, the rate of reduction is actually found to decrease with increasing temperature; above 800 °C, the reaction rate again increases with increasing temperature.[2,7]
Several factors that contribute to the slowdown in reaction rates have been proposed. In developing mathematical models to describe these high-temperature reduction rates, it was argued[8] that the slowdown in the nickel oxide reduction rate was associated with the sintering and densification of the product with increasing temperature and time. However, a comparison of the rates of reduction of NiO that had been partially reduced and then subjected to high-temperature thermal treatment in a neutral atmosphere demonstrated that there was no difference in rate with or without this treatment.[2] This experiment[2] shows that sintering the metal product (or physical changes) alone cannot account for the observed rate slowdown. Separate measurements[9,10] of the pore sizes of the Ni product have shown that, in the range 600 °C to 1000 °C, the changes taking place in the nickel product structure result in an increase in the pore size; the pore volumes, however, remained relatively constant. This indicates that the structural changes in the product took place with relatively little densification. These findings support the argument that the slowdown in the rate of NiO reduction is not associated with the physical changes occurring in the Ni product.
Other factors that have been proposed[2] as possible reasons for the slowdown in reduction rates are the surface segregation of sulfur to the nickel surface and the decrease in available chemical reaction sites on the nickel surface, or the formation of a thin sulfide film that coats the oxide surface. Experiments using dense synthetic nickel oxide and pure reducing gas (no sulfur present) have shown that, even in sulfur-free systems, a slowdown in the rate at temperatures above approximately 700 °C occurs.[7] The results clearly demonstrate that the cause of the slowdown is not from the interference of impurities, such as sulfur, with the reduction process. It is interesting to note, however, that the slowdown in the reaction rate was found to be accompanied by changes in the microstructure of the reduction product.[7]
A number of distinctly different types of nickel product structures developed during the gaseous reduction of NiO have been characterized,[9] namely, a coarse fibrous nickel with fissures, a fine porous nickel-planar interface, a coarse porous nickel-irregular interface, and a dense nickel layer. The conditions of the formation of these microstructures have been identified and their evolution with temperature and time has been systematically investigated.[10]
Although the reduction of nickel oxide appears superficially to be a simple reaction, the factors influencing the rates and extent of reduction are still not completely characterized. In the present article, the fundamental kinetics and mechanisms of nickel oxide reduction are examined. First, the observed rates of NiO are compared with the rates calculated assuming that the elementary process steps commonly found in a gas-solid reaction, i.e., the gas-film mass transfer, gas pore diffusion, and chemical reaction, are rate limiting. Next, this analysis is integrated with microstructural observations to provide further evidence of the mechanisms of NiO reduction. The implications of the findings for industrial practice are discussed in Section V of this article.
Experimental Techniques
The details of the experimental technique have been described in a previous publication by the authors.[9] Dense synthetic NiO samples with dimensions of 2 × 2 × 1 mm were used. Pure H2, H2-N2, and H2-H2O with various compositions were used as reductants. The reducing gas was injected into a quartz reaction tube with an approximately 10-mm internal diameter at a total flow rate of 1 l·min−1 (25 °C, 1 atm). When the effective cross section of flowing gas around the sample is 6.28 mm2, the linear flow rate of the reducing gas passing over the sample is approximately 2.65 m·s−1. Each sample was directly introduced into the hot zone of the reaction tube and was held in the tube for selected reaction times. For all of the experiments, the extent of the reactions of NiO to Ni are small, i.e., the product volume is small compared to the mass of the sample. Therefore, the amount of heat generated per unit total volume is quite small. The high gas velocity across the surface will also ensure that there are no large temperature excursions during the reaction. The sample was then quenched into a bath of liquid nitrogen. As a result, the morphology developed during the reduction process was preserved.
Two techniques were used for the preparation of samples for subsequent analysis of the reduction products. The first preparation technique was used for the internal kinetic measurement. The kinetic measurements were conducted by measuring the progress of the Ni-NiO interface on a cross section perpendicular to the sample surface. This was achieved by using conventional metallographic preparation techniques, i.e., mounting the samples in acrylic resin and polishing with SiC paper and diamond paste from 6 to 0.25 μm. The advantages of selecting the present technique over a thermogravimetric analysis approach have been presented in a previous article by the authors.[7] The second preparation technique was used to enable observation of the product pore structure without destroying the structure. This was achieved by fracturing the partially reduced samples in half by cutting them perpendicular to the sample surface using a scalpel. The samples prepared from both techniques were sputter coated with Pt (Eiko IB-5 sputter coater, Eiko Co. Ltd., Hitachinaka, Japan) before being analyzed using a scanning electron microscope (SEM).
Two types of SEM, a PHILIPSFootnote 1 XL-30 and a JEOLFootnote 2 6400F, were used in the present work. The PHILIPS XL-30 operating in backscattered electron mode was used for distinguishing the original nickel oxide and the nickel metal product; this is useful for obtaining information about the kinetics of the Ni product advancement. The JEOL 6400F, a cold field-emission SEM, on the other hand, was used to acquire high-resolution images of the morphology of the fractured, partially reduced samples.
Analysis of Kinetics of NiO Reduction
The NiO reduction by hydrogen gas takes place through a series of processes involving mass transport, chemical reactions, nucleation and growth. Each of the processes may be controlling the overall reduction process. In the case of the NiO reduction carried out at elevated temperatures and high hydrogen partial pressures, the process of the nucleation of the Ni metal proceeds rapidly. The high nucleation frequency results in the uniform coverage of the NiO surface by the Ni product. As the reducing gas constantly consumes the NiO, the Ni metal product layer is observed to continuously grow in a direction perpendicular to the solid surface; the Ni-NiO interface moves toward the NiO bulk. The measurements of the kinetics of the NiO reduction in various temperature and gas conditions have been presented in detail in a previous study by the authors.[7]
Under conditions in which the metal nucleation process takes place relatively rapidly, a variety of mass transport or chemical reactions may become rate-limiting steps in the overall reduction process (Figure 1). Figure 1(a) shows a reduction process that is controlled by mass-transfer of hydrogen gas from the gas bulk to the outer surface of the solid through a stagnant boundary layer or gas film surrounding the solid particle. In Figure 1(b), the stagnant gas-film layer does not impose any significant barrier for the reducing gas; however, the porous product layer limits the rate of transport of the reducing gas to the reaction interface. In cases in which the mass transport of the reducing and product gases are rapid, the rate-limiting steps of the overall process may be associated with the various chemical reactions at the metal-oxide interface and mass transfer in the condensed phases, as shown in Figure 1(c).
It has been shown previously that it is valid to consider that the thickness of the Ni product resulting from the reduction process is equal to the thickness of the NiO consumed by H2.[7] Consequently, the rate of the reduction process is proportional to the rate of the Ni product advancement. Because the synthetic oxide samples with plate geometry have been used in the present study and considering a one-dimensional (1-D) analysis, the following relationships apply:
where J O is the flux of oxygen removal (mole·m−2·s−1); n O represents the moles of oxygen (mole); t is time (s); s is the surface area of oxide (m2); x is depth of penetration (m), which equates to the thickness of the nickel oxide that is consumed by hydrogen gas, closely approximated with the thickness of the Ni product; and M NiO, ρ NiO, W NiO, and V NiO represent the molecular weight, density, sample weight, and sample volume, respectively, of NiO.
In this section, a detailed 1-D kinetic analysis is carried out. It is assumed that the oxide sample is an infinite plate and that only one side of the plate is considered for the analysis. Using this technique of analysis, advancements in the Ni product thickness under the influence of various controlling steps are evaluated. It is further assumed that each controlling step operates individually without the influences of other controlling step. The results of the analysis can be used to determine the rate-controlling processes of the NiO reduction for the conditions investigated in the present study. Several rate-limiting processes considered in the present analysis are summarized in Table I.
The partial pressure of hydrogen in equilibrium with Ni-NiO approaches zero (e.g., 0.51 pct H2-99.49 pct H2O at 700 °C); hence, it can be assumed that \( p_{{{\text{H}}_{ 2} ,eq}} \) equals 0. The mass-transfer coefficient (k m ) for a gas flowing over a sphere can be calculated using the Rantz–Marshall formula.[11] A characteristic dimension, e.g., an equivalent diameter for a nonspherical particle, is used for the Rantz–Marshall formula in the present calculations.
In the calculation of the effective binary diffusivity coefficient \( \left( {D_{{{\text{H}}_{ 2}\text{-}{\text{H}}_{ 2} {\text{O}},{\text{eff}}}} } \right), \) the porosity-to-tortuosity ratio (ε/τ) of approximately 0.06, as reported by DebRoy and Abraham,[12] is used in the calculation with the assumption that the value is independent of temperature. From the measurement of pore sizes,[10] it was found that the NiO reduction carried out in the present study takes place in the transition region between Knudsen and molecular diffusion.[13] In the transition region, both of the diffusion mechanisms are operative and the combined diffusivity of the Knudsen and molecular diffusions has been considered.
The data used to obtain the apparent chemical reaction rate constants were obtained for the NiO reduction in \( p_{{{\text{H}}_{ 2} }} = 100\,{\text{pct}} \) and 1 atm pressure in the range 227 °C to 700 °C, as measured using the cross-sectional analysis technique,[7] the direct observation of nuclei growth,[5] and thermogravimetric studies of thin foils.[6] All of these experiments are specifically designed to eliminate any limitation associated with gas-transfer processes; all give very similar rate data in this temperature range. The initial rates shown in Figure 2 were obtained using the cross-sectional analysis technique for times as short as 5 seconds. These initial rates, when the product layer is very thin, are the least likely to be influenced by gas-phase mass transfer or porous gas diffusion.
Measured initial rates of NiO reduction between 0 and 5 s using 100 pct H2 (1 atm) between 500 °C and 1000 °C[7]
It has therefore been assumed that the low-temperature data represent the “chemical reaction rate” and are described by the Arrhenius equation:
where k is the frequency factor (m·s−1), Q is the activation energy (J·mole−1), R is the gas constant (J·mole−1·K−1), and T is temperature (K). Using the Arrhenius law, the rates of NiO reduction above 700 °C are calculated using the activation energy measured from experiments between 500 °C and 700 °C (Q = 66.6 kJ·mole−1). The results of the calculation are shown as a dashed line in Figure 2. Finally, the progress of the Ni product thickness (x) as a function of time in the present analysis is determined from the initial reaction rates (k i ):
where k i (m·s−1) equals \( {{M_{\text{NiO}} k_{c} p_{{{\text{H}}_{ 2} , {\text{bulk}}}} } \mathord{\left/ {\vphantom {{M_{\text{NiO}} k_{c} p_{{{\text{H}}_{ 2} , {\text{bulk}}}} } {\rho_{\text{NiO}} {\text{R}}T}}} \right. \kern-\nulldelimiterspace} {\rho_{\text{NiO}} {\text{R}}T}}, \) obtained under the assumption that the rate of the overall process is controlled by chemical reaction.
A summary of the parameters and data used in the present analysis of the reaction kinetics is provided in the Appendix (Tables II and III). A kinetic analysis of the oxygen removal during the NiO reduction at various limiting conditions may give an indication as to which step controls the overall rate of the reduction process. To determine which subprocess is controlling the overall NiO reduction process, a comparison of the extent of the NiO reduction under several limiting conditions is carried out. By substituting Eq. [2] into each equation in Table I, a kinetic model of the Ni product thickness as a function of time for each individual controlling process can be acquired. Comparisons of Ni product thicknesses as a function of time in the NiO reduction using 100 pct H2 (1 atm) obtained from the actual experiments, and from kinetic models at various limiting conditions and temperatures are provided in Figure 3.
The thicknesses of the Ni product as a function of time in Figure 3 are presented in log-log scale. Using this presentation, the magnitude of each subprocess relative to others can be seen clearly. In all temperatures, it can be seen that the extent of the reduction under the influence of the gas-film mass transfer would be far greater than the extent of the reductions carried out under the influence of the gas pore diffusion or chemical reaction. This confirms the view that the present experimental technique is able to eliminate the limitations introduced by the stagnant gas film surrounding the solid sample. Hence, it is acceptable to rule out gas-film mass transfer from the list of processes that may limit the overall reduction process. Further discussion will be focused on the influence of the gas pore diffusion and chemical reaction.
At low temperatures, between 500 °C and 600 °C, the plots of the Ni thickness obtained from the actual measurements are identical to those from the results of the analysis assuming that the process is chemically controlled. This is because the chemical rate in the analysis was derived from the initial reaction rates of the actual measurements (Eq. [7]). It is also observed that the extent of the NiO reduction to any thickness up to approximately 100 μm under the rate of the gas pore diffusion is higher than the extent of the NiO reduction under the influence of a chemical reaction. The differences in the Ni thicknesses of both of the limiting conditions become less as the reaction temperature is increased.
Between 500 °C and 600 °C, the increase in the time of the reduction results in a decrease in the difference in the extent of the predicted reductions for the pore diffusion and chemical reaction limiting conditions. As temperatures approach 700 °C, the relative rates of the chemical reaction and gas pore diffusion become comparable in magnitude. It can be seen that, at 700 °C, the plot of the Ni thickness of the chemical-reaction-controlled reduction approaches that of gas-pore-diffusion-controlled reduction. Figure 3(c) shows that the extent of the NiO reduction for both mechanisms, if they are operating independently, will be equal at approximately 1 minute or a 292-μm product thickness. As the temperature increases, the time required for both limiting conditions to have the same extent of reduction becomes shorter. It can also be seen that, above 700 °C, the plots of the Ni thickness obtained from the actual measurement are all lower than those obtained from the theoretical calculations. It appears that 700 °C is the temperature at which changes in the rate-controlling reaction mechanism of the NiO reduction take place. From this analysis, it might be reasonably expected, based on observation in analogous systems, that the changes in the rate-controlling process takes place gradually, i.e., from chemical control at low temperatures and small product thickness, to mixed control at intermediate temperatures, and to gas-pore-diffusion control at high temperatures and large product thicknesses.
To investigate the possibility of changes in the apparent rates due to the transition in the rate-controlling process, kinetic analysis is carried out with respect to the NiO reduction process in 100 pct H2 (1 atm) at 800 °C; the influence of the mixed control of the chemical reaction and gas pore diffusion on the extent of the NiO reduction is considered. It has been shown that, in the mixed-control processes, the time required to reach certain stages of conversion will be equal to the sum of the times that would be needed if each resistance acted alone.[14] By using Eqs. [2], [4], and [7], the relation between the total time (t total) and the Ni thickness under the mixed control of the chemical reaction and gas pore diffusion can be determined from the following equation:
Based on Eq. [8], the nickel thickness resulting from the NiO reduction, using 100 pct H2 (1 atm) at various times under the mixed control of the chemical reaction and gas pore diffusion, can be calculated. The results of the calculations for the NiO reduction carried out at 800 °C using 100 pct H2 (1 atm) is provided in Figure 4(a). It can be seen that, when the mixed-control condition is considered, the rate of reaction under this condition is lower than each of the independent limiting steps. However, the measured extent of the reduction at all times is still much lower than was predicted assuming mixed-control conditions. The relative rates of the mass-transfer and chemical reaction steps for the NiO reduction have been calculated for a range of hydrogen partial pressures. The example of a calculation carried out for the NiO reduction at 600 °C using 26 pct H2-N2 (1 atm) is shown in Figure 4(b). The analyses of all these cases show that none of the limiting processes considered in the present analysis contributes to the slowdown in the apparent rate. The results of the calculations indicate that the anomalous behavior in the NiO reduction above 700 °C is not due to factors related to the influence of the chemical reaction and gas pore diffusion, or their combination.
Formation Mechanisms of Microstructure Developed During Gaseous NiO Reduction
Four types of nickel product structures developed during the reduction process were identified,[9] namely: (1) a coarse fibrous nickel with fissures, (2) a fine porous nickel-planar interface, (3) a coarse porous nickel-irregular interface, and (4) a dense nickel layer. The conditions for the formation of these structures have been identified and described in a previous study.[10] In this section, an investigation of the formation mechanisms of each product is carried out by considering the formation conditions, microstructural information, and elementary processes involved.
Coarse Fibrous Nickel with Fissures
The surface appearance of the coarse fibrous nickel with fissures resulting from a NiO reduction carried out at 300 °C using a pure H2 atmosphere is shown in Figure 5(a). It can be seen that circular, isolated Ni nuclei are formed with a star-shaped cracking pattern develop from the middle of the nucleus. The Ni nuclei appear to grow at the same rate in all directions, independent of the presence of fissures. A careful examination of the cross sections shows that the structure consists of nickel in the form of fibrous metal separated by fissures that appear to follow the direction of the growth of the metal nuclei (Figure 5(b)). The formation of a fissured structure has also been observed in other metal oxide systems. In the reduction of CoO in H2-N2 mixtures,[5] the Co nucleus with a star-shaped cracking pattern was observed after the CoO was reduced at 210 °C using 97 pct H2-N2. A similar structure was also found in the reduction of magnetite and tungsten oxide. Large radial fissures were observed at the middle of the iron nuclei during the reduction of magnetite below approximately 550 °C; in addition to the large radial fissures, the iron nuclei contained extensive micropores.[15,16] In the reduction of tungsten oxide,[17] it was found that, when the WO2.96 was reduced to metal with hydrogen gas between 500 °C and 700 °C, a star-shaped cracking pattern was formed on the solid surface. It was suggested that the star-shaped cracking pattern is formed due to a local contraction of the product during the reduction process.
Examination of the metal-metal oxide interface of partially reduced NiO (300 °C using 100 pct H2) was carried out using an SEM; the results of these observations are provided in Figure 6. An example of the structure of the Ni-NiO interface and Ni product is shown in Figure 6(a). A number of structures can be seen on this fractured interface. At locations 1 and 2 in Figure 6(a), the internal structure of the Ni product is exposed by the fracturing technique. Location 3, on the other hand, shows a portion of the surface of the Ni product adjacent to the large cracks or fissures in the product. The detail of location 1, shown in Figure 6(b), reveals the presence, at the Ni-NiO interface, of thin Ni plates approximately 10-nm thick oriented perpendicular to the interface, with a spacing of 15 to 50 nm. The presence of thin, aligned Ni plates within the product has been reported in a previous study.[10] It appears that the thin platelike Ni found in the coarse fibrous nickel with fissures is the initial structure developed by the reduction process. The formation of a platelike metal structure has also been observed in the gaseous reduction of solid calciowustites.[18] Since the edges of the platelike Ni products have a high curvature and the plates have relatively close spacing, they join/sinter together during the reduction process; this results in the geometry shown in location 2 (Figure 6(c)). In location 2, the thicker Ni product is apparently formed as a result of sintering of neighboring Ni plates. In order to reduce their surface energy, the edges of the Ni plates become rounded and progress to form equiaxed nickel particles/grains 0.02 to 0.1 μm as found in location 3 (Figure 6(d)). The rate of the NiO reduction at 300 °C using pure hydrogen was measured. It was found that the rate of the Ni-NiO interface advancement at this condition is approximately 1.9 μm·min−1. The distance between locations 1 and 2, that is, approximately 1.5 μm, indicates that the structure at location 2 has been exposed at 300 °C for approximately 1 minute. It appears that this relatively short holding time at this temperature is sufficient to sinter the thin Ni plates (location 1) to form Ni structures such as those at location 2. The rearrangement toward a more stable structure, in this case a nearly equiaxed grain structure, can be seen clearly near the Ni surface (Figure 7). The area close to the Ni surface has been exposed to heating for a longer time, i.e., 30 minutes. The grain size at 300 °C after the extensive holding time has increased to 0.1 to 0.2 μm diameter.
The sintering of the nickel during the course of reduction at these low temperature leads to a nickel product with a lower volume compared to the main oxide and also leads to the formation of fissures/cracks to compensate for the difference in volume. Although the agglomerates appear at low magnification to be dense, analysis by transmission electron microscopy[10] has shown that these structures are actually composed of fine loosely bound polycrystalline nickel. As a result, the fissures may propagate easily throughout this fragile metal product. The evidence appears to support the hypothesis[17] that the mechanism of the formation of starlike fissures is the result of local contraction in the metal product. The fissures were not observed in the NiO reduction at temperatures above 400 °C. It appears that, at high temperatures, nickel products are created with wider spacing. In this structure, the difference in the volume between the metal and the oxide is compensated by the formation of a porous network structure.
Microporous Structure
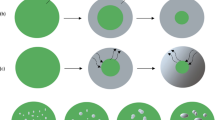
A transition in the microstructure from a structure of coarse fibrous nickel with fissures to a porous structure takes place as the reduction temperature is increased above 400 °C in pure hydrogen. The transition is also found to be dependent on the hydrogen partial pressure. In this porous structure, the metallic product consists of continuous networks of pores that provide direct contact between the gaseous reactant and the solid oxide. To investigate the mechanisms of formation of the porous structure, reduction experiments were carried out in low chemical driving force gas mixtures, in order to create a condition in which the reduction process is relatively slow and the progress of the experiment can be readily followed. The surface of the reduction product resulting from the reduction at 600 °C using 14.5 pct H2-H2O (equal to a Gibbs energy driving force of −53 kJ·(mole O2)−1) for 30 seconds is shown in Figure 8. Figure 8(a) shows the SEM secondary electron image of the product surface, while Figure 8(b) shows the SEM backscattered electron image of the same product surface. The SEM backscattered electron image is more sensitive to differences in the mean atom number of the phases, thus increasing the contrast between the NiO and Ni. It can be seen that two different types of instabilities are formed on the NiO surface, namely, instabilities that are surrounded by nickel metal and instabilities that are formed without nickel metal. The results suggest that the instabilities are initially formed on the NiO before the nickel metal starts to nucleate.
The formation of a porous structure that is initiated by the formation of instabilities has been proposed previously.[19] The mechanisms of the formation of instabilities themselves have been explained.[20,21] It was argued that, under conditions in which the chemical reaction rates are higher relative to the mass transport rates, the extensive removal of oxygen from the oxide surface takes place; this creates a high gradient of Ni ions between the surface and the bulk of the solid (Figure 9(a)). Because the rates of mass transport are relatively lower than the rates of chemical reaction, any perturbations on the oxide surface will result in a local variation in the mass transport process and, eventually, in differences in rates of Ni cation removal at that location. This condition leads to a nonplanar decomposition resulting in small tunnels or instabilities prior to the nucleation process (Figure 9(b)). Because the oxide surface surrounding the instabilities is supersaturated with Ni cations, nucleation of the Ni metal will occur (Figure 9(c)). The geometry of the tip of the instability provides a high flux of Ni removal and prevents the supersaturation of Ni ions at the tip. Consequently, direct contact between gaseous reactant and oxide phase will be maintained at the tip of the instability.
Instability Criteria
The critical conditions for the growth of instabilities on the oxide surface can be derived using the perturbation analysis approach. This approach has been used to describe the relative stabilities of solid surfaces during the solidification of metal alloys.[22] In alloy solidification, it is possible to obtain a planar solidification front or, cellular or dendritic growth morphologies. The possibilities for the growth and elimination of perturbations are examined by considering the degree of constitutional undercooling of the melt, which is directly related to the solute concentration, and the effect of the curvature of the solid interface. In the present study, a similar analysis can be undertaken by considering analogous parameters that are applicable to gas-solid reaction processes.
Figure 10 shows a schematic example of a perturbed solid surface developed after exposure to a reactive gas mixture for a particular reduction period. As a result of the different rates of mass transport and elementary reactions in the interface region, the tip of the concave instability contains a lower concentration of cations relative to that at the unperturbed surface. For the purpose of analyzing this phenomenon, it is assumed that the geometry (height (z)) of the perturbed surface follows a simple sinusoidal function:[22]
where ε is the amplitude of the perturbation and 2π/λ is the wave number.
Idealized model of interface perturbations at gas-solid interface[22]
The Gibbs free energy of the oxide at any point on the interface (G*), can be deduced from the assumption of local equilibrium:
where G b is the Gibbs free energy of the bulk oxide, C* is the difference in chemical composition between any given point on the interface and the bulk phase, \( {\left( {{{dG } \mathord{\left/ {\vphantom {{dG } {dC}}} \right. \kern-\nulldelimiterspace} {d C}}} \right)}_{ox}\) is the change in Gibbs free energy of the oxide associated with change in composition of the oxide, \( \bar{V} \) is the molar volume of the oxide, σ is the surface energy of the oxide, and K* is the curvature of the solid surface. In this equation, the second term on the right describes the change in Gibbs free energy of the oxide associated with compositional differences on the interface, the last term of the equation is the effect of shape of the interface on the Gibbs free energy of the oxide (Gibbs–Thompson effect[23]).
Considering the difference of the Gibbs free energy of the oxide between the tips (t) and depressions (d) of the interface, the following equation is obtained:
Assuming the Gibbs free energy of the oxide and concentration gradients in the oxide are unaffected by the presence of small perturbations, the difference of Gibbs free energy of the oxide between the tips and depressions can be expressed as follows:
where \( {\left( {{{dG } \mathord{\left/ {\vphantom {{dG } {dz}}} \right. \kern-\nulldelimiterspace} {dz}}} \right)}_{g}\) is the Gibbs free energy gradient in the oxide generated by the presence of the reactant gas. A similar analysis can be used to describe the difference of concentration between the tips and depressions:
The curvatures at the surface perturbation extrema can be determined from the second derivative of the Eq. [9] at y = λ/4 and 3λ/4:[22]
Note that the curvature is positive when the centers of its radii lie on the side of the solid (convex surface) and vice versa. By substituting Eqs. [12], [13], and [14] into Eq. [11], the following relationship is obtained:
and rearranging
where \( \Upphi_{I} = \left( {{{dG } \mathord{\left/ {\vphantom {{dG } {dz}}} \right. \kern-\nulldelimiterspace} {dz}}} \right)_{g} - \left( {{{dG } \mathord{\left/ {\vphantom {{dG } {dz}}} \right. \kern-\nulldelimiterspace} {dz}}} \right)_{ox} \) is the difference in thermodynamic gradients imposed by: i) the Gibbs free energy differences between the reacting gas and the solid, and ii) the composition differences in the solid. Consequently:
By using an analogous approach to instability formation to that adopted in solidification systems,[22] it can be shown that Eq. [16] describes the critical wavelength (λ c ) below which the curvature effect will dominate; as a result, any perturbations will be eliminated and the planar decomposition of oxide will result. If the wavelength is greater than this critical value, the interface will become unstable and the perturbation will grow in magnitude, i.e., the amplitude (ε) of the perturbation will progressively increase with time.
Transition between Fine Porous-Planar Interface and Large Porous-Irregular Interface
In the current study, two types of porous Ni product structures were observed, namely, a fine porous nickel-planar interface (Figure 11(a)) and large porous nickel-irregular interface structures (Figure 11(b)). The initiation of both structures may take place through the same process, which is schematically shown in Figure 9. The transition from one structure to the other is dependent on the characteristics and properties of the oxide and metal, and on the reduction parameters, namely, temperature, gas composition, and time.
The transition from the planar to the irregular growth interface during the gaseous reduction of nickel oxide can be explained as follows. The planar growth morphology corresponds to a reaction mechanism that involves a cooperative/coupled growth of the nickel metal product and the simultaneous decomposition of the oxide surface. The interface and the various subprocesses active during this reaction are shown in Figure 12. Oxygen is removed by chemical reaction from the surface of the oxide and from the nickel metal. The rate of oxygen removal is a function of the gas composition and temperature. The resulting excess of Ni cations at the oxide surface diffuses by surface and bulk diffusion mechanisms to the existing nickel metal that forms the metal pore walls.
Under steady-state conditions, the rate of oxygen removal by the chemical reaction at the interface will be equal to the diffusive flux of nickel cations to the pore wall; these are related directly to the interface velocity, V (m·s−1):
where \( {{M_{\text{NiO}} } \mathord{\left/ {\vphantom {{M_{\text{NiO}} } {\rho_{\text{NiO}} }}} \right. \kern-\nulldelimiterspace} {\rho_{\text{NiO}} }} \) is the molar volume of the NiO (m3·mole−1); k 1 and k 2 are the chemical reaction rate constants (mole·m−2·atm−1·s−1); Δμ is the chemical potential driving force for diffusion (J·mole−1); D is the diffusion coefficient of Ni cations (m2·s−1); R is the gas constant (J·mole−1·K−1); T is the absolute temperature (K); and δ/2 is the radius of the pores (m) formed at the interface, assuming cylindrical pores are formed. A similar equation of the growth mechanism of this steady-state situation has been proposed earlier by other researchers.[24] It can be seen that, for a given thermodynamic driving force, the rate of the chemical reaction will determine the pore size at the Ni-NiO interface. It is now possible to apply the previously described stability criteria to this situation.
Consider the situation for a fixed value of Δμ, in which the partial pressure of hydrogen in the gas mixture is decreased. Because in cooperative growth the chemical reaction rate and the cation diffusive flux are linked through Eq. [17], a reduction in the chemical rate will result in an increase in the pore diameter δ. At some value of \( p_{{{\text{H}}_{ 2} }} , \) the pore diameter will exceed λ c , the critical wavelength for stability of the interface given by Eq. [16]. At pore sizes greater than this critical value, the oxide surface is inherently unstable, i.e., the planar oxide surface will spontaneously move at different velocities at different points on the surface. The result will be the formation of instabilities or tunnels in the oxide; the resulting interface geometry is illustrated schematically in Figure 13. The onset of instabilities at the interface signals the end of the cooperative/coupled growth mechanism; the geometries of the instabilities become independent of the presence of the nickel metal.
The departure from the cooperative growth mechanism can also be the result of the increasing temperature of the reduction process. The activation energy of the solid-state diffusion processes is considerably greater than the chemical reaction. For example, the activation energy of the self-diffusion of Ni in NiO was found to be 191 kJ,[25] while the apparent activation energy of the chemical reaction obtained in this experimental work was 66.6 kJ. As a result, the changes in temperature will, in relative terms, affect the solid-state diffusion process to an extent greater than the chemical reaction. Based on Eq. [17], for a given interface velocity, an increase in the diffusion rate relative to the chemical rate will be compensated for by an increase in the pore diameter (δ); the increase in the pore diameter will eventually lead to the oxide surface becoming unstable, as explained earlier. The metallographic evidence demonstrating the increasing pore size of the planar coupled interface with increasing temperature and the transition from a fine porous nickel-planar interface to large porous nickel-irregular interface structures with increasing temperature is provided in previous work on this topic by the authors.[10]
The rate of growth of the pore tip of the perturbation or instability will, in each case, be determined by the diffusive flux of Ni2+ cations away from the free oxide surface and into the bulk oxide. Because the oxide surface is no longer planar, the diffusive fluxes move in all directions (three dimensions), depending on local concentration gradients. The pores may resemble linear or columnar tunnels or, if the tip of the perturbation becomes more distant from the nickel product, it is possible that the pore walls may also become unstable, forming side arms to the main instability. This latter structure closely resembles the analogous “dendritic” structures formed during alloy solidification.[22] In these scenarios, depending on the pore geometry, an increasing proportion of the available thermodynamic driving force for reaction is expended in driving the mass transport in the bulk solid oxide phase rather than the chemical reaction at the gas-solid interface. It is to be expected, therefore, that the overall rate of the reduction of the oxide will actually decrease relative to that expected from a purely “chemically controlled” rate as the result of the formation of interface instabilities at the oxide interface. In qualitative terms, this explains the observed slowdown in the apparent growth rate between 700 °C to 800 °C in 100 pct H2. In these cases, the nickel metal product is still formed on the oxide surface, but follows behind the tip of the perturbation.
Dense Nickel Layer
It has been shown[10] that, at low thermodynamic driving forces represented by gas mixtures containing low H2-H2O gas ratios, the oxide surface can become covered by a dense metallic nickel layer. If the conditions are maintained such that the dense Ni metal persists, then direct contact between the reducing gas mixtures and the oxide is lost and a catastrophic reduction in the overall rate of reduction of the oxide sample occurs. The detailed reaction mechanisms occurring in these situations are discussed in the following paragraphs.
A series of reduction experiments was carried out close to the limiting conditions between porous and dense Ni structures. Experiments were carried out at 800 °C using 12.5 pct H2-H2O. The micrographs of the reduction products are provided in Figures 14(a) through (d). It can be seen that, under this condition, the nickel nuclei are formed preferentially at the ledges of the faceted surface (Figures 14(b) and (c)). As the time progresses, the number of nuclei increase until the oxide surface is completely covered by nickel nuclei (Figure 14(d)).
The formation of a dense metal layer in the gaseous reduction of the metal oxide has been explained previously.[19] It was stated that, under atmospheres that have a low chemical driving force and in the presence of metal nuclei, chemical reaction rates on the metal surface are relatively faster than on the oxide surface. This condition promotes the removal of oxygen via the metal surface and, because this process is followed by the attachment of metal ions at the perimeter of metal nucleus, the growth of the metal nucleus predominantly occurs at the perimeter of the metal nucleus. If this continues to happen, the metal nucleus will spread on the surface of the original solid and the entire surface of the original solid will be covered by a dense metal product layer.
The experimental investigations carried out in the present study confirm that an individual metal nucleus may grow across the oxide surface by the attachment of cations at the perimeter of the nucleus; the evidence for this is shown by the circular nuclei observed in Figure 14. Apart from the lateral growth of metal nuclei, the results also suggest that the dense metal layer is promoted by a planar decomposition of oxide. As the planar decomposition of oxide slowly takes place, a number of metal nuclei are created (Figures 14(b) and (c)); the nuclei grow laterally and join together until the oxide surface is completely covered (Figure 14(d)).
The dense metal layer that covers the NiO surface prevents direct contact between the gaseous reactant and the oxide interface. The subsequent reduction of the oxide may only take place if the reactants (H or O) can diffuse through the dense metal product. If the O diffuses out through the dense layer from the metal-oxide to the metal-gas interface, there will not be any problem disposing of the gaseous H2O product from the reaction interface; however, when the H is the diffusing component, there will be issue with the disposal of steam produced from the reaction at the metal-oxide interface.
A two-stage reduction that involved both an initial reduction of NiO at 1000 °C in atmosphere that contains 5 pct H2-H2O for 30 minutes to form an initial dense nickel product on the surface (Figure 15(a)) and a subsequent reduction using 100 pct H2 (1 atm) was carried out to check the possibility of a subsequent reduction of NiO with a dense Ni product. It can be seen in Figure 15(b) that the introduction of pure hydrogen gas (1 atm) at the second stage of the reduction for 15 seconds results in the formation of bubbles on the metal surface. As the accumulation of steam under the dense metal layer proceeds, the rupture of the dense layer may occur, providing access for the reducing gas (Figures 15(c) and (d)). If the chemical driving force of the reducing gas is in the range of that required for the formation of porous structure, a secondary porous structure may be formed below the dense nickel layer. In Figure 16, it can be seen that the secondary porous structure develops extensively under the dense nickel layer. It appears that the passage for the reducing gas provided by the bursting of the dense metal layer is sufficient to create this extensive pore structure.
It has been demonstrated in the present study that the transitions in reaction mechanisms as a function of gas composition, temperature, and time are the result of relative changes in contributions from the elementary processes steps. Transitions in the reaction mechanism lead to changes in the reaction interface geometry and may influence the overall reduction rate. The present study shows that knowledge of the elementary processes is important in understanding the gaseous reduction of metal oxide.
Implications for Industrial Practice
A slowdown in the rate identified at temperatures above approximately 700 °C was observed during the reduction of Inco Goro NiO.[2] From the observations made in the present study, it appears likely that the slowdown in the reaction rate is associated with the large porous nickel-irregular interface structure. The transition from a continuous cooperative/coupled growth mechanism, in which simultaneous chemical reaction and mass transport processes occur, to a pore (instability) growth mechanism, in which the growth of its tips is determined by the rate of the solid-state diffusion of cations, appears to be the most likely explanation for the observed slowdown.
The entrapment of NiO by a dense Ni metal product during the reduction of BNC/NiO carried out in the BHP Billiton Yabulu refinery was reported by Rhamdhani et al.[26] The mechanism of formation of the dense metal layers is not straightforward. It was demonstrated that isothermal reduction at a high temperature and for a long time on its own was not sufficient to form the dense metal morphologies.[9,10] What was different about the studies carried out on the industrial BNC/NiO samples was that the reduction was carried out in nonisothermal conditions. It was shown by Rhamdhani et al.[3] that, under the conditions used, the reduction of NiO to Ni started at temperatures as low as 300 °C; it was only when temperatures reached 700 °C to 800 °C that the reaction was halted by the formation of a dense Ni product. The reason why the formation of this dense metal layer only occurs on nonisothermal reduction is now clearer, as a result of the present study. At low temperatures, the product microstructures are very fine, with nickel metal sheets of spacing 10 to 20 nm being formed. These structures are highly unstable and, as the temperature is progressively increased, they undertake a series of transformations driven by the high surface energy, curvatures, and defect concentrations in the product. The evolution of the microstructure leads to the densification of the Ni product and results in the encapsulation of the oxide.
The phenomenon explained here should not be confused with the dense nickel layer that can be formed at all temperatures as a result of the reduction process being carried out with a weakly reducing atmosphere. The coverage of the oxide surface with metal will, in this case, lead to the drastic lowering of the reduction rate, but the origins of these microstructures are very different.
The present study shows that there are many parameters that need to be considered in order to obtain a high-quality nickel product in the hydrogen reduction of NiO. As identified by Rhamdhani et al.[3,26] for a complete removal of oxygen at a relatively fast rate, it is recommended that the reactions are carried out by the following:
-
(a)
reducing at temperatures below 700 °C;
-
(b)
maintaining high concentrations of hydrogen in the atmosphere and ensuring the delivery of fresh reducing gas to the reaction interface; and
-
(c)
avoiding a slow heating rate in reducing atmospheres.
Summary
A detailed analysis has been undertaken on the kinetics of the reduction of dense nickel oxide by hydrogen in the temperature range between 500 °C and 1000 °C. It has been shown that the gas-film mass transfer and gas diffusion through the porous product cannot account for the observed anomalous low reaction rates in the temperature range 700 °C to 1000 °C. It is argued that the decrease in the rate in these conditions is associated with a breakdown in the cooperative/coupled growth mechanism. The transitions in the product microstructures between a coarse fibrous nickel with fissures, a porous planar (cooperative) growth, a porous-irregular interface, and a dense metal product are explained in terms of the relative rates of the chemical reactions and mass transport processes at the Ni-NiO interface.
Notes
PHILIPS is a trademark of Philips Electronic Instruments Corp., Mahwah, NJ.
JEOL is a trademark of Japan Electron Optics Ltd., Tokyo.
References
J.G. Reid and J.E. Fittock: Int. Laterite Nickel Symp., TMS, Warrendale, PA, 2004, pp. 599–618.
T.A. Utigard, M. Wu, G. Plascencia, and T. Marin: Chem. Eng. Sci., 2005, vol. 60 (7), pp. 2061–68.
M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 218–33.
J.T. Richardson, R. Scates, and M.V. Twigg: Appl. Catal. A-Gen., 2003, vol. 246, pp. 137–50.
W. Pluschkell and B.V.S. Sarma: Arch. Eisenhuttenwes., 1974, vol. 45 (1), pp. 23–31.
A.H. Rashed and Y.K. Rao: Chem. Eng. Commun., 1996, vol. 156, pp. 1–30.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 1–16.
J. Szekely and J.W. Evans: Chem. Eng. Sci., 1971, vol. 26, pp. 1901–13.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Miner. Eng., 2008, vol. 21 (2), pp. 157–66.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2009. doi:10.1007/s11663-009-9247-x
J. Szekely, J.W. Evans, and H.Y. Sohn: Gas-Solid Reactions, Academic Press, Inc., New York, NY, 1976, pp. 10–22.
T. DebRoy and K.P. Abraham: Physical Chemistry of Process Metallurgy: The Richardson Conf., Institute of Mining and Metallurgy, London, 1973, pp. 85–93.
C.N. Satterfield: Mass Transfer in Heterogeneous Catalysis, MIT Press, London, 1970, pp. 12–46.
O. Levenspiel: Chemical Reaction Engineering, John Wiley & Sons, Inc., New York, NY, 1999, p. 579.
W. Pluschkell and B.V.S. Sarma: Arch. Eisenhuttenwes., 1973, vol. 44 (1), pp. 161–66.
S.P. Matthew and P.C. Hayes: Metall. Trans. B, 1990, vol. 21B, pp. 153–72.
T.R. Wilken, W.R. Morcom, C.A. Wert, and J.B. Woodhouse: Metall. Trans. B, 1976, vol. 7B, pp. 589–97.
D.H. St. John, F. Nakiboglu, and P.C. Hayes: Metall. Trans. B, 1986, vol. 17B, pp. 383–93.
S.P. Matthew, T.R. Cho, and P.C. Hayes: Metall. Trans. B, 1990, vol. 21B, pp. 733–41.
D.H. St. John, S.P. Matthew, and P.C. Hayes: Metall. Trans. B, 1984, vol. 15B, pp. 709–17.
K.R. Lilius: Acta Polytech. Scand., 1974, vol. 118, pp. 1–17.
W. Kurz and D.J. Fisher: Fundamentals of Solidification, Trans Tech Publications, Aedermannsdorf, Switzerland, 1998, pp. 45–58.
C.H.P. Lupis: Chemical Thermodynamics of Materials, Elsevier Science Publishing Co., Inc., New York, NY, 1983, pp. 347–84.
B. Ilschner: Z. Metallkd., 1964, vol. 55, pp. 153–62.
J.S. Choi and W.J. Moore: J. Phys. Chem., 1962, vol. 66 (7), pp. 1217–1376.
M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 234–45.
O. Hirschfelder, C.F. Curtiss, and R.B. Bird: Molecular Theory of Gases and Liquids, John Wiley & Sons, Inc., New York, NY, 1967, p. 1110.
Acknowledgments
The authors thank the BHP Billiton Yabulu refinery and the Australian Research Council Linkage program (Grant number: LP0562201) for financial support, and AusAid for providing a scholarship. The authors also acknowledge Mr. John Fittock and Dr. Joy Morgan (BHP Billiton Yabulu) for their valuable help and critical discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 2, 2008.
Appendix
Appendix
In the present study, the combined diffusivity of the molecular and Knudsen diffusion is calculated from the following relationship:[13]
The Knudsen diffusivity of H2 is determined based on the following equation:[13]
where r is the radius of pores in the metal product, T is the temperature, and \( M_{{{\text{H}}_{ 2} }} \) is the molecular weight of hydrogen gas. While the binary diffusion coefficient of gases \( \left( {D_{{{\text{H}}_{ 2} K\text{-}{\text{H}}_{ 2} {\text{O}}}} } \right) \) is calculated using the Chapman–Enskog relation:[13]
where \( D_{{{\text{H}}_{ 2}\text{-}{\text{H}}_{ 2} {\text{O}}}} \) is the binary gas diffusion coefficient (m2·s−1), \( M_{{{\text{H}}_{ 2} }} \) and \( M_{{{\text{H}}_{ 2} {\text{O}}}} \) are the molecular weights of the two species, P is the total pressure (atm), \( \Upomega_{{{\text{H}}_{ 2}\text{-}{\text{H}}_{ 2} {\text{\rm O}}}} \) is the collision integral, and σ is the force constant in the Lennard–Jones potential function (Å). The parameters used in the calculation are provided in Tables II and III.
Rights and permissions
About this article
Cite this article
Hidayat, T., Rhamdhani, M.A., Jak, E. et al. On the Relationships between the Kinetics and Mechanisms of Gaseous Hydrogen Reduction of Solid Nickel Oxide. Metall Mater Trans B 40, 474–489 (2009). https://doi.org/10.1007/s11663-009-9239-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-009-9239-x