Abstract
In this study, a SiC/ZrB2 multilayer coating with a functional gradient structure was prepared on graphite to increase the ablation resistance. The SiC coating as the inner layer was applied by the reactive melt infiltration method. Then, a ZrB2 outer layer coating was applied by argon gas shrouded plasma spray. The effect of the shielding gas flow rate on the amount of oxides and quality of the ZrB2 coating was studied. To evaluate the ablation resistance of the coatings, the specimens were exposed perpendicular to an oxy-propane flame for 60 second with a temperature of 2473 K and heat flux of 3000 kW/m2. The single-layer SiC coating showed 24.24 pct mass loss. The results indicated that applying the ZrB2 coating significantly improved the ablation resistance and reduced the mass ablation rate. In the plasma spray process, by applying the argon shrouding with a flow rate of 150 L/min, the oxidation of ZrB2 phase decreased from 41.6 to 4.8 pct. In addition, the weight loss and mass ablation rate decreased from 12.79 and 1.857 × 10−3 g/cm2/s to 0.12 pct and 0.0393 × 10−3 g/cm2/s, respectively. The improvement of the ablation resistance of the coating prepared by argon gas shrouded plasma spray could be attributed to the lower amount of oxides and pores in the as-sprayed coating and better cohesion of splats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, new materials for hypersonic flight vehicles, re-entry vehicles, nozzles, etc., are required. These materials must be able to tolerate oxidizing and corrosive atmospheres at temperatures > 2270 K for long durations.[1,2,3] Carbon materials, including graphite and C/C composites, which are known as a class of advanced materials, are the best candidates for these applications.
Carbon materials possess a unique combination of desirable properties including low density, high toughness and high specific strength (strength to weight ratio), a very low coefficient of thermal expansion and mechanical property retention at high temperatures. However, the poor oxidation resistance of carbon materials above 773 K potentially limits their high-temperature applications.[4,5] Therefore, carbon materials must be protected against oxidation.
One of the most effective methods to improve the oxidation resistance of carbon materials is to use ceramic coatings. Among various ceramics, silicon-based ceramics such as SiC coatings show great potential for the protection of carbon materials from oxidation at high temperatures because of the beneficial formation of a SiO2 protective film that can efficiently prevent the diffusion of oxygen into the substrate.[6,7,8,9] Unfortunately, the SiC coatings have two main disadvantages: (1) Due to thermal expansion mismatches between the carbon substrate and SiC (αSiC = 3 × 10−6 – 5 × 10−6/C, αC = 2.2 × 10−6 – 3 × 10−6/C), poor adhesion is generally obtained along with a high crack density in the coating.[10,11] One of the approaches employed to solve this problem is the formation of the SiC coating with the transition interface. A functional gradient layer due to the formation of a material concentration gradient at the substrate/coating interface minimizes the influence of the CTE mismatch.[12,13]
In fact, a gradual change in the concentration from 100 pct silicon carbide on the surface to 100 pct carbon at the substrate and lack of a clear interface between the substrate and coating result in a gradual change of CTE from the surface toward the substrate. As a result, crack formation at the coating/substrate interface due to thermal shocks is prevented.[10,14,15] (2) The maximum working temperature of the SiC coating is limited to about 1873 K because of the active oxidation of SiC and volatilization of SiO2 at higher temperatures.[16]
To protect carbon materials at higher temperatures, the idea of using another ceramic material with high thermal resistance on the SiC has been raised. Due to the unique properties of ultra-high temperature ceramics (UHTC) in an oxidizing atmosphere > 2273 K, recent studies have been carried out on the application of these ceramics as coatings in thermal protection systems (TPS).[17,18]
Among the family of UHTCs, ZrB2 is one of the best choices for use in high-temperature applications because of its unique physical and chemical properties including a high melting point (3519 K), high thermal conductivity (58.2 W/m/K), good thermal shock resistance, a low coefficient of thermal expansion (5.8 × 10−6/C) and high hardness. Moreover, ZrB2 retains strength at high temperatures and is chemically stable in corrosive environments; compared with other UHTCs, it has a low density (about 6.1 g/cm3). In past years, some studies have been done on ZrB2-based composite coatings applied by different methods such as reactive melt infiltration (RMI),[10,19] slurry,[20] precursor infiltration pyrolysis (PIP)[17] and plasma spray.[21]
Compared with other methods, plasma spray-based methods can produce thick coatings and can be used to fabricate components with complex geometries. Also, this method is appropriate for applying ultra-high-temperature coatings. Different plasma spray methods are atmospheric plasma spray (APS), vacuum plasma spray (VPS), inert gas shrouded plasma spray, low-pressure plasma spray (LPPS), supersonic plasma spray and solid inert barrier shroud plasma spray (SSPS). Due to the possibility of complete oxidation of particles during the spray process, the use of the APS method is not feasible. On the other hand, controlled atmosphere plasma spray methods (i.e., vacuum plasma spray and low-pressure plasma spray) are expensive. This restriction limits the application of the vacuum plasma spray (VPS) and low-pressure plasma spray (LPPS) methods, especially in industrial applications. Shrouded plasma spray (SPS) is an alternative method that is promising enough for use to reduce production costs. In this route, the contact of particles with oxygen by an inert gas such as argon and nitrogen is limited, and as a result the oxidation of powders is reduced. Therefore, the argon gas shrouded plasma spray method is suitable because of it allows creating thick and compact coatings with low porosity.
Up to now, many studies have been conducted on ultra-high-temperature coatings prepared by the atmospheric plasma spray method.[21,22,23,24] However, preparation of the ultra-high-temperature coatings by a plasma spray under argon shielding gas and optimizing the spray parameters, especially the flow rate of the shielding gas, has not been studied. Therefore, in this study, to reduce the cost of ultra-high-temperature coating preparation, an inert gas shrouded plasma spray method was used instead of the VPS and LPPS processes.
The purpose of this study was to improve the ablation resistance of graphite at temperatures > 2073 K by applying a SiC/ZrB2 multilayer coating. The study consists of two parts. In the first part, a functional gradient SiC coating was applied as the transition layer on the graphite substrate. The SiC inner coating creates a suitable chemical and physical matching with the graphite substrate and reduces the coefficients of thermal expansion mismatch between the ZrB2 coating and graphite. In the second part, the ZrB2 coating was deposited by the inert gas shrouded plasma spray method on the SiC-coated graphite substrates. Then, the flow rate of the shielding gas was optimized to apply ZrB2 coatings with low porosity and oxide. The purpose of applying the ZrB2 layer in this study was to create a dense stable thermal layer on the SiC surface so that it could protect the specimen against the high flow rate of hot gases during the ablation test.
2 Experimental Procedure
2.1 Coating Preparation Process
In this study, graphite specimens with dimensions of 25 × 25 × 10 mm3 and density of 1.82 g/cm3 were used as substrate. The SiC functional gradient coating was applied as the inner layer on the graphite by the reactive melt infiltration method.[10,15] Then, the ZrB2 outer coating was created on the SiC coating using the plasma spray method.
2.1.1 Preparation of the SiC Coating
The surfaces of the graphite substrates were polished and cleaned in an ultrasonic bath with ethanol. Then, they were dried at 373 K for 0.5 hour in an oven. Al2O3, C and Si powders were used as bed materials to produce the SiC FG coating by the reactive melt infiltration method. To achieve a uniform distribution of the particles, they were mixed in a polymer container in a jar mill using alumina balls for 2 hour. The graphite specimens were placed in a graphite crucible with the pack mixture and were heat-treated at 1873 K under an argon atmosphere for 2 hour.
2.1.2 Preparation of the ZrB2 Coating
To prepare the ZrB2 coating, atmospheric plasma spray equipment (Plasma Technik A-3000 S) with an F4 plasma gun (Sulzer Metco, Switzerland) was used. Argon and hydrogen were used as the main and secondary gases for the plasma spray process, respectively. The movement of the workpiece and the gun was performed automatically to ensure repeatability and uniformity. In this research, the type and the flow rate of the shroud gas were considered as the variable parameters. In Table I, the plasma spray parameters are presented.
In this study, zirconium diboride powder with purity > 99 pct and particle size < 10 μm was used. Before the coating process, the ZrB2 powder was mixed with the PVA and then heated at 423 K. The resultant mass was subjected to grinding and sieving to obtain a powder in the range of 50 to 150 μm to make it suitable for the spray process.
2.2 Single-Line Scan Spray Experiments
To determine the condition of deposition under inert gas shrouding, a single-line scan-spray experiment was used according to Table I. In this experiment, glass specimens with dimensions of 1.5 × 1.5 cm2 at 500 mm/s speed were passed in front of a spray torch to form a thin layer on them. By microstructural characterization of this layer using an optical microscope and scanning electron microscope (SEM), optimized parameters for deposition of ZrB2 were obtained.
2.3 Flame Test
The aim of the flame test was to determine the ablation resistance of the coated samples against the heat generated by a flame with a stable temperature and thermal flux. Oxygen and propane gas with flow rates of about 2 and 1 L/min, respectively, were used to form the flame. The heat flux of the flame perpendicular to the sample surface was approximately 3000 kW/m2. The distance between the nozzle and sample surface was 10 mm, and the flame was applied perpendicularly to the surface of the samples for 60 second. The surface temperature of the sample was measured during the test with an optical pyrometer, which ultimately reached 2473 K. The experimental set-up of the flame ablation test is shown in Figure 1.
The mass of the samples was measured before and after the test, and the mass change percentage was calculated according to Eq. [1]:
The mass ablation rate (Rm) was calculated by Eq. [2]:
In this equation, Δm is the mass change of the samples before and after the ablation test, t is the ablation time, and S is the surface area of the sample that has been exposed to the flame.
2.4 Characterization
An X-ray diffractometer (XRD, Philips, PW1730) equipped with a copper X-ray source (λ = 1.5406 Å) was used for the phase analysis of the coatings. The Rietveld refinement method was used to calculate the relative contents of phases in the coating. Elemental distribution and morphology of the coatings before and after the ablation test were analyzed by an optical stereo microscope, scanning electron microscope (SEM, VEGA3 TESCAN) and MIRAII TESCAN field emission scanning electron microscope (FE-SEM) equipped with energy-dispersive spectroscopy (EDS).
3 Results and Discussion
3.1 Phase and Microstructural Characterization of the SiC Coating
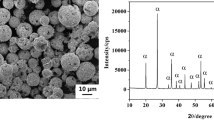
Figure 2 shows the X-ray diffraction pattern and SEM micrograph of the SiC coating produced by the RMI method. As shown in Figure 2(a), the peaks corresponding to β-SiC appear as the main phase of the coating. In addition to the SiC peaks, the Si peaks are also seen because of the high percentage of silicon in the packed powder. Figure 2(b) shows that the coating has good continuity without any cracks and voids.
Figure 3 shows the cross-sectional SEM image and linear EDS analysis of the SiC graded coating. This figure indicates that the thickness of the coating is about 600 μm, and no delamination or cracks are detected. As can be seen, the concentration of Si gradually increases from the substrate to the coating surface, and the concentration of carbon is reduced. This indicates the development of a gradient interface (FG coating). This FG coating causes a slight change in the coefficient of thermal expansion in the component; as a result, the cracks caused by the temperature change during the cooling from high to room temperature are prevented in the coating/substrate interface.[15]
3.2 Effect of the Inert Gas Shrouding on the Deposition of ZrB2 Particles
Figure 4 shows the FE-SEM images of the ZrB2 particles on the glass substrate in single-line scan-spray experiments at different argon gas flow rates. As can be seen, the morphology of the layer formed on the glass substrate consists of either fully or partially molten droplets and fine spherical particles. The formation of the splats after cooling is a function of the process parameters such as the powder size distribution, velocity, temperature, and solidification rate of in-flight particles as well as the substrate temperature and its material.
From Figure 4, it can be concluded that the argon gas has an effective role in reducing the fine and semi-molten particles and increasing the number of molten particles and their flattening (with the increase in the shielding gas flow rate). This could be due to higher heat transfer to the ZrB2 particles at a higher argon gas flow rate.
According to Figure 4 and by comparing the images at various argon gas flow rates, a flow rate < 150 L/min is probably insufficient to prevent the air entrance into the plasma flame. Also, flow rates > 150 L/min cause turbulence in the plasma jet; therefore, argon gas flow rates up to 150 L/min in this study have been investigated. The layers formed on the glass specimens in the single-line scan-spray experiment showed that the highest argon gas flow rate that can reduce the air entry inside the plasma jet is about 150 L/min.
Figure 5 shows the length of the plasma jet at different argon gas flow rates (0 to 150 L/min). It can be seen that the length of the plasma flame increases with increasing argon gas flow rate. As a result, the powder particles have a longer residence time in the high-temperature region of the plasma flame; thus, the temperature of the particles increases. Therefore, as shown in Figure 4, the amount of splats increases with increasing argon gas flow rate.
Also, to investigate the effects of the type of shielding gas, both argon and nitrogen were studied. Figure 6 shows the FE-SEM images of ZrB2 deposits formed in single-line scan-spray experiments on glass specimens for Ar and N2 gases. As can be seen, when argon is used, there is a slight improvement in the splats compared with nitrogen shielding gas.
Regarding the lower specific heat capacity of argon (0.52 j/g K) compared with nitrogen (1.04 j/g K), it seems that the argon gas absorbs less heat from the plasma jet and reduces the temperature of the plasma flame less than nitrogen does. On the other hand, Matthews[25] investigated the effect of the external shielding gas (argon and nitrogen) on the plasma spray process of Ni-20Cr coatings and reported that there is no significant difference in the type of the shielding gas. Due to the lower melting point of Ni-20Cr, it seems that the difference between the plasma flame temperature in two conditions of using argon or nitrogen shielding gas does not have a remarkable effect on the melting of this material. However, for ZrB2, which has a high melting point, this difference is evident.
The ZrB2 coating was deposited on the SiC-coated graphite in a similar condition to the single-line scan-spray experiment with argon gas shrouding. In Figure 7, the surface SEM image of the ZrB2 coating is presented, which confirms the results of the single-line scan-spray experiment. As seen in this figure, with increasing argon flow rate, the number of splats and their degree of flattening are increased and the number of spherical particles decreased. Also, in the constant argon flow rate, the number of splats and their degree of flattening are increased compared with the single-line scan-spray experiment because of the heat transfer to the particles and the substrate during the multiple passes of the plasma torch. In the single-line scan-spray experiment, the heat transferred to the particles after the deposition is ignored.
Figure 8 shows the cross-sectional SEM images of the SiC/ZrB2-coated graphite at two different magnifications for samples coated under argon gas flow rates of 0 and 150 L/min.
A 600-μm-thick graded SiC coating with a 200-μm-thick ZrB2 outer layer coating can be observed. The mechanical locking at the substrate/coating interface has occurred properly, and there is no crack or discontinuity in the interface of the coatings. As can be seen, in the sample under the 150 L/min argon gas flow rate, the coating has a denser structure than other samples, which indicates better melting of the particles and decreasing oxidation of particles during spraying. Figure 9 presents the zirconium, boron and oxygen elemental mapping at the cross section of the ZrB2 coating. As can be seen, increasing the argon flow rate of argon from 0 to 150 L/min decreases the oxygen content. In other words, the oxidation of ZrB2 has been limited.
These results show that an increase in the argon flow rate (up to 150 L/min) resulted in a significant reduction in particle oxidation.[26,27] The phase analysis of the samples at different argon flow rates is presented in Figure 10. As can be seen, all samples contain ZrB2 and ZrO2 phases with different percentages. The results of the phase analysis confirm the results of the map analysis of the elements and the effect of argon gas flow rate on the reduction of ZrB2 particle oxidation. As can be seen, ZrO2 is decreased from 41.6 to 4.8 pct by increasing the argon gas flow rate from 0 to 150 L/min. In Figure 11, the Williamson-Hall diagram is plotted for each coating. The diagram slope for all coatings is positive, which indicates the formation of the tensile residual stress in the coatings after the coating process.[28] Also, increasing the ZrO2 content increased the amount of residual stress from 132 to 264 MPa.
3.3 Ablation Behavior of the SiC/ZrB2 Coatings
The results of the flame test including weight change (ΔW pct) and mass ablation rate (Rm) are presented in Table II. As can be seen, the weight change and mass ablation rate of the SiC single-layer coating are greater than those of the SiC/ZrB2 coating, which indicates the lower ablation resistance of the SiC coating. The weight loss and mass ablation rates for the SiC coating were 24.24 pct and 6.64 × 10−3g/cm2/s, respectively. The results show that using the SPS method with a higher argon gas flow rate increases the ablation resistance remarkably. The lowest erosion rate (0.0393 × 10−3 g/cm2/s) was obtained at an argon gas flow rate of 150 L/min.
In Figures 12 and 13, the macroscopic images of the SiC/ZrB2 and single-layer SiC samples after the flame test are shown. A comparison of the images shows that the SiC single-layer coating completely disappeared after 60 second and the graphite substrate was ablated. In the bilayer coatings, the ZrB2 coating has been converted to ZrO2 and then delaminated after the ablation test during cooling. In the next section, the mechanism of each sample is described separately.
3.3.1 Investigation of ablation resistance of the single-layer SiC coating
In Figure 13, the SEM image and EDS analysis of the SiC coating after the ablation test are shown. This image indicates that the coating is completely destroyed and the graphite substrate has been exposed to the flame.
In the early stage of the ablation test, SiC converts to the SiO2 glassy phase because of the flame heat according to Reactions [3] and [4]. The formation of the SiO2 phase can partially fill the surface defects and closes the paths of oxygen penetration into the substrate, but in the central part of the sample, which is directly exposed to the flame, because of the high pressure of the flame, part of the glassy phase flies out of this region and thus can no longer play its protective role in the central ablation region[29] (SEM image in Figure 14).
With increasing ablation time, according to the surface temperature of the sample, which is measured to be up to 2473 K, the SiO2 glassy phase tends to evaporate or decompose according to Reactions [5] and [6]. Also, according to Reaction [7], the free Si undergoes active oxidation under the ablation test condition. In other words, Si is transformed to SiO gas instead of converting to molten SiO2.[29]
On the other hand, the active oxidation of SiC might occur and generate SiO because of the high temperature of the flame according to Reaction [8].[30]
One of the main problems of SiC single-phase coatings in ablative environments is the mechanical instability of the protective layer against the shear force produced by the flame pressure.[29,31] In particular, when the working temperature of the sample increases, not only does the SiO2 protective phase evaporate, but also its fluidity increases remarkably. This causes the protective layer formed on the surface to be easily removed from the surface.[29,31]
If the ablation process continues, the entire protective film in the central ablation region separates from the surface through a layer-by-layer mechanism[29]; finally, the graphite substrate is directly exposed to the flame because of the protective film scouring (according to Reactions [3] through [8]). This suggests that the SiC single-layer coating cannot resist temperatures > 2073 K and therefore cannot protect the graphite substrate in this condition. For this reason, to protect the carbon substrate at temperatures > 2073 K, UHTC coatings were deposited on the SiC coating. The ablation process of the SiC coating is illustrated schematically in Figure 15.
3.3.2 Effect of argon gas flow rate on the ablation resistance of the SiC/ZrB2 coating
Figure 12 shows the SiC/ZrB2 coatings after the flame test. A white layer is formed on the surface of the samples. This white layer is zirconium oxide, formed by the oxidation of ZrB2. The results of the ablation test in Table II show that applying the ZrB2 coating increased the ablation resistance of the coating because of the formation of the ZrO2 protective layer on the surface, which can prevent oxygen penetration into the substrate. The comparison of the mass change of the SiC/ZrB2 coatings shows that, with increasing argon gas flow rate, the ablation resistance of the coating improved, so the ZrB2 coating prepared at the argon gas flow rate of 150 L/min exhibits the best ablation resistance. It should be noted that during the ablation test, delamination does not occur in the coating; therefore, the coating retains its protective role. The coating delamination occurs after the ablation test and during the cooling owing to the phase changes and the volume changes caused by the transformation of Mon-ZrO2 → Tet-ZrO2. Also, as shown in the macroscopic images of the samples in Figure 13, the coating delamination decreased by increasing the argon gas flow rate (due to the decrease in the percentage of ZrO2 phase).
In general, increasing the flow rate of the shielding gas resulted in the following:
-
1.
Reduction of oxidation of ZrB2 particles during the spray process: In fact, with an increase in the flow rate of the shielding gas, according to the XRD pattern (Figure 10) and analysis of elemental distribution (Figure 9), the oxidation of the ZrB2 phase reduced from 41.6 to 4.8 pct.
-
2.
ZrO2 undergoes phase transformation when coated samples cool from coating temperature to room temperature accompanied by volume expansion, thus leading to the formation of residual stresses in the coating. As shown in Figure 11, this stress is tensile and results in the formation of cracks in the coating. These cracks lead to a reduction in the ablation resistance of the coating.
-
3.
Since the coefficient of thermal expansion of ZrO2 is greater than that of ZrB2, according to Eq. [9], an increase of ZrO2 content in the coating increases the mismatching of the coefficients of thermal expansion, which can lead to increasing the residual stresses in the coating. Also, increasing the amount of the residual stresses increases the number of cracks, which, on one hand, leads to increasing the oxygen penetration paths and, on the other, increases the coating delamination after the ablation test, which is confirmed by Figure 12. The surface FE-SEM image of the SiC/ZrB2 coating (shielding gas flow rate of 30 L/min) in Figure 16 shows the coating delamination clearly.
$$ \sigma \; = \;\Delta T \cdot \alpha [E/(1 - \nu )] $$(9)where ΔT is the difference between the ambient temperature and testing temperature, α is the CTE difference between the coating and the substrate, and E and ν are Young’s modulus and the Poisson ratio of the coating, respectively.
-
4.
As explained above, an increase in the flow rate of the shielding gas increases the degree of flattening, and therefore the adhesion of the coating improves. One of the advantages of increasing the coating adhesion is its greater resistance to cracking. In other words, by increasing the coating adhesion, the delamination of the coating (due to crack propagation) decreases. Therefore, the protective role of the coating will be greater.
In Figure 17, the surface FE-SEM image of the ZrB2 coating prepared under the shielding gas flow rate of 150 L/min is shown after the ablation test. In this image, the relatively dense structure of the ZrO2 coating is visible.
The voids created during the ablation test are also found on the surface of the coating. The reason for the formation of these voids is the release of gases caused by the oxidation of the coating according to Reaction [10]:
Since B2O3 has a high vapor pressure, it rapidly evaporates and exits from the system at temperatures > 1273 K,[28] resulting in void formation on the coating surface. By creating these voids, the oxygen penetrates the SiC layer, and therefore the oxidation rate of the SiC layer increases (Reactions [3], [4] and [8]).
In the FE-SEM image of the ablated surface of the SiC/ZrB2 coating (Figure 18 ), there are dark phases containing Si and O elements. According to the XRD results (Figure 19), these regions are SiO2 glassy phase formed by SiC phase oxidation. This glassy phase probably penetrates from the subsurface layers to the outer layer and fills the cracks created on the surface; it inhibits the oxygen penetration toward the substrate. The bright areas in Figure 18 are also related to the ZrO2 phase.
The SiO2 glassy phase surrounds the ZrO2 particles and creates a mosaic structure. The formation of the mosaic structure prevents the ZrO2 particles from scouring and thus inhibits oxygen penetration into the sample. As a result, it increases the ablation resistance. On the other hand, the SiO2 phase acts as a binder and keeps the ZrO2 particles together. It also increases the adhesion of the ZrB2 layer and effectively inhibits the ablation of the protective layer.[31] The ablation process of the SiC/ZrB2 coating is illustrated schematically in Figure 20.
Figure 21 shows a network of cracks created on the surface of the ZrB2 coating under shielding gas flow rates of 30 and 150 L/min at different magnifications after the ablation test. As mentioned above, if the cohesion between the splats is weak, when the coating undergoes ablation because of the difference in the thermal expansion coefficients, residual stress will be created in the coating, and this stress causes spallation. However, if the adhesion of the ZrB2 coating to the SiC-coated substrate and cohesion between the splats are high, thermal expansion coefficient mismatch does not allow interface destruction, and thus the residual stresses will be released in the form of surface network cracks. The cracks on the surface of these coatings seem to have been formed for this reason. According to the SEM images, the number of these cracks at a shielding gas flow rate of 150 L/min is higher than that at 30 L/min.
The reason for the presence of these cracks in the 150 L/min specimen is the greater cohesion between the splats. In other words, the greater the number of these cracks is, the better the cohesion between the splats and the better adhesion of the coating to the substrate.
4 Conclusions
In this research, a SiC/ZrB2 multilayer coating was prepared on graphite by the reactive melt infiltration and plasma spray methods. A SiC functionally gradient coating was prepared as an inner layer coating and ZrB2 as an outer layer. The effect of the flow rate of the argon gas on the coating quality and the ablation resistance of the specimens was investigated. Results showed that with increasing the flow rate of the shielding gas from 0 to 150 L/min, the oxidation of the particles in the plasma jet decreases from 41.6 to 4.8 pct. Also, argon gas inhibits the air entering into the plasma jet. By the formation of a barrier against the air, the velocity and temperature of the particles increase. As a result, the coating quality improves. The results of the ablation test showed that the ZrB2 coating produced under the external shielding gas flow rate of 150 L/min had the lowest ablation rate (about 0.0393 g/cm2/s) and the ZrB2 coating produced in air had the highest ablation rate (about 1.857 g/cm2/s). This is due to the high quality of the coating and the proper cohesion between the splats under an argon gas flow rate of 150 L/min.
Change history
27 October 2021
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11661-021-06489-x
References
G.W. Meetham, M.H. Van de Voorde: Materials for high temperature engineering applications, Springer, New York 2012.
K. Upadhya, J. Yang, W. Hoffman: Am. Ceram. Soc. Bull., 1997, vol. 76, pp. 51-56.
M. Tului, G. Marino, T. Valente: Surf. Coat. Technol., 2006, vol. 201, pp. 2103-08.
J. Pourasad, N. Ehsani, Z. Valefi, S.A. Khalifesoltani: Surf. Coat. Technol., 2017, vol. 323, pp. 58-64.
C. Hu, Y. Niu, H. Li, M. Ren, X. Zheng, J. Sun: J. Therm. Spray Technol. 2012, vol. 21, pp. 16-22.
X. Yang, Q. Huang, Z. Su, X. Chang, L. Chai, C. Liu, L. Xue, D. Huang: Corros. Sci., 2013, vol. 75. pp. 16-27.
Y.-L. Zhang, H.-J. Li, Q.-G. Fu, K.-Z. Li, J. Wei, P.-Y. Wang: Surf. Coat. Technol., 2006, vol. 201, pp. 3491-95.
W. Shi, Y. Tan, J. Hao, J. L: Ceram. Int., 2016, vol. 42, 17666-72.
L. Cheng, Y. Xu, L. Zhang, X. Yin: Carbon, 2000, vol. 38, pp. 1493-98.
A. Abdollahi, N. Ehsani, Z. Valefi: Mater. Chem. Phys., 2016, vol. 182, pp. 49-61.
J. Li, R. Luo, Y. Chen, Q. Xiang, C. Lin, P. Ding, N. An, J. Cheng: Appl. Sur. Sci, 2008, vol. 255, pp. 1967-74.
A. Abdollahi, N. Ehsani, Z. Valefi, A. Khalifesoltani, J. Mater. Eng. Perform., 2017, vol. 26, pp. 2878-89.
J. Kim, W.-J. Kim, D. Choi, J. Park, W.-S. Ryu: Carbon, 2005, vol. 43, pp. 1749-57.
C. Cairo, M. Graca, C. Silva, J. Bressiani: J. Eur. Ceram. Soc., 2001, vol. 21, pp. 325-29.
A. Abdollahi, N. Ehsani: Metall. Mater. Trans. A, 2017, vol. 48, pp. 265-78.
D. McKee: Carbon, 1987, vol. 25, pp. 551-557.
E.L. Corral, R.E. Loehman: J. Am. Ceram. Soc., 2008, vol. 9, pp. 1495-502.
G. Pulci, M. Tului, J. Tirillò, F. Marra, S. Lionetti, T. Valente: J. Therm. Spray Technol., 2011, vol. 20, pp. 139-44.
J. Pourasad, N. Ehsani: J. Alloy. Compd., 2017, vol. 690, pp. 692-98.
X. Yang, L. Wei, W. Song, Z. Bi-feng, C. Zhao-hui: Composite B, 2013, vol. 45, pp. 1391-96.
C. Bartuli, T. Valente, M. Tului: Surf. Coat. Technol. 2002, vol. 155, pp. 260-73.
B. Xu, R. He, C. Hong, Y. Ma, W. Wen, H. Li, T. Cheng, D. Fang, Y. Yang: J. Alloy. Compd., 2017, vol. 702, pp. 551-60.
Y. Zhang, Z. Hu, H. Li, J. Ren: Ceram. Int., 2014, vol. 40, pp. 14749-55.
C. Fourmond, G. Da Rold, F. Rousseau, C. Guyon, S. Cavadias, D. Morvan, R. Mévrel: J. Eur. Ceram. Soc., 2011, vol. 31, pp. 2295-302.
S. Matthews: Surf. Coat. Technol., 2014, vol. 249, pp. 56-74.
G. Espie, A. Denoirjean, P. Fauchais, J. Labbe, J. Dubsky, O. Schneeweiss, K. Volenik: Surf. Coat. Technol., 2005, vol. 195, pp. 17-28.
C.C. Berndt, K.A. Khor, E.F. Lugscheider: ASM International, 2001.
A. Abdollahi, Z. Valefi, N. Ehsani, S. Torabi: Int. J. Appl. Ceram. Technol., 2018, vol. 15, pp. 1319-33.
A. Abdollahi, S. Torabi, Z. Valefi, N. Ehsani: Corros. Sci., 2019. vol. 159, pp. 1-16.
P. Wang, S. Zhou, P. Hu, G. Chen, X. Zhang, W. Han: J. Alloy. Compd., 2016. vol. 682, pp. 203-07.
A. Abdollahi, N. Ehsani, Z. Valefi, J. Alloy. Compd., 2018, vol. 745, pp. 798-809.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 9, 2019.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11661-021-06489-x
About this article
Cite this article
Torabi, S., Valefi, Z. & Ehsani, N. RETRACTED ARTICLE: Ablation Behavior of SiC/ZrB2 Multilayer Coating Prepared by Plasma Spray Method. Metall Mater Trans A 51, 1304–1319 (2020). https://doi.org/10.1007/s11661-019-05566-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-019-05566-6