Abstract
The effects of carbon on the phase structure and on the yield stress σ 0.2 in the temperature range from 873 K to 1073 K (600 °C to 800 °C) of the Fe3Al type aluminides alloyed by Zr are analyzed. Four alloys with Zr and C in ranging from 1.0 to 5.0 at. pct of additives were used. The appearing of either Laves phase (Fe,Al)2Zr and/or carbides depend on the difference in concentrations, c Zr − c C. This parameter (c Zr − c C) has been selected instead of the concentration ratio c Zr/c C used in previous works since it exhibits a significantly better correlation with the Laves phase concentration which influences the high-temperature yield stress, σ 0.2, of the tested alloys. The presence of Laves phase or eutectic (matrix—Laves phase), respectively, enhances the value of the yield stress σ 0.2. The amount of Laves phase is decreased by the presence of C due to the affinity of carbon to Zr.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The alloying by zirconium is a method to enhance HT mechanical properties of Fe3Al alloys. Very low solid solubility of Zr in the Fe-Al initiated investigations of the influence of Zr addition to Fe3Al and FeAl alloys. The beneficial effect of zirconium and niobium addition on e.g. creep resistance was first reported in papers by McKamey and Maziasz.[1–3] Significant attention was payed to the effect of Zr on the phase structure and high-temperature (HT) mechanical properties of iron aluminides. Especially the formation of Laves phase (Fe, Al)2 Zr and (Fe, Al)2 Nb was referred.[4–10]
Aside are left the large additions of Zr (i.e., up to 30 at. pct[4,5]). It is important to determine the effect of carbon (present in the raw iron used for the preparation of iron aluminides for structural uses). This may modify the mechanical properties, corrosion process, etc., by the influence of, e.g., the Laves phase. The presence of carbon in iron aluminides with zirconium modifies the formation of Laves phase in the Fe corner of the ternary equilibrium diagram.[6] Low solubility of Zr in D03/B2 Fe-Al results in the formation of Laves phase (Fe,Al)2Zr.
Recently, Kratochvil et al.[11] showed in iron aluminides alloyed by Cr that the ratio of zirconium and carbon influences the phase structure of Fe3Al-type alloys. This was documented by the effects of the ratio c Zr/c C on both the yield stress 0.2 and the creep rate at high temperatures.
Laves phase λ1 (Fe,Al)2Zr and ZrC particles were found primarily along grain boundaries, with some distribution in the grains as well. Their strengthening role is limited to blocking the grain boundaries’ motion because the effect of these particles inside the grains (owing to large distance between the particles) was very small. Partly such effects of low concentrations of Zr and C have been mentioned by Kejzlar et al.[12] The appearing of metastable phases, (Fe1−x Al x )3Zr and Fe2Zr, which originate during the HT deformation[10] was also detected in the iron aluminides alloyed by Cr.
It is therefore the purpose of the present paper to verify the effect of the ratio of zirconium and carbon—described by the difference c Zr − c C—in an alloy without chromium on the structures of Fe3Al alloys and on their HT yield strengths. The difference c Zr − c C was chosen as it better describes the amount of Zr left for the formation of Laves phase, if any, compared with the ratio of both concentrations. This fact is obvious from a significantly better correlation with eutectic (matrix—Laves phase) in case of c Zr − c C compared with c Zr/c C.
2 Experimental Procedure
The alloys were prepared by a vacuum melting and casting under argon. The chemical compositions are given in Table I. The actual compositions of the alloys were established by wet chemical analysis at the Research and Testing Centre, Plzen. The concentrations were chosen to get alloys with different values of c Zr − c C. The alloys contain (25.6 to 26.2) at. pct Al. Concentrations of impurities (from the metals used for the preparation of the alloys) were: 0.1 at. pct Cr, 0.01 at. pct B, 0.1 at. pct Mn.
All alloys were tested in the as-cast state. Samples (parallelepipeds 6 × 6 × 10 mm) for compression test at temperatures ranging from 873 K to 1073 K (600 °C to 800 °C) were prepared from the alloys via electrical discharge machining (EDM). The compressive yield stress was evaluated using a digitally controlled testing machine (INSTRON 1186R). The deformation rate was 1.2 × 10−4 s−1.
The temperatures ranging from 873 K to 1073 K (600 °C to 800 °C) were chosen because these temperatures are mostly above the yield strength anomaly. This is an increase in the strength with the increasing temperature, typically observed for Fe-Al-based alloys with a maximum at about 823 K (550 °C). All the investigated alloys are at these temperatures, B2-ordered, which allows direct comparison of their yield strengths.
Microstructural phase compositions were studied using a field emission-scanning electron microscope (FE-SEM)—Zeiss Ultra Plus—equipped with energy-dispersive spectrometer (EDS)—OXFORD X-MAX 20—and with a detector for electron backscatter diffraction (EBSD)—OXFORD NordlysNano. For the characterization of the carbides, EDS combined with analysis of Kikuchi patterns was used. Analyses were carried out at an accelerating voltage of 20 kV, and the typical primary electron beam diameter was 2 nm.
3 Experimental Results and Discussion
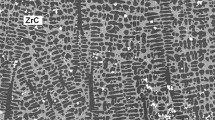
The sizes of the grains of all the investigated as-cast alloys were ranging from 100 to 400 µm. The phase structures of the alloys are summarized in Table I. In alloy 1143 (c Zr − c C = −0.67 at. pct), the volume fraction f v of ZrC is 1.9 pct. ZrC are plates partly seen edgeways. The rest of the carbon forms perovskite AlFe3C0.5 (f v = 4.5 pct) as documented in Figure 1 or is dissolved in the lattice. Porovskite is forming also plates, contrast of which is not excellent. In alloy 1144 (c Zr − c C = 0.53 at. pct), ZrC particles (f v = 2.3 pct) and Laves phase (Fe,Al)2Zr (f v = 4.2 pct) appear. The Laves phase is a part of an eutectic with matrix, Figure 2.
The structures of alloys 1181 and 1182 are characterized by a dendritic arrangement of eutectic (matrix plus Laves phase) immersed in the matrix, Figures 3 and 4. ZrC carbide particles are seen inhomogeneously dispersed, and its volume fraction f v =1.2 pct in 1181 alloy. In 1182 alloy, f v reaches the value of 3.9 pct. The values of f v of the eutectic Laves phase plus matrix are 41.7 and 34.7 pct, respectively, in the both the alloys. The basic point of the above observations is that the eutectic strengthens the tested alloys.
The compression 0.2 yield stresses as a function of temperature was used to describe HT mechanical properties of the tested iron aluminides, which are summarized in Table II. Experimental errors are 3 pct (valid also for yield stress 0.2 values in Table II). The increasing value of HT yield stress 0.2 with the increasing volume fraction of the eutectic (Laves phase λ1 (Fe,Al)2 Zr with matrix) is obvious as seen in Figure 5. Due to the fact that carbon forms ZrC, it lowers the amount of Zr available for the formation of Laves phase λ1 (Fe,Al)2 Zr. Figure 5 shows the comparison of the curves (1144, 1181, and 1182) with c Zr − c C > 0 with the curve of alloy 1143 with c Zr − c C < 0. This relationship (the influence of concentration of zirconium and carbon) between the four alloys studied is kept even during further deformation, i.e., up to the maximum stress (given also for all alloys and temperatures in Table II). Maximum stress is reached between 5 and 10 pct. compressive strain. The lengths of error bars are comparable with the sizes of symbols, and the bars in Figure 5 are not plotted. The effect is less pronounced at 1073 K (800 °C) probably due to the softness of the matrix. The main effect of softening by the exhaustion of Zr in the aluminide by formation of the ZrC remains.
The solubility of Nb in iron aluminide Fe3Al is low compared with Zr. For the similarity of ternary diagrams in respect of Fe-Al-Zr and Fe-Al-Nb with Laves phase in Fe corners, see References 13 and 14. The phase structure and the HT strength of the Fe-26Al-4Nb-1C and Fe-26Al-2Nb-1C alloys are compared in References 15 and 16. The structures of both alloys include NbC carbide and (FeAl)2Nb Laves phase. Only nominal values of c Nb − c C for the mentioned alloys are available. Thus, it correlates to the above reasoning that the presence of the Laves phase is reported only in the Fe-26Al-4Nb-1C alloy, for which higher values of yield stress 0.2 at HT were observed.
4 Conclusions
The amount of the Laves phase λ1 (Fe,Al)2 Zr in Fe3Al aluminides is influenced by the presence of carbon (great affinity of C to Zr). The formation of ZrC carbides has a negative effect on the HT strength of Fe3Al type aluminides, while it lowers the amount of Zr available for the formation of the Laves phase, strengthening the aluminide. The eutectic of Laves phase plus matrix enhances the yield stress 0.2 at high temperatures.
The behaviors of Nb-and C-alloyed iron aluminides reported elsewhere can be understood in the same way. For the use Zr or Nb as an additive to iron aluminides in structural materials, the presence of even small concentrations of carbon must be taken in account.
References
C.G. McKamey, P.J. Maziasz, J.W. Jones, J. Mater. Res., 1992, vol. 7, pp. 2089–2106.
C.G. McKamey, P.J. Maziasz, G.M. Goodwin, T. Zacharia, Mater. Sci. Eng.,1994, vol. A174, pp. 59–70.
C.G. McKamey, P.J. Maziasz, Intermetallics, 1998, vol. 6, pp. 303–314.
A. Wasilkowska, M. Bartsch, F. Stein, M. Palm, K. Sztwiertnia, G. Sauthoff, and U. Messerschmidt, Mater. Sci. Eng., 2004, vol. 380A, pp. 9–19.
A. Wasilkowska, M. Bartsch, F. Stein, M. Palm, G. Sauthoff, and U. Messerschmidt, Mater. Sci. Eng., 2004, vol. 381A, pp. 1–15.
F. Stein, M. Palm, G. Sauthoff, Intermetallics, 2005, vol. 13, pp. 1275–1285.
D.G. Morris, M.A. Muñoz-Morris, L.M. Requejo, Acta Mater., 2006, vol. 54, pp. 2335 – 2341.
D.G. Morris, I.Gutierrez-Urrutia, M.A. Muñoz-Morris, Scripta Mater., 2007, vol. 57, pp. 449–452.
D.G. Morris and M.A. Muñoz-Morris, Mater. Sci. Eng., 2012, vol. 552, pp. 134–144.
D.G. Morris, L.M. Requejo, M.A. Muñoz-Morris, Intermetallics, 2012, vol.13. pp. 862–871.
P. Kratochvil, F. Dobeš, J. Pešička, P. Málek, J. Buršík, V. Vodičková, P. Hanus, Mater. Sci. Eng., 2012, vol. A548, pp. 175–182.
P. Kejzlar, P.Kratochvíl, R. Král, V. Vodičková, Metall. Mater. Trans. A, 2014, vol.45A, pp. 335–343.
F. Stein, G. Sauthoff, M. Palm, Z. Metallk., 2004, vol. 95, pp. 469–476.
M. Palm, J. Alloy Compd., 2009, vol. 475, pp. 173–177.
A. Schneider, L. Falat, G. Sauthoff, G. Frommeyer, Intermetallics, 2003, vol. 11, pp. 443–450.
L. Falat, A. Schneider, G. Sauthoff, G. Frommeyer, Intermetallics, 2003, vol. 13, pp. 1256–1262.
Acknowledgment
The paper is based on the work supported by the Grant Agency of the Czech Republic within the Project 108/12/1452.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 10, 2015.
Rights and permissions
About this article
Cite this article
Kratochvíl, P., Vodičková, V., Král, R. et al. The Effect of Laves Phase (Fe,Al)2Zr on the High-Temperature Strength of Carbon-Alloyed Fe3Al Aluminide. Metall Mater Trans A 47, 1128–1131 (2016). https://doi.org/10.1007/s11661-015-3309-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3309-2