Abstract
A method for measuring the full liquid width of a braze joint during TLPB was developed based on enthalpy of solidification values obtained from DSC and microstructural measurements. This differs from most techniques in the literature which only measure the centreline eutectic width using metallographic methods. Comparison of full liquid width and eutectic only measurements confirmed that the rate of isothermal solidification and gap widening due to dissolution is underestimated, particularly at higher braze temperatures, when relying on eutectic measurements only. However, the time for complete isothermal solidification is the same whether considering the full liquid width or eutectic width. The underestimation of the IS rate by eutectic centreline measurements increases as the braze temperature increases above the filler liquidus temperature. The DSC enthalpy measurements were used to build a TLPB processing map for a range of braze temperatures and starting filler metal interlayer thickness. This map can predict the degree of gap widening and the time for complete isothermal solidification for this gap. Results indicate that an optimum braze temperature exists where the apposing influences of gap widening and increased IS rate combine to give the shortest IS completion time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several researchers including Ojo et al.,[1] Idowu et al.,[2] Tokoro et al.,[3] Mosallaee et al.,[4] Egbewande et al.,[5] Jalilvand et al.,[6] and Pouranvari and Mousavizadehz[7] have reported the influence of brazing temperature on the TLPB behaviour of Ni based superalloys. These studies have examined “sandwich” joints, which have a constant gap thickness, and made metallographic measurements of the centreline eutectic width remaining in the joint after specified brazing times. Amongst these studies,[4,5,6] only report the time required to produce no centreline eutectic (i.e., the complete Isothermal Solidification (IS) time tIS) as a function of braze temperature. References 1,2,3, 7 report the width of the centreline eutectic as a function of braze time and temperature, creating the potential to determine not only tIS but the rate of IS (i.e., kIS).
More specifically, Reference 6 examined IN738LC/BNi-1a braze couples with a 50 μm braze foil insert and temperatures from 1100 °C to 1150 °C. With the observation that tIS increased from 1100 °C to 1150 °C, they conclude that the rate of IS decreases with an increase in braze temperature. Reference 5 investigated IN600/BNi-9 braze couples with 40 μm inserts and braze temperatures from 1080 °C to 1220 °C. They also conclude that the rate of IS decreases with an increase in braze temperature using tIS measurements. Reference 4 studied IN738LC/BNi-3 with 25.4 μm inserts using temperatures from 1100 °C to 1250 °C. They observed a decreased in tIS from 1100 °C to 1150°C but a large increase in tIS occurring from 1200 °C to 1250°C.
Ojo et al.[1] and Idowu et al.[2] examined IN738LC/BNi-9 braze couples in the temperature range from 1070 °C to 1175 °C. The rate of centreline eutectic removal increased with temperature up to 1145 °C, but decreased up to 1175 °C. Reference 3 investigated Waspaloy/BNi-9 couples from 1065 °C to 1225 °C. The rate of centreline eutectic removal increased with temperature up to 1145 °C (i.e., tIS decreased). Only tIS was reported above 1145 °C. The rate of centreline eutectic removal increased with temperature up to 1145 °C, with its value increasing with increased temperature. Reference 7 report on IN718/Ni-2 from 1000 °C to 1150 °C, using a 50 μm insert. The rate of the centreline eutectic removal increased and tIS decreased, with an increase in braze temperature.
The above studies seem to contradict each other, with some reporting tIS increasing and a few with tIS decreasing as temperature increases. Others indicate an optimum, intermediate temperature where tIS reaches a minimum. In addition, some researchers conclude that the rate of IS decreases with increasing temperature while others state that the IS rate increases as the braze temperature increases.
A challenge in measuring the influence of temperature on TLPB that may be contributing to the apparent contradiction in the literature has been articulated in an earlier study by Tuah-Poku et al.[8] In examining TLPB in relatively simple binary eutectic systems, they point out that the time for complete isothermal solidification depends on the degree of base metal dissolution and rate of diffusion of the melting point depressant (MPD) in the base metal. Base metal dissolution generally increases with increasing temperature leading to liquid gap widening. A wider initial liquid gap should contribute to an increase in tIS. However, the rate of diffusion of the MPD generally increases with increased temperature, contributing to a reduction in tIS. The opposing influences of dissolution and MPD diffusion vs. temperature can lead to unexpected variations in tIS as a function of braze temperature. Measuring only tIS vs. temperature, while serving a practical purpose, cannot lead to a full understanding of the influence of temperature on TLPB.
1.1 Disadvantages of Using Centreline Eutectic Measurements to Interpret Isothermal Solidification
The above studies, which measure the width of the centreline eutectic as the Athermally Solidified Zone (ASZ), do not include a measure of dissolution or gap widening and the true liquid width as a function of braze temperature. This can be explained in detail with the aid of Figure 1. Figure 1(a) depicts a hypothetical eutectic like binary phase diagram. A base metal with a composition of Co = 0, in contact with a eutectic filler with a composition Ce is assumed, where the original thickness of the filler metal insert is Wo. If the braze temperature Tb is at, or near the eutectic temperature Te, negligible dissolution of the base metal would take place since the liquid composition CL1 = Ce. The liquid width would be well represented by the centreline eutectic width measured metallographically on a post braze sample, since all the liquid solidifies as eutectic.
If the braze temperature Tb is increased to be significantly above Te, the solubility of BM elements in the liquid increases when the liquid composition becomes CL2. Solubility also decreases in the solid BM from CS1 to CS2. This leads to BM dissolution and gap widening where Wliq. > Wo (Figure 1(b)). Since the composition of the liquid is now hypoeutectic, when the braze joint is cooled from Tb under non-equilibrium conditions, primary (or pro-eutectic) phase first solidifies by epitaxial growth from the solid/liquid interface, forming dendrites labelled “D” in Figure 1(c). Following this, interdendritic eutectic solidification occurs.
For a given alloy system, the relative amount of liquid which solidifies as primary proeutectic phase or eutectic phase, depends on the cooling rate and the brazing temperature (which determines the solidus and liquidus compositions at the solid/liquid interface). For example, if cooling is very slow such that it approximates equilibrium, then the situation of Tb and Cs2 would produce no eutectic, since Cs2 is less that Cmax (or CS1). In practice, the cooling from a TLPB process would occur under non-equilibrium conditions where the Scheil equation is most commonly used;
where fs is the fraction of solid formed at a given temperature below Tb, Cs is the composition of the solute at the solid/liquid interface, Co is the bulk composition of the alloy and k is the partition coefficient which is typically assumed constant with temperature and equal to k = Cs/Cl or Cs1/Ce in Figure 1(a). Local equilibrium is assumed at the solid/liquid interface such that, during cooling fs increases, causing Cs to also increase. The last portion of the solid to freeze occurs as eutectic and its fraction fe can be calculated using Eq. [1] when Cs = Cmax (or Cs1, the maximum solid solubility according to Figure 1(a)), Therefore, for a given alloy system, fixed braze temperature and cooling rate, the ratio of the full liquid width that solidifies as pro-eutectic and eutectic phase would be constant. The full liquid width continues to reduce in thickness due to isothermal solidification (IS). The Scheil equation is not applicable to this process. However, during cooling after a specific braze time, the liquid width remaining solidifies under non-equilibrium conditions which can be represented by the Scheil equation. Therefore, as the full liquid width reduces over the braze time due to IS, the absolute value of both the pro-eutectic and centreline eutectic forming from this liquid width as the ASZ also reduces, but at a fixed ratio.
Metallographically, the athermally solidified region (Wliq. in Figure 1(c)) is sometimes difficult to distinguish from the isothermally solidified zone (ISZ) since both have a similar structure. The centreline eutectic is typically more visible since it usually has a markedly different microstructure. As Figure 1(c) indicates, measuring only the centreline eutectic width WE, at room temperature, after brazing at Tb > Te would underestimate the actual liquid width present at the braze temperature. This underestimation would increase as the liquid composition becomes more hypoeutectic with increasing temperature. The studies of References 1,2,3, 7 all report the centreline eutectic width vs. braze time at different temperatures. The initial width of this eutectic is either observed to be insensitive to temperature or shows a decrease as the temperature increases. This trend does not properly reflect dissolution and gap widening because of the hypoeutectic solidification phenomena.
The presence of a centreline eutectic in the joint is very detrimental to mechanical properties. Therefore, knowledge generated by the above studies for the rate of eutectic reduction and the time for its complete removal is of great importance. However, dissolution and gap widening during brazing is also an important practical parameter to obtain. In addition, interpretation of the true rate of the isothermal solidification as a diffusion driven process should be done using the actual liquid width. Measurements of the rate of centreline eutectic width cannot be used to accurately measure the true rate of IS particularly as it varies with temperature.
Partz and Lugsceider,[9] Nakao et al.,[10] Sakamoto et al.[11] and Arafin et al.[12] investigated TLPB at various braze temperatures using wedge gap type specimens. In this approach, a variable gap from 0 to 250 μm is created by the wedge geometry. Several samples are brazed for different times at each temperature and the maximum gap width at which no centreline eutectic is observed is determine by post metallography. This is termed the maximum brazing clearance (MBC). The advantage of this approach is that the dissolution or gap widening is inherently measured and there is no reliance on measuring the centreline eutectic width as a function of braze time. In all these studies the rate of IS was observed to increase with an increase in braze temperature which would be expected since it is dependent on MPD diffusivity. A disadvantage of this approach, which it shares with the parallel “sandwich” method in the studies above, includes the need to perform many experiments, with each data point obtained needing a full brazing cycle and separate sample. In addition, it is difficult to reference the dissolution and gap widening back to a more industrially relevant room temperature, or parallel set gap.
Therefore, it would be of great value to develop a method that could measure and predict the degree of dissolution and gap widening in reference to a starting set gap, true rate of liquid width removal and the complete isothermal solidification time, tIS, as a function of braze temperature.
Recently,[13,14] developed a technique based on differential scanning calorimetry (DSC) capable of measuring the rate of liquid removal and MBC as a function of braze time. Using this technique, the full rate of IS at a given temperature could be determined from a single DSC experiment. The cyclic method employed was able to produce a dense data set with measurements of IS in 10 to 15 braze time intervals. However, this technique was only applicable to brazing at the liquidus temperature of the filler metal, where negligible dissolution of the base metal occurred. The goal of the current study was to explore the potential of extending the DSC technique to determine the degree of dissolution, true rate of IS and the time for complete IS over a range of braze temperatures and degrees of dissolution. While each temperature would require a separate DSC experiment, it would still represent a significant reduction in experimentation.
2 Experimental Methods
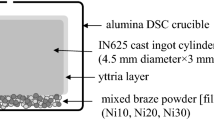
Inconel 625 (IN625; Ni-21Cr-9Mo-4Fe-3.7Nb) base metal cylinders were provided by Pratt and Whitney Canada (P&WC) with dimensions of 4.5 mm in diameter and 3 mm in height. TLPB was simulated within the DSC using a half joint arrangement depicted in Figure 2, where cylinders of IN625 were placed in contact with a powder bed of PALNICRO-36M (Ni-36Pd-10.5Cr-3B-1Si wt pct) braze alloy. IN625 was chosen as a base metal because it has a relatively simple solid-solution microstructure and is an important and widely used superalloy. The TLPB behaviour of IN625 using the filler metals of BNi-2 and PALNICRO-36M have also been documented using the DSC method for the case of negligible dissolution.[13,14] PALNICRO-36M was chosen as a filler metal because of its commercial use but also because of its high Palladium content. Pd is not contained within the IN625 base metal and therefore measurements of the Pd concentration within the liquid layer proved to be an effective way to measure the degree of base metal dissolution as a function of braze temperature. The weight of the powder bed used was in the range of 7.03 mg ± 1 pct. The weight of the base metal cylinders was in the range of 296 mg ± 4 pct. To limit the area of interaction of the braze material to the bottom of the base metal (BM) surface facing down (i.e., the faying surface), the sides and top of the cylinders were painted with yttria. The flat faying surface was polished using a 500 grit SiC paper and ultrasonically cleaned in acetone to create a clean, unoxidized surface to allow wetting by the molten braze filler. Upon melting, the weight of the BM against the crucible bottom created a “capillary channel”. The high degree of wettability of the molten filler with the BM faying surface resulted in a uniform spread of the molten filler within the capillary channel creating a filler spread fully and evenly across the base metal faying surface. At 1000 °C, the liquidus temperature for PALNICRO-36, the filler metal reached a thickness of 40 µm ± 3.5 pct, representing a case of negligible BM dissolution.
Differential scanning calorimetry (DSC) on the IN625/PALNICRO-36M couples was performed in a DSC 404 F1, produced by Netzsch Gmbh. The DSC tests were performed under a 50 mL/min. flowing Ar (99.999 pct) atmosphere and were heated and cooled at 20 °C/min. In a recent study,[13] developed a cyclic DSC method capable of measuring TLPB behavior of Ni based superalloys using boron-based filler metals. This method was adapted for the current investigation, where half TLPB braze joints were heated to the braze temperature, held for 5 min. then cooled to 700 °C (approx. 100 degrees below the measured solidus of PALNICRO-36M) then immediately reheated to the same braze temperature and held for 5 minutes. This cycle was repeated until there was no evidence of solidification measured on the cooling trace. The braze temperatures investigated included 1050 °C, 1100 °C, 1130 °C and 1175 °C. Two separate samples at each temperature were completed to determine reproducibility. This body of data was compared to recent work at 1000 °C, which is described in more detail elsewhere.[15] This previous work confirmed that brazing at 1000 °C resulted in limited dissolution, or gap widening, of the base metal.
The extent to which the solidification of a hypoeutectic liquid phase forms proeutectic and eutectic phases according to the description of Figure 1, is dependent of the non-equilibrium cooling rate as mentioned earlier. In most of the TLPB studies referenced, where centreline eutectic was measured metallographically to determine liquid width, brazing was done in a vacuum furnace with furnace cooling. Ghasemi and Pouranvari[16] report that vacuum furnace cooling results in a cooling rate of 15 °C/min in the temperature range over which solidification and ASZ formation occur. This is consistent with the studies of References 4 and 11 which report average furnace vacuum cooling rates of 10 and 8 °C/min. References 1,2,3, 5, 9, 10, 12 do not specify a cooling rate but use vacuum furnace brazing which likely resulted in vacuum furnace cooling and rates similar to that reported by others. Therefore, in the current study, the rate of 20 °C/min. used in the DSC, is a close representation of the cooling rate present in other TLPB studies.
In the cyclic method, a cumulative liquid duration time is measured which is analogous to the braze time. As described in detail by Reference 13, this method involves determining the time the braze couple spends above the solidus temperature for each cycle in the thermal profile. These cumulative times are then added to give the total liquid duration or braze time.
Temperature and enthalpy calibrations of the DSC were carried out using 5 pure metals (i.e., In, Sn, Bi, Al, Ag) under conditions identical to that used for the sample experiments, but without the presence of the IN625 base metal cylinder. This included the use of an empty Al2O3 crucible on the reference side of the DSC sample carrier. An enthalpy correction which considered the presence of the base metal cylinder on the sample side is described in Section III–B) below.
Microstructural examination on post DSC treated samples was performed using both a Hitachi S-4700 FEG SEM with an Oxford INCA EDS analysis system for phase composition, and a Zeiss optical microscope. Samples for this analysis were hot mounted in a conductive resin and polished to a mirror-like finish using SiC papers, diamond suspension and colloidal silica. Samples included those that were fully cycled to cause complete isothermal solidification and samples that were held at the braze temperature for shorter times, such that the progression in isothermal solidification could be observed microstructurally.
3 Results and Discussion
3.1 DSC Assessment of Centreline Eutectic Removal
Figure 3(a) presents the first DSC cooling cycle for samples heated to 1050 °C, 1100 °C, 1130 °C and 1175 °C and immediately cooled to room temperature. This represents liquid duration (or braze) times of 19.5, 24, 27, 30.6 minutes respectively. Since this value represents the time spent above the solidus temperature of 830 °C, it makes sense that it increases with increased braze temperature. All samples exhibit a clear eutectic solidification peak. The magnitude of the peak, in terms of its integrated area and enthalpy of solidification, decreases with an increased braze temperature as will be quantified in more detail below.
Figure 3(b) presents the DSC cooling curves for the sample heated to 1050 °C only, but plotting all the cycles, with the 1st cycle at the top and increasing cycles presented in decreasing order. The magnitude of the solidification enthalpy decreases with increased liquid duration, until no solidification is present on the 7th cooling cycle. Samples brazed at higher temperatures showed similar overall trends with liquid duration time.
Figure 4 summarizes the enthalpy of eutectic solidification as a function of square root of braze time for all the samples of this study and including the 1000 °C sample that was previously investigated by Reference 15 A line representing a linear regression analysis is included for each braze temperature to help delineate the trends with temperature. Note also that the enthalpy data is an average from two separate braze experiment at each temperature. On average the variation in enthalpy measurements was in the range of ± 10 pct.
Reference 15 has shown that the magnitude of the solidification enthalpy correlates to the amount of centerline eutectic present in the joint for the IN625/PALNICRO-36M braze couple. The data of Figure 4 indicates that the amount of centreline eutectic remaining in the joint for a given braze time, deceases with an increase in braze temperature. This contradicts the expectation that increasing dissolution and the associated gap widening should increase the amount of liquid present with increasing temperature. The rate of isothermal solidification also appears to decrease with temperature, which is contradictory to the expectation that the rate should increase, since the diffusion process it relies on increases with increasing temperature. These discrepancies will be discussed in more detail in a later section.
The isothermal solidification time (i.e., the time at which the eutectic enthalpy reduces to zero) was determined to be 190, 156, 132, 143 and 145 minutes for braze temperatures of 1000 °C, 1050 °C, 1100 °C, 1130 °C and 1175 °C respectively. Therefore, there is an optimum braze temperature at which the complete removal of centreline eutectic is the shortest. This trend agrees with the studies of References 1,2,3,4.
As described in the introduction, it is argued that a measurement of centreline eutectic does not properly measure the rate of liquid removal and the occurrence of dissolution and gap widening. This argument is reinforced by the microstructural results of Figure 5 where the half joint microstructure for samples immediately cooled from the braze peak temperature are depicted for the 1050 °C, 1100 °C and 1130 °C. The samples represent liquid duration times (or braze times) of 19.5, 24 and 27 minutes respectively, representing the first data point in the curves of Figure 4.
All samples exhibit the microstructural regions expected from a TLPB joint that has not undergone complete IS. This includes the unaffected base metal (BM) away from the faying surface, the diffusionally affected zone (DAZ) just below the faying surface where B has migrated
and precipitated as metal borides within the BM, an isothermally solidified zone (ISZ) where diffusion has created a γ-(Ni) solid solution layer and an athermally solidified zone (ASZ) where the remaining liquid solidified during cooling from the braze temperature to form brittle borides and a “centreline eutectic”. This region is labelled ASZ in the figure and exhibits a two-phase microstructure of light and medium grey. The ISZ region etches dark grey while the DAZ appears black. The thickness designated with the label W 1ISZ will be discussed in a later section.
The interface between the ISZ phase and the ASZ is “wavy” in structure and this unevenness of the interface increases as the braze temperature increases. Protrusions labelled with the letter “D” in Figure 5 exhibit the dendritic structure that would be expected when a hypoeutectic liquid undergoes solidification during cooling, as previously explained in Figure 1. The Pd concentration within the (γ-Ni) solid region for a joint held at 1175 °C for 62 minutes and until complete IS (144 minutes) is plotted in Figure 6.
At this temperature, the ISZ grows isothermally with a uniform composition of approximately 16 wt pct Pd. This is the composition measured throughout the ISZ when the joint is completely solidified before cooling. In the sample cooled before complete IS, the (γ-Ni) region that resembles the ‘D’ areas in Figure 5 has a progressively increased Pd composition. This is due to the microsegregation that would be expected in the (γ-Ni) during hypoeutectic athermal solidification as described in Figure 1. During cooling the primary (γ-Ni) or pro-eutectic phase solidification composition would shift from CS2 to CS1 resulting in an enriched solute content, Pd in the current case. Therefore, the compositional variation measured in Figure 6 supports the concept of primary dendrite formation in the ASZ. This type of microsegregation in an identified “D” region was present in the joints brazed at all temperatures.
Figure 5 also points out the difficulty in measuring the centreline eutectic using metallography, particularly as the braze temperature increases and the solidification process includes more pro-eutectic dendrites. For example, for the 1100 °C sample, some of the pro-eutectic dendrites nearly extend through the full liquid width. Associated with this, the centerline eutectic width (labelled as the ASZ region in the figure) ranges from a 1 or 2 μm to over 50 μm in thickness along the length of the half joint depicted. Such a large thickness variation greatly diminishes the value of centreline eutectic as a measure of liquid width remaining.
3.2 Influence of Braze Temperature on BM Dissolution and Gap Widening
Figure 5 illustrates that dissolution and gap widening increases as the braze temperature increases. To quantify this process further, over 30 thickness measurements of the gap thickness wmax were made along the width of the ½ joint samples that were cycled to complete IS. The average wmax is plotted in Figure 7vs. temperature, presented as full gap width 2wmax. The standard deviation of the thickness measurements was in the range of ± 10 to 15 pct. As noted by Reference 15, joints brazed at 1000 °C exhibited minimal base metal dissolution, such that the 2wmax value for this couple (80 μm) is close to the original thickness of the filler insert. Dissolution increases slowly in the temperature range up to 1100 °C and then increases more rapidly from 1100 °C to 1175 °C.
Full gap, 2wmax width as a function of braze temperature measured from the micrographs of Fig. 5
Table I presents the average composition of the filler region of the joint after immediately cooling from the peak braze temperature. This was determined using EDS box scans of the region above the DAZ, encompassing the thickness wmax noted in Figure 5. In the case of Pd, which is not present in the BM, increased dilution systematically decreases its concentration within filler region (i.e., ISZ/ASZ). For elements present in the BM but not in the filler (i.e., Fe, Nb and Mo) there is a systematic increase in ISZ/ASZ concentration with temperature. Cr is present at higher concentrations in the BM compared to PALNICRO-36M and therefore also increases in the ISZ/ASZ as temperature increases. Si and Ni remain approximately constant likely due to there very low and high respective concentrations overall. The data clearly supports the systematic increase in dissolution with increased temperature.
3.3 Determining Total Athermal Solidification Enthalpy
Figure 8(a) presents the DSC cooling traces for the sample brazed at 1175 °C after a series of increasing braze times. Both the lower temperature ternary eutectic solidification peaks and the higher temperature primary solidification peaks are visible. The ‘hook’ like shape immediately upon cooling and the sloped baseline are characteristics of the DSC measuring head.
One advantage of the cyclic DSC method is that, when complete IS has occurred, the DSC trace only includes the characteristics of the measuring head. That means that this last cycle (cycle 5 in Figure 8(a)) acts as an internal baseline correction for the sample. Therefore, subtracting this curve from cycles 1 to 4 produces a flat baseline from which the enthalpy of solidification of both the primary and ternary eutectic can be measured, as shown in Figure 8(b). Therefore, these DSC traces would allow a measure of the full athermal solidification event and a true measure of the liquid fraction remaining in the braze joint, as a function of braze time. Examination of the DSC cooling traces for the other braze temperatures (see Figure 9) exhibited little evidence of a primary solidification event. This is due to the broad range over which this solidification event occurs, leading to only a shallow deviation in the baseline between 1000 °C and 1100 °C. In these cases, the curve subtraction method used for the 1175 °C case could not be used. However, the centerline eutectic peak was always clear, with enthalpies easily measured.
Based on these observations, a method was developed to examine the possibility of using the enthalpy measured from the centreline eutectic peak to calculate the full liquid fraction. This assumed that, for a given hypoeutectic alloy composition and cooling condition, the fraction of solidification that takes place as eutectic should remain constant. A method based on this assumption involves several steps as described below.
As Section III–B above indicates, the composition of the filler metal changes at each brazing temperature due to increasing dissolution of the BM. The wt pct of BM (or IN625) dissolved into the liquid layer can be estimated as;
where MPdT is the mass pct of Pd in the ISZ/ASZ region of the filler at a specified braze temperature (i.e., the values of Table I) and MPdo is the original Pd mass pct in the PALNICRO-36M braze alloy (measured as 38.5 wt pct using SEM-EDS). Using Eq. [2] and the values of Table I gives MBM wt pct IN625 dissolution values ranging from 5.3 to 55.9 wt pct for 1000 °C to 1175 °C respectively.
To simulate this BM dissolution and determine the solidification behaviour of the various hypoeutectic compositions it creates, powder mixtures of PALNICRO-36M with increasing amounts of IN625 powder were produced. These mixtures were heated in the DSC to 1250 °C three times to ensure homogenization in the molten state. During the last cooling segment, enthalpy and temperature measurements were made. All samples consolidated into a dense braze ball. Following the three heat/cool cycles, samples were mounted and polished and their compositions measured using the same SEM-EDS condition used to measure the ISZ/ASZ composition of the braze joints.
Table II presents the compositions of the braze alloys as a function of IN625 wt pct introduced into the mixture. The trends in composition are very similar to those measured for dilution of the braze joints as a function of temperature (See Table I). The Cr, Fe, Mo and Nb increase in value, Pd decrease in value and the Si and Ni remain nearly constant. Also, the levels of dilution experienced by the joints, as measured by the Pd content (i.e., 36.46 to 16.98 wt pct) are captured within the range of Pd contents present in the prepared braze alloys (i.e., 36.28 to 13.5 wt pct).
Figure 10 plots the solidification behaviour measured for the various PALNICRO-36M+IN625 braze ball mixtures. Even the PALNICRO-36M filler with no IN625 added, exhibits a small primary solidification peak prior to the large centreline eutectic solidification event. This alloy also has a small solidification event onsetting near 800 °C. This solidification event represents less than 4 pct of the total solidification enthalpy for PALNICRO-36M. This 800 °C peak was visible on some brazed joint DSC traces but was too small to obtain an accurate enthalpy measurement.
With increasing IN625 content, the overall solidification temperature range increases, with associated reductions in the magnitude of the centreline eutectic enthalpy and an increase in the primary solidification enthalpy. This increasing hypoeutectic solidification behaviour is supported by the solidified microstructure presented in Figure 11 for the PALNICRO-36M alloy and mixtures with 6.3 and 20 wt pct IN625 added. Increasing IN625 content increases the fraction of primary dendrites in the microstructure.
The Netzsch thermal analysis software allows a partial integration of the total solidification behaviour evident in Figure 10. This resulted in a determination of eutectic enthalpy as a
percentage of the total solidification enthalpy. This value is plotted in Figure 12vs. the pct Pd measured in each braze ball. The pct enthalpy (pct EUT) that is eutectic, decreases linearly with a decrease in Pd composition (i.e., increase in dilution). The least squares fit to this experimental data can be used to scale the measured eutectic enthalpy from the braze joints, ΔHeut, to the full solidification enthalpy ΔHfull, knowing the Pd composition in the ISZ/ASZ joint at each braze temperature, pct PdISZ;
Percentage of the total solidification enthalpy that is eutectic enthalpy for the range of PALNICRO-36M + IN625 braze ball mixtures. Data is plotted vs. the Pd content reported in Table II
This determination of the full solidification enthalpy in the braze joints is plotted in Figure 13 along with the previously determined eutectic solidification enthalpy previously reported in Figure 4. Included is an enthalpy value for the 1175 °C sample directly determined by integration of the area under the curve subtraction peak that was plotted in Figure 8(b). The close agreement between the subtraction data and that calculated using Eq. [3] validates the eutectic scale up approach.
Both the full and eutectic enthalpy measured for IN625/PALNICRO-36M half braze joints as function of the square root of liquid duration (increasing DSC cycles) for all braze temperatures explored. Closed symbols indicate the full solidification enthalpy. Open symbols indicate only the eutectic enthalpy
The full enthalpy is significantly larger than the eutectic enthalpy, particularly at the higher braze temperatures. This make sense since the liquid composition shifts to a more hypoeutectic composition vs. temperature, increasing the amount of primary phase solidification. Interpretations of the rate of isothermal solidification with respect to braze temperature is also changed from the analysis based on the eutectic enthalpy only. For example, when considering the full solidification enthalpy, the rate of IS is highest at 1175 °C and lowest at 1000°C, which would be expected based on the temperature dependence of diffusion. The exact opposite is true when only considering the eutectic enthalpy. Figure 13 indicates that the predicted isothermal solidification time tIS is the same for both eutectic only and full solidification enthalpy plots. Therefore, it validates that the approach used by many authors to predict tIS using metallographically determined centreline eutectic is correct. However, it also points out that errors would arise in using this same measurement to determine the true rate of isothermal solidification and the extent of gap widening and dissolution.
3.4 Determining the Liquid Width and Isothermal Solidification Rate
Reference 13 developed an equation for converting enthalpy measurements obtained from DSC to a liquid width 2WL;
where ΔHt are the liquid width and enthalpy of solidification respectively, measured from the ½ braze joint assembly in the DSC at a specified braze time. The ½ braze joint gap width developed at the braze temperature due to dissolution is Wmax. Equation [4] is expressed based on a full joint width (i.e., 2WL and 2Wmax) assuming symmetry through the joint centreline. ΔHf is the enthalpy of solidification of 100 pct braze filler. This value is measured with the experimental set up described in Figure 1, accept that the faying surface of the base metal (BM) is also coated with yittria paint. Therefore, the filler metal melts and solidifies under the identical thermal dampening influence of the BM experienced with the ΔHt measurements, but without any metallurgical or diffusional solidification contribution. In the current case, the enthalpy measured for the PALNICRO-36M filler on its own (ΔHo) was 165.6 J/g, while ΔHf was 120.9 J/g. Therefore, the presence of the BM lowers the measured enthalpy by approximately 27 pct (i.e., ΔHf = 0.73*ΔHo).
Moreau and Corbin[14] further expressed the width of the isothermally solidified zone WISZ as;
In the studies of References 13,14,15, brazing was done at the filler metal liquidus temperature where BM dissolution was negligible. In the current case, significant dissolution of the BM occurs which increases with temperature. In terms of analysis and Eqs. [4] and [5], dissolution is partially accounted for through the 2Wmax term. However, dissolution also impacts the DSC enthalpy measurements. First, the enthalpies plotted in Figure 13 are based on the original mass of the PALNICRO-36M filler powder used in the half joint. However, dissolution of the base metal increases the filler mass. Second, dissolution also changes the composition of the filler metal, which could change its enthalpy of solidification, ΔHo and consequently, ΔHf. The braze ball preparation described above allows both these factors to be determined as outlined in Figure 14. The weight pct of PALNICRO-36M in the filler and the enthalpy of solidification are both plotted as a function of the weight pct of Palladium determined for the braze ball using EDS analysis. Included in the graph is the 185 J/g enthalpy of solidification determined for a 100 pct IN625 composition.
Plots of the enthalpy of solidification and the wt pct PALNICRO-36M in the filler of the PALNICRO-36M + IN625 braze balls mixtures of Table II. Both data sets are plotted as a function of the Pd concentration measured in the braze mixture by EDS
Given that the enthalpy of the of IN625 and PALNICRO-36M are within 20 J/g, the enthalpy dependence on the filler composition is low and can be approximated using a rule of mixture with a linear regression analysis of;
where ΔH Do is the enthalpy of 100 pct of the new diluted filler alloy and wt pct Pd is the palladium in the filler alloy.
As expected, the pct of the filler mixture that is PALNICRO-36M is 100 when the Palladium composition is 38.5 wt pct and falls to 0 pct for the 100 pct IN625 composition. The linear regression of this data series is;
where pct CPW is the weight percent of original PALNICRO-36M in the filler. This is a direct measure of the mass-based dissolution which can be used to correct for the increased mass of the filler metal.
The wt pct Pd in the filler metal at each braze temperature reported in Table I can then be use in Eqs. [6] and [7] to determine the dissolution parameters ΔH Do and pct CPW. In the case of enthalpy, the thermal attenuation of the BM is applied as before (ΔH Df =0.73*ΔH Do ). This leads to the dissolution modified equations for the liquid width and isothermally solidified width;
Figure 15 plots the liquid width determined from Eq. [8a] vs. square root of liquid duration time for all the temperatures explored. Recall that in all cases, the original PALNICRO-36M filler metal thickness was 40 μm (or 2WL = 80 μm), so the influence of dissolution on gap widening, as the braze temperature increases, is clearly illustrated by the data. It is also clear that the rate of isothermal solidification systematically increases with braze temperature. The time to achieve isothermal solidification, tIS was determined to be 189, 156, 132.5, 143, 145 minutes (i.e., 13.77, 12.49, 11.51, 11.96, 12.05 (min.)1/2) at 1000 °C, 1050 °C, 1100 °C, 1130 °C and 1175 °C respectively. The opposing influences of gap widening and the increased rate of isothermal solidification, kIS on tIS results in an optimum braze temperature of 1100 °C producing the shortest IS time.
Full liquid gap width vs. the square root of liquid duration time for all braze temperatures. The plots are a result of the application of Eq. [8a] to each DSC enthalpy measurement
A disadvantage of plotting the data according to liquid width is that it is specific to a starting gap width or braze interlayer thickness. Reference 14 has shown that if the data is plotted as the thickness of the isothermally solidified layer, predictions of the IS time can be made for any starting gap size. It also allows a deeper analysis of the mechanisms of TLPB.
Figure 16 plots the DSC data in this way using Eq. [8b]. The closed symbols for each temperature are from the DSC experimental results. A linear regression (LR) analysis, indicated by the dashed lines, was performed on the data. The slope of LR which represents the rate of isothermal solidification, kIS and the y-intercept 2WISZo for each braze temperature are given in Table III. Work by Ruiz-Vargas et al.[17] and others[13,14,15] indicate that no diffusion between the filler and superalloy base metal occurs before the solidus temperature, which represents zero liquid
Full isothermally solidified width vs. the square root of liquid duration time for all braze temperatures. The plots are a result of the application of Eq. [8b] to each DSC enthalpy measurement
duration in the current study. Rather, a rapid transient growth of the ISZ layer occurs when heating between the solidus and braze temperature. Once the braze temperature is reached, the rate of ISZ growth stabilized to have a linear dependence on the square root of time.
The LR analysis allows a prediction of the 2WISZi when the braze temperature is reached, which is shown in Figure 16 as an open symbol for each temperature. This value is also given in Table III. The time at which IS was complete for the joints explored with specific 2Wmax values, is shown as an open symbol at the opposite end of the data set. Note that in some cases the experimental data covered this IS completions time and in other cases it was determined by extrapolation using the LR equation.
The increasing rate of IS with temperature and the optimum braze temperature of 1100 °C, where tIS is a minimum, is clearly illustrated in Figure 16 and Table III. The data also points out that the initial rapid growth of the IS layer is dependent on temperature where 2W iISZ increases from 26 to 112 μm from 1000 °C to 1175 °C respectively.
Microstructural validation of 2W iISZ , the hypoeutectic ASZ microstructure and the MBC analysis of Figure 16 is available in Figure 5. The value of W 1ISZ shown in this figure is from the first solid data point in each of curves of Figure 16. In each case, this thickness marks the point where the joint microstructure transitions from a continuous solid-solution layer to the dendritic “D” protrusions + centreline eutectic region. Therefore, the DSC measurements and analysis leading to the MBC curves quantitatively supports the concept that the ASZ forms from the solidification of a hypoeutectic composition which includes the primary dendrites. It also correctly measures the true ISZ layer formed and confirms the temperature dependence of the initial width of the ISZ thickness.
This rapid ISZ growth that occurs between the solidus and isothermal braze temperature was explored in detail for a BNi-2/IN625 braze joint at 1050 °C by Moreau and Corbin[14] and was termed the Initial Boron Uptake (IBU). It occurs through diffusion of boron into the base metal in order to establish an interface dictated by phase equilibria, consisting of a DAZ and ISZ layer.
As demonstrated by Reference 14 the MBC curves of Figure 16 can be used to predict the isothermal solidification time for any starting gap. Furthermore, Zhang and Shi[18] have demonstrated that the dilution factor, Df or gap widening for a TLPB joint at a given braze temperature is independent of the starting gap size or interlayer thickness. These ideas allow the construction of a TLPB processing map depicted in Figure 17. Note that the time axis in this case is in minutes rather than the square root of minutes.
The data points linked by a solid line are predictions for the same starting PALNICRO-36M braze interlayer thickness. The position where the data point intersects the MBC braze temperature predictions, shown as a dashed line, marks the gap widening as a result of dissolution at a specific temperature, and the time for complete isothermal solidification for that gap. The use of a 50 μm gap is very common industrially. Brazed at 1000 °C this gap would reach complete IS after a braze time of 60 minutes. This starting gap would widen to 55, 57, 107, 141 μm and undergo complete IS in 53, 35, 30 and 38 minutes for braze temperatures of 1050 °C, 1100 °C, 1130 °C and 1175 °C respectively.
Therefore, optimizing the braze temperature can have a significant impact on the time for IS over all levels of starting gap thickness, reducing it in half in the 50 μm case considered. For gaps below 65 μm, the shortest IS time occurs at a temperature of 1130 °C, whereas with higher gaps, a temperature of 1100 °C leads to the shortest IS. This transition is due to the competing influence of IBU and gap widening. The higher IBU and rate of IS at 1130 °C compensates for gap widening in the case of smaller gaps.
The rate of IS measured as a function of temperature in the current study is compared to the rate derived from a selection of other researchers in Figure 18. All the literature data was derived from metallurgical measurements of the centreline eutectic width from sandwich joints or through the gap width where no centreline eutectic appears in wedge samples. A comprehensive study of the influence of braze temperature on IS for a range of base metal/filler combinations was completed by Reference 10. This included IN600, M007, IN713C and MAR-M247. Pouravnari et al.[19] has recently pointed out that the time for complete isothermal solidification is longer for base metals with a low boride former content (Cr + Mo + Nb + Ta + W). The alloys study by Reference 10 have relatively low boride former contents in the summed ranged of 15 to 21 wt pct. This is the reason why the overall rate of IS is low for these alloys, in the range of 2.5 to 4 μm/(min.)1/2.
This may also be the reason for the relatively low sensitivity of IS rate to braze temperature in these alloys. However, it may also be because the centreline eutectic was measured rather that the full liquid width. The IN625 alloy of the current study has a high boride former content of 33.5 wt pct which explains its relatively high overall rate of IS compared to the study of Reference 10. The only previous study of the influence of braze temperature on the rate of IS for IN625 was that of Reference 11 who used a filler alloy with a composition of Ni-15wt pct Cr-3.5 wt pct B. Because this alloy has a similar liquidus temperature and boron content as PALNICRO-36M, its is expected to be a good comparison. Figure 18 shows that the rate measured in the two studies are close at lower braze temperatures where base metal dissolution in the current study was shown to be low. The rates of Sakamoto et al.[11] significantly deviate from the current work at higher temperatures. Since Sakamoto’s study only measures the centreline eutectic width, it makes sense that as dissolution, gap widening and the increasing formation of a hypoeutectic liquid composition magnifies at higher temperature, disagreement with the current study increases.
Reference 7 measured the rate of IS vs. temperature for a IN718/BNi-2 braze joint. Since IN718 also has a high boride former content, it exhibits a similar rate of IS at lower temperatures to the current study. However, like the work of Reference 11 it begins to deviate from the current study at higher temperatures. Based on these comparisons, it can be concluded that, when brazing is performed at temperatures close to the liquidus temperature of the filler metal, dissolution is relatively low and measuring the centreline eutectic provides accurate measurements of the rate of IS. However, as dissolution increases, an accurate measure of the rate of IS can only be achieved if a method capable of measuring the entire liquid width is used.
4 Conclusions
In this work, a method for measuring the full liquid width of a braze joint during TLPB was developed based on enthalpy of solidification values obtained from DSC and microstructural measurements. This differs from most techniques in the literature which only measure the centreline eutectic width using metallographic methods. The importance of measuring the full liquid width increases when examining the influence of braze temperature on TLPB. As the braze temperature increases above the eutectic temperature (or liquidus of the filler) base metal dissolution increases. This causes liquid width gap widening and a change in the liquid composition, shifting towards a hypoeutectic composition. Upon cooling, the liquid remaining in the joint solidified first through primary dendrites and then through eutectic formation. It is generally very difficult to distinguish the actual ISZ layer from the primary dendrites metallographically. Thus, measuring centreline eutectic is typically what is done. However, this measurement will underestimate the amount of liquid present at a particular braze time. This level of underestimation should increase with an increase in braze temperature.
Through comparison of full liquid with and eutectic only measurements, the current study has confirmed that the rate of isothermal solidification and gap widening due to dissolution is significantly underestimated when relying on eutectic measurements only. However, the time for complete isothermal solidification is the same whether considering the full liquid width, or eutectic width. The underestimation of the IS rate by eutectic centreline measurements increases as the braze temperature increases above the filler liquidus temperature.
The DSC enthalpy measurements can be used to build a TLPB processing map for a range of braze temperatures and starting filler metal interlayer thickness. This map can predict the degree of gap widening and the time for complete isothermal solidification for this gap. Results indicate that an optimum braze temperature exists where the opposing influences of gap widening and increased IS rate combine to give the shortest IS completion time.
References
O.A. Ojo, N.L Richards, M.C. Chaturvedi, Sci. Tech. Weld. Joining, 2004, 9(6), pp. 532-40.
O.A. Idowu, N.L. Richards, M.C. Chaturvedi, Mat. Sci. and Eng. A, 2005, 397, pp. 98-112.
K. Tokoro, N.P. Wikstrom, O.A Ojo, M.C. Chaturvedi, Mat. Sci. & Eng. A, 2008, 477, pp. 311-318.
M. Mosallaee, A. Ekrami, K. Ohsasa, K. Matsuura, Met. & Mater. Trans. A, 2008, 39A, pp. 2389- 2402.
A.T. Egbewande. C. Chukwukaeme, O.A. Ojo (2008) Mater. Charact. 59:1051–58.
V. Jalilvand, H. Omidvar, M.R. Rahimipour, H.R. Shakeri, Materials and Design, 2013, 52 pp. 36–46.
M. Pouranvari, S.M. Mousavizadeh, Mater. Tech., 2015, 49: 247-251.
I. Tuah-Poku, M. Dollar, T.B. Massalski, Metallurgical Transactions A, 1988, 19: 675-686.
K.D. Partz, E. Lugscheider, Welding Journal, 1983, 62, pp. S160-S164.
Y. Nakao, K. Nishimoto, K. Shinozaki, C. Kang, Trans. Jpn. Weld. Soc., 1989, 20: 60-65.
A. Sakamoto, C. Fujimara, R. Hattori, S. Sakai, Welding Journal, 1989, 68, pp.63-67.
M.A. Arafin, M. Medraj, D.P. Turner, P. Bocher, Mat. Sci. Eng. A, 2007, 447:125-133.
D.C. Murray, S.F. Corbin, J. Mater. Proc. Tech., 2017, 248, pp. 92-102.
E.D. Moreau, S.F. Corbin, Met. Mater. Trans. A., 2020, 51: 2882-2892.
C. Tadgell, S.F. Corbin, Can. Met. Quart., 2020, 59:3, pp. 288-296.
A. Ghasemi, M. Pouranvari, Sci. Tech. Weld. Joining, 2018, 23: 441-448.
J. Ruiz-Vargas, N. Siredey-Schwaller, P. Bocher, A. Hazotte, J. Mater. Proc. Tech., 2013, 213, pp. 2074-80.
X.P. Zhang, Y.W. Shi, Scripta Materialia, 2004, 50, pp. 1003-1006.
M. Pouranvari, A. Ekrami, A.H. Kokabi, Sci. & Tech. Welding and Joining, 2018, 23: 13–18.
Acknowledgments
Thanks to Thomas Georges, Francesco D’Angelo and Elizabeth Kendrick of Pratt and Whitney Canada for the contributions and guidance with this work.
Funding
This study was funded by the Government of Canada under the Natural Sciences and Engineering Council and Pratt and Whitney Canada under the Industrial Research Chair program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The research described in this paper was partially funded by Pratt and Whitney Canada (P&WC). The work was carried out with guidance and communication with P&WC personnel
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 14, 2020; accepted December 21, 2020.
Rights and permissions
About this article
Cite this article
Corbin, S.F., Tadgell, C.A. Determining the Influence of Braze Temperature on the Dissolution Behaviour and Kinetics of Isothermal Solidification During Transient Liquid Phase Bonding (TLPB) Ni-Based Superalloys. Metall Mater Trans A 52, 1232–1247 (2021). https://doi.org/10.1007/s11661-021-06143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-021-06143-6