Abstract
The corrosion behavior of selective laser melted Ti–6Al–4V alloy (SLM Ti–6Al–4V) was assessed in 0.1 M lactic acid + 0.1 M NaCl environment (pH 2.5) after 1150 hours of immersion at 37 °C and was compared with that of wrought Ti–6Al–4V alloy. Corrosion potential Ecor (Ecor = 0.083 ± 0.02 V) and corrosion current icor (icor = 0.145 ± 0.05 μA cm−2) of SLM Ti–6Al–4V alloy, estimated from anodic polarization tests, are under minimum recommended values for biomaterial surgical applications. Based on open-circuit potential (OCP) investigations, one may infer that this good corrosion resistance is due to the formation of a fast and stable protective oxide layer. According to X-ray photoelectron spectroscopy (XPS) results, this protective oxide layer is mainly formed from TiO2. From electrochemical impedance spectroscopy (EIS) investigations, a slightly lower corrosion resistance was observed at SLM Ti–6Al–4V alloy compared to the wrought one. Thus, the R1 associated with barrier film resistance of SLM Ti–6Al–4V is 339.1 kΩ cm2 whereas that one of wrought Ti–6Al–4V is 780.1 kΩ cm2. These results are conspicuous ones because they point out that SLM technique, which allows obtaining easily customized implants without expensive costs, is a valid alternative for obtaining new alloys for medical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium and its alloys are recognized as metallic biomaterials with high corrosion resistance and biological affinity in the environment of body fluids and human tissues. The excellent properties of these alloys are mainly due to the self-passivation and re-passivation ability of the metal surface.[1,2,3] Among the different alloys employed for dental and orthopedic implants, the Ti–6Al–4V might be regarded as an excellent candidate.[4]
It is well known that this alloy might be processed by conventional techniques, as well as by the additive manufacturing (AM) techniques.[5] By using this latter method, the Ti–6Al–4V alloys with a porous microstructure are obtained. According to literature, this peculiar morphology of the alloys appears to provide higher corrosion resistance and better mechanical properties compared to those ones obtained by classical technologies.
Furthermore, by using traditional techniques, the Ti–6Al–4V products with complex shapes cannot be obtained without subsequent machining, resulting inevitably in a large amount of material waste, high manufacturing cost, and long lead time.[6] Conversely, by employment of the AM techniques, such as the SLM method, customized implants, like bones, dentals, and skulls with different shapes,[7] can be achieved relatively facilely without involving secondary machining process.
Moreover, as a variety of AM techniques are available, the features of the alloys used for biological implants might be more easily tailored for a specific target compared to the classical one. Thus, in the case of Ti–6Al–4V alloy, its properties (e.g., high strength-to-weight ratio, fatigue resistance, biocompatibility, low elastic modulus, good corrosion resistance) which are of great importance for their use as implants (e.g., for the repair of bone tissue), can be easily tuned by SLM method. Apart from this, due to the microstructural variations induced by the manufacturing process, the corrosion behavior of SLM Ti–6Al–4V might be affected and different than the wrought.[8]
In order to tailor the properties of Ti–6Al–4V alloy for a certain implant, the biological environment should not be disregarded because it has a major influence on the biomaterial properties. In this respect, many researchers[8,9,10] have studied the corrosion behavior of Ti–6Al–4V in biofluids with different chemical compositions, and they noticed that the degradation of these alloys is generated by some chemical species present in the biofluid (like calcium chloride, fluoride ions, lactic acid). For example,[11] the presence of fluoride ions in artificial saliva (AS) yields the formation of a small amount of HF (hydrogen fluoride) in the solution that subsequently interacts with TiO2 film, leading to the formation of TiF4. This chemical dissolution of the titanium oxide film exposes the substrate to the solution attack, yielding a significant decrease of the resistance corrosion of Ti–6Al–4V. A similar phenomenon was observed in the presence of CaCl2 in AS. Thus, the corrosion process begins with the complexation of the oxide film with Cl− ions. The [Ti(OH)3]Cl complex, hence formed, locally decreases the pH, and an acceleration of the oxide film dissolution is likely to be observed.
Lactic acid, which is secreted by the bacteria present in oral cavity, has a significant role in the corrosion behavior of Ti–6Al–4V dental alloy. The influence of lactic acid on corrosion processes of dental alloy was also studied,[8,12] and the results revealed a decrease of corrosion resistance in this environment. The literature reports attest that the presence of lactic acid in AS induces a low pH in the biofluid environment that inherently reduces the corrosion resistance of the titanium dental implants. This lower corrosion resistance observed at Ti–6Al–4V alloy, in the presence of lactic acid, mainly originates from the reaction of lactate ions (L−) with the hydrated titanium oxide [Ti(OH)3]Cl in the presence of chloride ions.[8]
In this context, the corrosion behavior of SLM Ti–6Al–4V was investigated in 0.1 M lactic acid + 0.1 M NaCl solution at 37 °C after 1150 hours of immersion. For comparison, the corrosion behavior of Ti–6Al–4V, obtained by wrought technique, was studied under the same experimental conditions. To our knowledge, there are very scarce reports about the corrosion behavior of SLM Ti–6Al–4V in the presence of lactic acid. Keeping in mind that the corrosion processes are slow ones, we consider that, by investigating the corrosion resistance of SLM Ti–6Al–4V after long time immersion, more accurate information about its corrosion behavior might be gained. No data about the corrosion resistance of this material, obtained by SLM method after long time immersion (more than 30 days), were reported. However, there are reports[1,8,13] about the corrosion behavior of Ti–6Al–4V obtained by both types of techniques after short time immersion. The results pointed out that the titanium passive oxide film is unstable in the presence of lactic acid, leading to the decline of the alloy corrosion resistance. In order to better understand the corrosion behavior of SLM Ti–6Al–4V, the structural, wettability, and chemical composition of the samples were investigated as well.
2 Experimental
2.1 Specimens Preparation
Two types of Ti–6Al–4V alloy specimens were used in this study: one was manufactured by selective laser melting (SLM Ti–6Al–4V) and the other in the wrought state (wrought Ti–6Al–4V) as a control.
An EOS EOSINT M270 laser-sintering machine was used to manufacture parts from gas atomized Ti6Al4V powder under a high-purity Ar atmosphere. Small cubes (10 mm × 10 mm × 10 mm) were manufactured using a laser power (at the part bed) of 200 W (λ = 1.06 µm), with a laser beam diameter of 35 µm, and a laser scan speed of 1250 mm s−1. The hatch spacing (distance between scan lines) and the layer thickness were 100 µm. The direction of scanning was rotated through 90 deg between successive layers. In terms of chemical composition, the alloys belong to the same class of Ti–6Al–4V alloys and the small deviations are within the limits allowed by the product standard for Titanium grade 5[14] (Table I).
The wrought Ti–6Al–4V specimens were cut with dimensions of 30 × 10 × 5 mm from a bar. Both types of specimens, wrought and cubes produced by SLM, were successively sanded with SiC paper up to 1200 grit, and finally, they were ultrasonically cleaned in deionized water and rinsed in ethanol.
2.2 Surface Characterization
2.2.1 Microstructural characterization
The structural analysis of specimens was performed using an F50 Inspect SEM (FEI Company, Brno, Czech Republic) equipped with an energy-dispersive X-ray spectrometer EDAX APEX 2i with SDD Apollo X detector. To highlight the differences in the chemical composition of the α and β phases of the Ti sample, backscattered electron images (vCD detector—low voltage high contrast detector) were used, while for the investigation of the microstructural features, secondary electron images (ETD detector—Everhart–Thornley detector) were used.
The microcompositional analysis was performed by scanning electron microscopy (SEM)–energy-dispersive X-ray spectroscopy (EDS) on the backscattered electron images acquired on the specimen’s surface. The qualitative analysis was performed using an acceleration voltage of 30 kV, spot size of 4 nm, working distance of 10 mm, and an acquisition of 60 seconds.
2.2.2 Surface chemistry
X-ray photoelectron microscopy (XPS) measurements were performed on a K Alpha spectrometer from Thermo Fisher, equipped with a monochromated X-ray Source (Al Kα, 1486.6 eV) with a spot size of 400 µm. The hemispherical analyzer was operated in CAE (constant analyzer energy) mode, with pass energy of 200 eV and a step of 1 eV for the acquisition of surveys spectra, and pass energy of 50 eV and a step of 0.1 eV for the acquisition of narrow spectra. A “dual beam” flood gun was used to neutralize the charge build-up. In order to avoid contamination, before the XPS analysis, all samples were ultrasonically cleaned in distillated water, acetone, and ethanol and subsequently dried under a cold air stream. The spectra obtained were analyzed by means of the Casa XPS software. A Shirley-type background subtraction was used, and the peak areas were normalized using the Scofield sensitivity factors. The binding energies were calibrated against the C 1s binding energy set a 284.8 eV. The peaks were analyzed using mixed Gaussian–Lorentzian curves (70 pct of Gaussian character).
2.2.3 Surface wettability
The surface hydrophobicity was characterized using Drop Shape Analysis System apparatus, DSA1 model (FM40 Easy Drop) from KRÜSS GmbH Germany for the water contact angle (WCA) measurements. The samples were placed on a plane support, and drops of deionized water with a volume of 3 µL each were put on the surface with a dispensing micro-syringe. The contact angle values were collected in static regime at room temperature, initially and at 30 seconds after the drop of water was placed on the sample surface. Static contact angle was recorded using Sessile Drop Fitting method for the angles in between 30 and 90 deg and Circle Fitting method for those less than 30 deg. Over seven measurements were averaged for each sample.
2.3 Electrochemical Characterization
Both types of specimens, SLM and wrought Ti–6Al–4V alloy, were tested after 1150 hours of immersion in 0.1 M lactic acid and 0.1 M NaCl solution, (pH 2.5) at 37 °C, to evaluate their electrochemical behavior and corrosion resistance. All electrochemical measurements were carried out in a conventional three-electrode cell using a potentiostat/galvanostat PARSTAT 4000. Ti–6Al–4V alloy specimens were employed as working electrodes, a platinum sheet as counter electrode, and Ag/AgCl as a reference electrode. The surface area of the SLM and wrought Ti–6Al–4V alloy used as working electrodes was 2.0 and 3.0 cm2, respectively. All reagents were analytical grade (Sigma-Aldrich), and the solutions were prepared using bidistilled water.
Electrochemical impedance spectroscopy (EIS) was carried out at the OCP potentiostatically, with an AC amplitude of 10 mV over the frequency range of 100 kHz to 0.01 Hz, after 1150 hours of exposure in artificial biofluid. Potentiodynamic polarization was accomplished in the potential range from − 0.25 V vs. OCP to + 2.5 V vs. Ag/AgCl, with a scan rate of 1 mV s−1. CorrView and ZView specialized software were used to analyze the electrochemical parameters (Ecor, icor) and EIS data. Long-term monitoring of open-circuit potential (OCP) was performed according to the static immersion method.[15] The stationary potential, corresponding steady-state spontaneously established at the interface between the Ti–6Al–4V surface and the solution (0.1 M lactic acid + 0.1 M NaCl), was monitored for 1150 hours. The samples were immersed in a volume of 60 mL at 37 °C in a Thermocal 20 incubator. Potential values were recorded every 10 minutes in the first hour of immersion, then every hour for 10 hours, then daily. The volume of the solution was kept constant by the addition of warm distilled water.
In order to certify the reproducibility, three identical samples of each material (SLM Ti–6Al–4V and wrought Ti–6Al–4V) were tested.
3 Results and Discussions
3.1 Surface Morphology (SEM)
Although AM is one of the most promising and interesting technologies for many areas, such as medicine, the AM technique could bring out a series of issues, like the microstructural inhomogeneity in the build of material.[16] Therefore, an analysis of the surface morphologies of wrought and SLM Ti–6Al–4V alloys was performed. The SEM micrographs of specimens were recorded after etching the samples in Kroll's reagent.[17]
In order to establish whether the alpha and beta phases are present within the microstructures of samples (Figure 1), the EDS method in backscattered electron signal was used. It is well known that the Al and V stabilize α and β phases of Ti–6Al–4V alloy, respectively. Based on the fact that the mean atomic number (Z) of each different phase appears as a discrete variation in gray level, the phases might be distinguished from backscattered electron images of Ti–6Al–4V specimens. Thus, in the present case, α phase appears darkest whereas the β phase appears brightest. From SEM images, it appears that the wrought and SLM Ti–6Al–4V alloys show bi-phasic composition, i.e., α and β phases (Figure 1).[18,19,20]
However, a major difference in the microstructural morphology of these two types of specimens was observed (Figures 1(a) and 1(b)). The wrought material has an α phase epitaxial grain structure, with scattered β phase islands, while the specimen obtained by SLM has a microstructure consisting of α phase fine laths separated by linearly interphase arranged β phase islands. Moreover, it appears that the concentration of β phase is higher at SLM Ti–6Al–4V. These results are in line with the literature reports,[8,18] which highlighted a restriction of α phase and an extended β phase formation as a result of a high cooling rate during the SLM process.
3.2 Surface Chemistry (XPS)
It is well documented[18,21] that Ti–6Al–4V alloy obtained by classical technologies and AM techniques could show compositional differences in the surface, depending on the technology. Therefore, XPS investigations were carried at wrought and SLM Ti–6Al–4V specimens after their immersion in 0.1 M (lactic acid + NaCl) solution at 37 °C for 1150 hours. The survey spectra of wrought and SLM Ti–6Al–4V, illustrated in Figures 2(a) and (c), indicate mainly the presence of carbon, oxygen, nitrogen, titanium, aluminum, and vanadium on the surface of both specimens. The constituents of the wrought surface are 25.1 pct Ti 2p, 2.1 pct Al 2p, 0.28 pct V 2p, 35.3 pct O 1s, 35.0 pct C 1s, and 1.22 pct N 1s while the constituents of the SLM surface are 21.16 pct Ti 2p, 1.96 pct Al 2p, 0.68 pct V 2p, 32.59 pct O 1s, 36.7 pct C 1s, and 6.4 pct N 1s. These results point out that Ti, O, and C prevail on the surface of both specimens. Moreover, the ratios of Ti:O (0.71 for wrought Ti–6Al–4V and 0.64 for SLM Ti–6Al–4V) are cca. 11 times higher than Al:O (0.06 for wrought and SLM Ti–6Al–4V), regardless of the type of specimen. These findings, which are in good agreement with other reports,[16] suggest that at both types of specimens, a surface film, composed mainly from titanium oxide, is formed.
High-resolution spectra of both specimens were recorded in the Ti 2p region (Figure 2), and the parameters are summarized in Table II. The Ti 2p spectra of both specimens were fitted by assuming four doublets and assert the presence of Ti 2p metallic, TiO, Ti–OH,[22] and TiO2 species (see Table II). From the relative fractions of these chemical species, depicted in inset Figures 2(b) and (d), one may consider that titanium mainly prevails on the surface as TiO2 at both types of samples, i.e., 82.38 pct for wrought Ti–6Al–4V and 79.6 pct for SLM Ti–6Al–4V.
Small amounts of the Ti metallic, TiO, and Ti–OH species are also present on the surface of both specimens (see insets Figures 2(b) and (d)). Thus, one may conjecture that the Ti–6Al–4V alloy is covered by a protective layer originated mainly from TiO2. Based on these findings (see inset Figures 2(b) and (d)), it appears that, in fact, the technology used for obtaining the Ti–6Al–4V does not bring out significant changes in the chemistry of the protective layer formed on the alloy surface during its immersion.
3.3 Surface Wettability
According to data reports,[23] protein and cell adhesion, proliferation, and calcification are mainly influenced by the surface properties of implants, such as surface wettability. Since surface wettability offers information about how easily the implant is integrated into the biological tissue, the wettability of Ti–6Al–4V surface will be explored by WCA. From the wettability properties of material, information about the roughness of material can be gained because the wettability is significantly influenced by the material roughness.[24] Therefore, a material which has a rough surface shows a high WCA as well.
From WCA values, by using Eq. [1], the thermodynamic work of adhesion, Wa, was estimated as well. This parameter also brings out information about the surface of a biomaterial in terms of wettability.
where γL is the liquid surface tension and θ is the measured contact angle. For distilled water γL = 72.86 mN m−1 at 20 °C.[25] Calculating the work of adhesion for water, one can attain information about changes in the hydrophilic/hydrophobic properties of the surface. According to Eq. [1], the water adhesion increases with decreasing the contact angle of the studied surfaces (see Table III).
The contact angle measurements of Ti–6Al–4V, obtained by wrought and SLM techniques in 0.1 M lactic acid and 0.1 M NaCl solution, were carried out and the results are presented in Table III. Before immersion, at SLM Ti–6Al–4V surface, the WCA is 94.5 ±1.5 deg (Table III) whereas at wrought Ti–6Al–4V, the WCA is 84 ± 2 deg (Table III), revealing that SLM Ti–6Al–4V surface is hydrophobic compared to that one of wrought Ti–6Al–4V which is hydrophilic. It appears that the laser-sintering method provides a rough surface of material, leading to a slight hydrophobicity of it. These findings could raise some inquiries about the possibility of using SLM Ti–6Al–4V for implant applications.
In order to have a clear response, the WCA of both types of specimens was measured after 1150 hours of immersion as well. From comparison of WCA obtained for SLM Ti–6Al–4V specimens, before and after immersion, a significant decrease of WCA from about 94.5±1.5 to 70.7 ± 2.20 deg, is noticed (Table III). This behavior reveals that after 1150 hours of immersion, the wettability of surface dramatically changes from a hydrophobic surface to a hydrophilic one. One may assert that the presence of oxide layer formed during immersion significantly decreases the hydrophobicity of SLM Ti–6Al–4V, making them suitable for medical applications.
Conversely, at wrought Ti–6Al–4V, a slight decrease of WCA is observed from 85.4 ± 1.5, before immersion to 73.7 ± 1.8, after immersion (Table III), suggesting that the wettability properties of the surface modestly change during the immersion process. These results reveal that at this specimen, the oxide layer grown on surface, during 1150 hours of immersion, does not significantly amend the condition of the surface alloy compared to SLM Ti–6Al–4V where dramatic changes have been evidenced. We may presume that the oxide film formed on the wrought Ti–6Al–4V surface does not bring out changes in the roughness of the surface and wettability as well.
These observations are also well highlighted from the adhesion strength parameter calculated for both specimens, before and after immersion. As it can be seen in Table III, at SLM Ti–6Al–4V specimen, the adhesion strength significantly enhances, i.e., 33 pct, after immersion whereas at wrought Ti–6Al–4V, a slight increase of the adhesion strength is noticed, i.e., 15 pct after immersion.
In conclusion, the contact angle measurements, performed after 1150 hours of immersion, clearly point out that both types of specimens show similar moderate hydrophilicity (Table III) which make them suitable for surgical applications.
3.4 Electrochemical Characterization
The corrosion behavior of surgical implants, and of all devices that come in contact with living tissue, is particularly important in terms of the effects that ions or molecules, released by these devices, can have on biological fluids. Therefore, the corrosion behavior of titanium alloys is strongly influenced by many factors like chemical composition, microstructure, and the thermo-mechanical history of the alloy and the biological environment as well.[26] In this respect, different electrochemical techniques like the OCP monitoring, EIS, and anodic polarization methods were employed for studying the corrosion behavior of wrought and SLM Ti–6Al–4V alloys.
3.5 OCP Evolution
The OCP of a surface is an important parameter which offers information about the thermodynamic tendency of those materials to electrochemically corrode with the surrounding medium. It is well known that chemical systems might not be at equilibrium even at OCP. Therefore, in time, the OCP might not remain constant, and its change most probably originates from the reactions that occur on different regions of the surface. According to the Pourbaix diagrams,[27] the OCP evolution toward the electropositive values is a sign of thermodynamic stabilization of the surface by passivation whereas its evolution toward the electronegative values is a sign of the surface activation.
Figure 3 illustrates the OCP evolution of both specimens, during 1150 hours of immersion. As can be seen in Figure 3, both specimens show the same passivation tendency in the first 200 hours of immersion, i.e., the potentials shift with more than 300 mV toward electropositive values. In the first 100 hours of immersion, a relative fast change of the potential, i.e., cca. 3 mV h−1, toward electropositive values is observed (Figure 3), suggesting a fast rate of the oxide formation on the surface of the specimens. Based on literature reports and our XPS findings, one may assert that this oxide layer is mainly titanium oxide. Taking into account that this protective oxide layer is relatively fast formed on surface of both specimens, one may assert that their utilization for medical implants is a good option.
After 200 hours, the OCP remains relatively constant for both specimens, revealing that the oxide layer formed on both types of surface is stable. As Figure 3 illustrates, the OCP is shifted with 60 mV towards positive potentials at SLM Ti–6Al–4V, compared to wrought one (i.e., from 170 ± 10 mV for SLM Ti–6Al–4V to 100 ± 10 mV for wrought Ti–6Al–4V), suggesting that at SLM Ti–6Al–4V, the dissolution of the oxide layer is slightly sluggish. However, all OCP values remain in a domain in which TiO2 is thermodynamically stable[27] pointing out that the oxide layer formed on the surface hampers the corrosion processes.
Based on these findings, which revealed that the oxide layer provides protective properties of surface against corrosion at both specimens, it is quite plausible to assume that these types of alloys are good candidates for different medical applications.
3.6 Electrochemical Impedance Spectroscopy
In order to better understand the corrosion behavior of SLM Ti–6Al–4V alloy, the EIS investigations, in 0.1 M NaCl + 0.1 M lactic acid, were performed potentiostatically at OCP, after 1150 hours of immersion. For comparison, the EIS investigations at wrought Ti–6Al–4V were carried out.
From Bode representation, within the frequency range from 0.5 to 0.01 Hz, an increase of impedance Z at wrought Ti–6Al–4V compared with SLM Ti–6Al–4V was observed (Figure 4(a)), suggesting better resistance corrosion at wrought Ti–6Al–4V.
From phase angle plots (Figure 4(b)), a maximum value of phase angle of 82 deg was found regardless of the type of specimen. This behavior indicates that the oxide film formed on the surface during immersion shows an almost capacitive response.[28,29]
From Nyquist plots of SLM and wrought Ti–6Al–4V (Figure 4(c)), an incomplete semicircle, regardless of the type of sample, can be observed. We consider that an equivalent electrical circuit (EEC) with one time constant, which takes into account the capacitive and charge transfer characteristics of the oxide layer formed on alloys, is appropriate for fitting the impedance spectra.[8] Therefore, the EEC, shown in inset Figure 4(c), was used to fit the impedance spectra (Figure 4), and the values of the EEC elements for all samples are exhibited in Table IV. As a certain degree of electrode roughness and inhomogeneity is expected to be observed due to the complex structure of the oxide layer formed on Ti-based alloys,[28,30] a constant phase element (Q1) instead of a capacitance[31] was more suited for describing the capacitive characteristic of the oxide layer.
As a general remark, one may infer that a high barrier resistance of the film (a high value of R1) indicates a very good surface protection against corrosion processes.[26] From the fitted results (Table IV), one may state that the R1 of wrought Ti–6Al–4V, i.e., 780.1 kΩ cm2, is cca. two times higher than that of SLM Ti–6Al–4V, i.e., 339.1 kΩ cm2. These findings, which are in line with other reports,[8,32] revealed a relative better corrosion resistance of the film formed on wrought Ti–6Al–4V, indicating that at this type of alloy, the rate of oxide layer dissolution is slightly lower. The literature reports[26] attested that the alloys with high porosity exhibit a low resistance of the film and high value of the constant phase element of the film. Therefore, one may assume that the lower R1 and higher Q1, evidenced at SLM Ti–6Al–4V specimen compared with wrought one (Table IV), could be the result of a higher porosity of the SLM Ti–6Al–4V which inherently endorses the corrosion attack. Actually, higher porosity of the SLM Ti–6Al–4V material is not unexpected to be observed because, according to some reports,[26] the SLM process induces certain porosity in the structure of the material.
However, one may consider that both types of samples show appropriate good corrosion resistance because the R1 value of both types of samples is of the order of hundreds of kiloohm (Table IV). These results are conspicuous ones because in our experimental conditions, in which the alloys are immersed for 48 days in an aggressive environment (pH 2.5), the corrosion resistance of the film formed on Ti–6Al–4V is comparable with that one reported in the literature[1,8,32] for other Ti–6Al–4V alloys in similar aggressive environment but immersed for a shorter period of time (i.e., around 2 weeks). It is well known that, in lactic acid and NaCl solution, a dissolution of the TiO2 layer occurs and this process is controlled by the presence of both chloride (Cl−) and lactate (L−) ions.[1,8]
These results highlight that both specimens show good surface protection, suggesting that both alloys are good candidates for dental implant applications.
3.7 Anodic Polarization
Anodic polarization of wrought and SLM Ti–6Al–4V was performed after 1150 hours of immersion in lactic acid and sodium chloride solution at 37 °C, and the results are shown in Figure 5 and Table V.
Based on the previous XPS, OCP, and EIS results, a protective oxide film, which inhibits corrosive attack of the environment, was supposed to be formed on both types of alloys.
At SLM Ti–6Al–4V, a current density (i) less than 25 µA cm−2 was noticed between 0.083± 0.02 and 1.015 ± 0.03 V vs. Ag/AgCl (see Figure 5 and Table V), suggesting that in this potential range, the protective oxide film remains stable. According to XPS results, this protective oxide layer is mainly formed from TiO2. At potentials higher than 1.015 V, the current density significantly increases up to 950 µA cm−2. These observations (Figure 5) indicate that, at potentials higher than 1.015 V, a dissolution of this protective film takes place because of the presence of lactic acid and the high acidity of the solution. Based on Pourbaix diagrams,[27] one may assume that the dissolution of this oxide film arises from activation of the Ti–6Al–4V surface.
Conversely, the protective oxide layer formed on wrought Ti–6Al–4V surface remains stable in a potential range of about 1.62 V, i.e., from − 0.097 to + 1.520 V, in which the current density is less than 25 µA cm−2. Despite the fact that the stationary potential of this sample slightly moves to electronegative values (Figure 5), the anodic polarization tests point out that the oxide layer remains stable in a higher potentials range at wrought Ti–6Al–4V compared to SLM Ti–6Al–4V. At potentials higher than 1.52 V, a slight increase of current densities was observed, i.e., up to 57 µA cm−2 (Figure 5), at wrought Ti–6Al–4V surface, as well. As this increase of current densities is much insignificant at wrought Ti–6Al–4V sample, i.e., around nine times lower, compared with SLM Ti–6Al–4V, one may assert that the oxide film formed on wrought Ti–6Al–4V surface is more stable.
The corrosion potential (Ecor) and the corrosion current (icor) of both types of samples were estimated from the extrapolation of the Tafel curves (inset Figure 5). The Ecor of SLM Ti–6Al–4V is 0.083 ± 0.02 V vs. Ag/AgCl, and the Ecor of wrought Ti–6Al–4V is − 0.097 ± 0.03 V vs. Ag/AgCl. Since no significant changes in Ecor are observed at both types of samples, no reliable information about the oxide layer stability and corrosion resistance might be advanced from this corrosion parameter. The potential range, in which the dissolution of oxide layer is hampered, is lower at SLM Ti–6Al–4V, i.e., ΔE = 930 ± 5 mV, compared to wrought Ti–6Al–4V, i.e., ΔE = 1620 ± 5 mV (Table V), pointing out that the wrought Ti–6Al–4V provides better corrosion resistance. These findings are supported by the EIS results which revealed higher barrier film resistance of wrought Ti–6Al–4V alloy.
Based on the literature reports,[30] which attest that the presence of a lower amount of α phase induces lower resistance corrosion of the material in acidic media, one may consider that slowing down of the passivation process observed at SLM Ti–6Al–4V could be also due to the presence of a larger content of β phase in the SLM Ti–6Al–4V alloy structure (see Figure 1). This phenomenon was also reported by Hamza et al.[8] for SLM Ti–6Al–4V in AS with lactic acid.
Although these preliminary results show that wrought Ti–6Al–4V has slightly better protective properties, from icor determined for both samples, which is less than 1 µA cm−2 (see Table V), one may undoubtedly state that both types of alloys are perfectly resistant from corrosion point of view.[33]
These results could be considered as promising ones because in our aggressive biofluid (pH 2.5) icor is lower than those reported for other Ti–6Al–4V alloys, obtained by cast[34] (i.e., i = 0.206 µA cm−2), EBM[35] (i.e., i = 1.5 µA cm−2) and SLM[32] (i.e., i = 3.42 µA cm−2) methods, in biofluids less aggressive. It appears that the corrosion resistance of these biomaterials is a very good one in spite of the fact that the corrosion tests were performed after long time immersion in an aggressive media. It is well known that an aggressive environment, which contains a high concentration of oxidizing species such as chloride ions (Cl−), promotes the corrosion processes, leading to a lower corrosion resistance of biomaterials.[8,32] This good resistance corrosion might also reside in the appropriate hydrophilicity character of the oxidized material. The wettability investigations revealed that the hydrophilicity of these two types of alloys is a moderate one (see Table III).
These results clearly point out that although wrought Ti–6Al–4V shows slightly better corrosion resistance, as attested from EIS and anodic polarization tests, both types of samples might be successfully used for medical implant. Considering that the SLM technique used for the manufacturing of Ti–6Al–4V alloy has a tremendous advantage compared with the classical ones, as, for example, porous structures with the complex geometry are obtained in a short time with minimal waste, one may conjecture that SLM Ti–6Al–4V alloy, including the one used by us, might be regarded as the next generation of biomaterials. In this respect, detailed researches about their use as implants are required, such as corrosion resistance, mechanical properties, and biocompatibility studies. We consider that the results herein presented bring new light on the possibility of using SLM Ti–6Al–4V for medical implants which can open new perspectives in the fabrication of implants with great importance in this field.
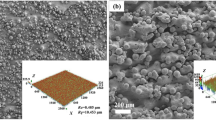
3.7.1 Surface morphology after corrosion tests
From the EDS chemical analysis of Ti–6Al–4V alloys non-immersed (Table I) and after immersed in lactic acid solution (Table VI), the presence of oxygen and carbon elements was observed after the immersion. It can be also noticed a decrease of weight percentage (wt pct) of titanium element after the exposure of the alloys to this aggressive environment. These findings indicate that on the surface of these Ti–6Al–4V alloys, besides the oxide layer, evidenced from XPS results, some chelate compounds[1] might also be formed regardless of the technology used for the preparation of the samples. Since these chelate compounds are involved in the dissolution of the TiO2 film,[8,32] their presence on the surface might be regarded as an important factor in the corrosion processes. However, the weight percentage (wt pct) of carbon element on the surface of both types of samples is low, i.e., 6.3 pct for wrought Ti–6Al–4V and 12.1 pct for SLM Ti–6Al–4V alloys, indicating that the oxide layer has good corrosion resistance. These findings are supported by EIS data and anodic polarization curves which attested that the oxide layer formed on the surface has good corrosion resistance, although some corrosion processes are present on the surface of these alloys. Additionally, the SEM micrographs of the non-immersed and immersed samples were recorded. The SEM images (Figure 6) revealed a significant modification of their surface morphology after immersion. These observations are congruent with EDS elemental analysis which pointed out the formation of a protective oxide layer and of most probably some chelate compounds on their surface.
4 Conclusions
The corrosion behavior of SLM Ti–6Al–4V alloy was investigated in 0.1 M lactic acid + 0.1 M NaCl solution at 37 °C after 1150 hours of immersion and compared to a classical one, i.e., wrought Ti–6Al–4V. From anodic polarization tests, it was clearly evidenced that SLM Ti–6Al–4V has suitable resistance from corrosion point of view. Therefore, the icor of SLM Ti–6Al–4V is 0.145 μA cm−2 which is much lower than 1 μA cm−2. These results could be considered as promising ones because, in our middle aggressive biofluid (pH 2.5), the icor is lower than those reported for other Ti–6Al–4V alloys, obtained by different methods, including SLM ones, in biofluids less aggressive. The good corrosion resistance of this alloy mainly originates from the formation of a protective oxide layer, which according to XPS results, is mainly formed from TiO2. Based on OPC investigations, this protective oxide layer is relatively fast formed on alloy surface, i.e., 3 mV h−1. Furthermore, this protective layer is a stable one because the dissolution of this layer is inhibited on a large potential window (around 1000 mV). Based on other reports and our results, one may conjecture that the dissolution of this film is mainly due to the complexation of titanium oxide film with chloride ions (Cl−) from the solution, leading to the formation of complexes. These complexes most probably further interact with the lactate ions (Lac−), yielding the formation of chelate compounds which promote the dissolution of the oxide film.
From OCP and XPS findings, the formation of a protective layer mainly formed from TiO2 was also observed at wrought Ti–6Al–4V.
However, from EIS investigations, which evidenced high barrier resistance of the film, R1, at wrought Ti–6Al–4V compared with SLM one, and anodic polarization results, which revealed at wrought Ti–6Al–4V a larger potential range in which the dissolution of protective oxide film is hampered, one may state that at wrought Ti–6Al–4V the oxide layer is more stable and provides better protective properties against corrosion.
Although the SLM Ti–6Al–4V appears to have slightly lower corrosion resistance compared to classic one, the SLM Ti–6Al–4V alloy could be regarded as a valid alternative when aiming to develop new alloys for medical applications because by using this new method, customized implants with different shapes are obtained relatively easily without expensive costs. The wettability investigations of both types of specimens clearly support our previous assertions. Wrought and SLM Ti–6Al–4V alloys show similar moderate hydrophilicity which made them appropriate for these types of applications. In other words, they are enough hydrophobic in order to prevent the corrosion process and at the same time enough hydrophilic in order to be helpful to cell adhesion and proliferation. The WCA, determined after 1150 hours of immersion, is around 70 deg no matter the type of sample.
References
Q. Qu, L. Wang, Y. Chen, L. Li, Y. He, and Z. Ding: Materials, 2014, vol. 7, pp. 5528–42.
C. Vasilescu, S.I. Drob, P. Osiceanu, J.M. Calderon Moreno, M. Prodana, D. Ionita, I. Demetrescu, M. Marcu, I.A. Popovici, and E. Vasilescu: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 513–23.
C. Vasilescu, P. Osiceanu, J.M.C. Moreno, S.I. Drob, S. Preda, M. Popa, I. Dan, M. Marcu, M. Prodana, I.A. Popovici, D. Ionita, and E. Vasilescu: Mater. Sci. Eng. C, 2017, vol. 71, pp. 322–34.
D.M. Gordin, R. Ion, C. Vasilescu, S.I. Drob, A. Cimpean, and T. Gloriant: Mater. Sci. Eng. C, 2014, vol. 44, pp. 362–70.
F. Toptan, A.C. Alves, O. Carvalho, F. Bartolomeu, F. Silva, G. Miranda, and A.M.P. Pinto: J. Mater. Process. Technol., 2019, vol. 266, pp. 239–45.
R. Huang, M. Riddle, D. Graziano, J. Warren, S. Das, S. Nimbalkar, J. Cresko, and E. Masanet: J. Clean. Prod., 2016, vol. 135, pp. 1559–70.
T.M. Pollock, A.J. Clarke, and S.S. Babu: Metall. Mater. Trans. A, 2020, vol. 51A, pp. 6000–19.
H.M. Hamza, K.M. Deen, and W. Haider: Mater. Sci. Eng. C, 2020, vol. 113, p. 110980.
D. Mah, M.H. Pelletier, V. Lovric, and W.R. Walsh: Ann. Biomed. Eng., 2019, vol. 47, pp. 162–73.
M.P. Licausi, A. Igual, V. Munozand, and A. Borras: J. Mech. Behav. Biomed. Mater., 2013, vol. 20, pp. 137–48.
J.C.M. Souza, S.L. Barbosa, E. Ariza, J.P. Celis, and L.A. Rocha: Wear, 2012, vol. 292–293, pp. 82–88.
A. Banu, M. Marcu, C. Juganaru, P. Osiceanu, M. Anastasescu, and L. Capra: Arab. J. Chem., 2019, vol. 12, pp. 2007–16.
Y. Okazakia and E. Gotoh: Biomaterials, 2005, vol. 26, pp. 11–21.
ASTM B977, ASTM F1108-97 Standard Specification for Ti6Al4V Alloy Castings for Surgical Implants (UNS R56406) ASTM F2924-14 Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion.
ISO 10271/2001-Dental Metallic Materials-Corrosion Test Methods.
J. Vaithilingam, E. Prina, R.D. Goodridge, R.J.M. Hague, F.R.A.J. Rose, S.D.R. Christie, and S. Edmondson: Mater. Sci. Eng. C, 2016, vol. 67, pp. 294–303.
A. Tamadon, D.J. Pons, K. Sued, and D. Clucas: Metals, 2017, vol. 7, p. 423.
M. Fousova, D. Vojtech, J. Kubasek, E. Jablonska, and J. Fojt: J. Mech. Behav. Biomed. Mater., 2017, vol. 69, pp. 368–76.
K.S. Chan, M. Koike, R.L. Mason, and T. Okabe: Metall. Mater. Trans. A, 2013, vol. 44A, pp. 1010–22.
L.L. Dinca Boulven, A. Banu, G.N. Soylu, G. Dobri, and M.E. Mocirla: MATEC Web of Conferences 299 01005 MTeM 2019, 2019.
F. Variola, J.H. Yi, L. Richert, J.D. Wuest, F. Rosei, and A. Nanci: Biomaterials, 2008, vol. 29, p. 1285.
M. Jenko, M. Goren, M. Gotec, M. Hodnik, B. Setinabati, C. Donik, J.T. Grant, and D. Dolinar: Appl. Surf. Sci., 2018, vol. 427, pp. 584–93.
L. Ponsonnet, K. Reybier, N. Jaffrezic, V. Comte, C. Lagneau, M. Lissac, and C. Martelet: Mater. Sci. Eng. C, 2003, vol. 23, pp. 551–60.
Y. Yan, E. Chibowski, and A. Szcześ: Mater. Sci. Eng. C, 2017, vol. 70, pp. 207–51.
X. Zhu, J. Chen, L. Scheidler, and J. Geis-Gerstorfer: Biomaterials, 2004, vol. 25, pp. 4087–4103.
F.X. Xie, X.B. He, S.L. Cao, X. Lu, and X.H. Qu: Corros. Sci., 2013, vol. 67, pp. 217–24.
M. Pourbaix: Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed. Pergamon Press, New York, 1966, pp. 243–45.
R. Chelariu, G. Bolat, J. Izquierdo, D. Mareci, D.M. Gordin, T. Gloriant, and R.M. Souto: Electrochim. Acta, 2014, vol. 137, pp. 280–89.
L. Preda, C. Negrila, M.F. Lazarescu, M. Enache, M. Anastasescu, A.M. Toader, S. Ionescu, and V. Lazarescu: Electrochim. Acta, 2013, vol. 104, pp. 1–11.
J. Yang, H. Yang, H. Yu, Z. Wang, and X.A. Zeng: Metall. Mater. Trans. A, 2017, vol. 48A, pp. 3583–86.
D. Wang, P. Li, K. Kang, C. Zang, J. Yin, M. Jing, and X. Zeng: Surf. Coat. Technol., 2016, vol. 300, pp. 128–34.
A. Sharma, M.C. Oh, J.T. Kim, A.K. Srivastava, and B. Ahn: J. Alloys Compd., 2020, vol. 830, p. 154620.
M. Eliaz: Materials, 2019, vol. 12, p. 407.
R. Ion, C. Vasilescu, P. Drob, E. Vasilescu, A. Cimpean, S.I. Drob, D.M. Gordin, and T. Gloriant: Mater. Corros., 2014, vol. 65, pp. 593–604.
E. Almanza, M.J. Pérez, N.A. Rodríguez, and L.E. Murr: J. Mater. Res. Technol., 2017, vol. 6, pp. 251–57.
Acknowledgments
This work was supported by Romanian Ministry of Research in the frame of National Project PN-III-P2-2.1-PED-2019 Contract No. 329PED/2020. Some investigations were performed within the framework of the “Electrochemical preparation and characterization of active materials with predetermined features” Research Project of the “Ilie Murgulescu” Institute of Physical Chemistry of the Romanian Academy. Contact angle measurements were performed using the research infrastructure acquired under POS-CCEO2.2.1 Project EU (ERDF) INFRANANOCHEM—No. 19/01.03.2009 and Romanian Government. The authors thank Dr. Alexandru Paraschiv, COMOTI—Romanian Research and Development Institute for Gas Turbines, Bucharest, Romania for SEM and EDS analysis.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banu, A., Preda, L., Marcu, M. et al. Electrochemical Behavior of SLM Ti–6Al–4V Alloy After Long Time of Immersion in Lactic Acid Environment. Metall Mater Trans A 53, 2060–2070 (2022). https://doi.org/10.1007/s11661-022-06648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-022-06648-8