Abstract
Low carbon steel was plasma pack aluminized at 973 K (700 °C) for 5, 10, and 15 minutes in vacuum of 15 mbar. A paste of aluminum was packed around the samples, and the process was carried out in glow discharge of argon gas in one step without further diffusion processes. The aluminized samples were oxidized at 973 K (700 °C) and 15 mbar for 60 minutes in synthetic air. The aluminized and oxidized layers were characterized for iron-aluminum intermetallic phases, local composition, morphology, and hardness of the phases. At 973 K (700 °C) after 5-, 10-, and 15-minute treatments, FeAl3 and Fe2Al5 intermetallic compounds were observed in the surface layers. After 60-minute oxidation, Fe3Al and FeAl compounds appeared at the interface between the compound layers and the oxide scale. Oxidation products were mainly Al2O3 and a few amounts of Fe3O4 and Fe2O3. The thicknesses of the compound layers on the samples were up to 350 μm. The maximum hardness of the aluminized layers was 980 HV0.5.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aluminizing is an effective surface-modification process to improve oxidation and corrosion resistances of steels as well as the hardness of these alloys. The combination of steel and aluminum provides superior properties such as good formability, corrosion resistance, coating brightness, and long service life. The aluminized steel is widely used in applications such as automotive exhaust system parts, heat reflector plates, etc. Hot-dip aluminizing and pack aluminizing are most widely used to develop iron aluminide coatings on steels by enriching the surface layers with a high concentration of Al at different ranges of dipping times and diffusion temperatures.[1] The common aspect of these processes is the diffusion phenomenon during the formation of iron aluminide coatings at the steel-aluminum interface.[2] It has been reported that the iron compounds form by the diffusion of iron toward the coating and the diffusion of aluminum in the opposite direction. Other researchers suggested that the diffusion reactions are principally governed by the interdiffusion of the reactants to form the compound layers.[3] Overall, the dipping or coating is usually performed at temperatures as low as 873 K (600 °C) and then followed by diffusion annealing at temperatures as high as 1273 K (1000 °C) for more than several hours.[1–3] Holding at such temperatures might induce distortion into the workpieces, carbide precipitation, and grain coarsening of the steel matrix. Therefore, lowering the aluminizing temperatures or minimizing the time of process is of great significance in useful application of these techniques.[4]
We have developed a new process called plasma pack cementation in the pilot scale which has been explained elsewhere.[5] In this process, the samples are packed with a paste of powder elements and heated by irradiation of a plasma gas. The necessary heat for quick mixing and simultaneous interdiffusion of elements is provided by the high rate of energy transfer from the interaction of electrons and ions in the plasma gas with the surface. The energetic particles bombard the surface of the samples during aluminizing treatment. This bombardment generates large amounts of dislocations and vacancies on the surface, which accelerates the diffusion of aluminum inside the sample and form the intermetallic compounds in 5 or 10 minutes.[6]
The advantage of plasma pack aluminizing over the conventional hot-dip aluminizing process is lower processing time (5 to 15 minutes) without any further diffusion annealing.
In this study, low carbon steel was packed with aluminum powder without any other activator and heated under argon irradiation for 5, 10, and 15 minutes. The phases, microstructure, morphology, and hardness of the phases were studied using optical and electron microscopy, EDX, XRD, and microhardness testing.
2 Experimental Methods and Materials
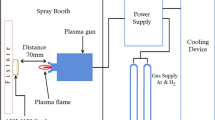
A commercial low carbon steel with the composition (at. pct) of 0.11C, 0.01Si, 0.10Mn, 0.020S (max), 0.01P (max), and remaining Fe was used as the substrate material in this study. The samples were cut with the dimensions of 20 mm × 10 mm × 2 mm from a low carbon steel plate. The samples were ground by SiC papers to remove the surface oxides and contaminants and then washed with alcohol and acetone. The samples were packed with the paste of aluminum pack and placed in the vacuum chamber (Figure 1). The specimens were subjected to glow discharge plasma of argon gas at 15 mbar. The samples were heated under the irradiation of glow discharge to reach the required temperature and held for 5, 10, and 15 minutes. The oxidation of the samples was performed at 973 K (700 °C) for 60 minutes at 15 mbar in synthetic air (79 pct nitrogen and 21 pct oxygen) (Table I). The degradation of untreated low carbon steel after 60 minutes plasma air oxidation was approximately equal to half the thickness of the samples. From an economic point of view, plasma air oxidation was chosen instead of long atmospheric air oxidation. For metallographic examination, the specimens were sectioned across the layers and mounted. The cross sections were mechanically ground using emery papers of grades 120-1500. The polishing was performed using the solution of water and aluminum oxide. The polished specimens were etched using 2 pct Nital at room temperature. The microhardness of plasma pack-aluminized specimens was measured under the load of 500 gf on distinct places of the surface layers. The real measurements were carried out in far enough (from 7 to 10 times of the indentation diameter) separate places across the surface layers to be in accordance with standards. The indentation impressions shown in the figures were just for comparative observation of the hardness values. The indentations on all samples and on tilted places were made several times and averaged after measuring both diameters of the indention impressions.
The surface layers were also studied using SEM and EDX analyses to differentiate and measure the compositions of the phases. XRD with Cu k α radiation was used to identify the exact composition of the phases.
3 Results and Discussion
3.1 Aluminizing Observations
Plasma pack aluminizing of low carbon steel at 973 K (700 °C) for 5, 10, and 15 minutes did not result in significant variations in the microstructure and phases of the compound layers. However, there were reasonable differences in the thickness and irregularity of the surface layers. This trend has also been observed in other investigations.[7] The treatment time was the most important parameter that affected the variation of phases. Figure 2 shows the microstructure and the compositions of the phases on the surface of the sample aluminized for 5 minutes. The optical micrograph of the cross section of the sample (Figure 2(a)) consists of the substrate material (A), a compound layer with a finger-type front inside the substrate (B), and a top layer of aluminum which contains irregular precipitates or platelet (C). The microhardness tests showed a low hardness value (48 HV0.5) for the top surface layer (C) in comparison with the compound layer (950 HV0.5) and the substrate material (106 HV0.5). At the first instance, it was concluded that the top surface layer is composed of the remaining aluminum after treatment. This was confirmed later by EDX analysis. EDX point analyses of the sample in the BSE micrograph (Figure 2(b)) revealed that the compound layer (B) was composed of approximately 72 at pct aluminum, while the dispersed irregular or acicular precipitates in the top Al layer (C) were composed of more than 76 at. pct aluminum. These concentrations corresponded to Fe2Al5 for compound layer (B) and FeAl3 for precipitates (C) according to the binary phase diagram of Fe-Al.[8]
(a) Optical micrograph shows the cross section of the plasma pack-aluminized sample at 973 K (700 °C) for 5 min, Vickers indentations’ impressions and hardness values obtained under 500 gf. (b) BSE micrograph shows the place of EDX line and point analyses and the values for the relevant phases in the sample
Line EDX analysis (Figure 3) showed a gradual increase in the amount of aluminum in the range approximately from 330 to 530 μm of the analysis distance along the arrow shown in Figure 2(b). This result implied the presence of a mixture of Fe2Al5 and FeAl3 compounds.[1–3,8] However, after approximately 530 μm, there is a sharp increase in the aluminum content which remains at high value across the top surface layer. This indicated the presence of mainly aluminum element on the top surface.
It is very likely that the FeAl3 has precipitated in the top layer by diffusion and mixing of a few amount of iron into the aluminum. The solubility of Fe in solid Al is very low. Consequently, in the outer layer, FeAl3 precipitates are easily formed during the eutectic solidification of liquid Al. It should be mentioned that in our experiments, argon irradiation raised the surface temperature of the samples to range from 923 K to 973 K (650 °C to 700 °C) after a few seconds. Therefore, the solid aluminum on the samples transformed into a transient liquid-like phase which was active enough for fast interdiffusion and mixing of iron and aluminum.[6]
The chemical composition of FeAl3 phase is very close to that of the Fe2Al5 phase. Hence, the identification with only EDX analysis may be difficult. Also there was not a significant difference between the intensities of both phases in the BSE image as shown in Figure 2(b). XRD analysis of the 5-minute-treated sample (Figure 4) clearly confirmed the existence of aluminum and two intermetallic phases of FeAl3 and Fe2Al5. However, the intensity of FeAl3 peaks was low. It can be proposed that the amount of FeAl3 phase was very low or in a mixture of intermetallic compounds.
Plasma pack aluminizing of low carbon steel at 973 K (700 °C) for 10 minutes converted all aluminum to intermetallic compounds. Optical microscopy of this sample (Figure 5(a)) showed only an intermetallic compound, and no aluminum was present on the surface. The interface between the intermetallic compounds and the substrate[9] had larger finger-type structure than that of the sample treated for 5 minutes. The microhardness tests showed approximately the same value for the substrate material (A) (103 HV0.5), while the hardness of the compound layer (B) was slightly higher (980 HV0.5) than that of the 5-minute-treated sample (960 HV0.5). EDX point analysis of the intermetallic compound showed approximately 71 at. pct aluminum and 28 at. pct iron which was assigned to Fe2Al5 compound such as that for the 5-minute-treated sample (Figure 5(b)).
(a) Optical micrograph shows the cross section of the plasma pack-aluminized sample at 973 K (700 °C) for 10 min, Vickers indentations’ impressions, and hardness values obtained under 500 gf. (b) BSE micrograph shows the position of EDX line and point analyses and the values for the relevant phases in the sample
Line EDX analysis (Figure 6) showed a sudden increase of aluminum after approximately 350-μm distance of analysis along the arrow as shown in Figure 5(b). It is obvious that there was no gradual variation in the composition at the interface between the substrate and the compound layer. In fact, the nucleation and growth of Fe2Al5 occurred in the first few minutes of the treatment, and the entire aluminum was converted to Fe2Al5.[10] However, EDX point analysis at the edge of finger-type protrusions of the compound layer showed approximately 31 pct aluminum and 68 pct iron which is referred to an iron rich compound such as Fe3Al (Figure 5(b)). This may be correlated to the first stages of aluminum dissolution in iron which is then converted to Fe2Al5, but partly remained in the short treatment time of 10 minutes.[3,8]
XRD analysis (Figure 7) on the surface of 10-minute-treated sample revealed the peaks of Fe2Al5 compound with a strong peak of Fe2Al5 (002). This indicated the preferred orientation along the Fe2Al5 (002) planes which can also be correlated to the high hardness (980 HV0.5) of the compound layer.[11]
The XRD result did not show any indication of Fe3Al or any other compound. This may be due to the very low amount of iron rich compound of Fe3Al at the finger-type interface of the compound layer and the substrate.
However, Fe2Al5 has been reported as a brittle phase and its hardness was high in the compound layers, No cracks, embrittlement and lack of adhesion were observed between the compound layers and the substrate. This is favorable for the application of the aluminized steel.
By the time extension of plasma pack aluminizing to 15 minutes, the overall appearance of the compound layer did not vary and only the irregular finger-type interface became smoother (Figure 8) in comparison with 10-minute-treated sample (Figure 5). The interface between the substrate and the compound layer was sharp which indicated on the sudden changes from the substrate phases to the compound layer.
(a) Optical micrograph shows the cross section of the plasma pack-aluminized sample at 973 K (700 °C) for 15 min, Vickers indentations impressions and hardness values obtained under 500 gf. (b) BSE micrograph shows the place of EDX line and point analyses and the values for the relevant phases in the sample
In spite of these variations, no mismatching or cracking was observed at the interface. If the interface between the compound layer and substrate is investigated clearly (Figure 8(a)), it is observed that a little grain growth has occurred in some portions of the substrate in comparison with the sample treated for 10 minutes (Figure 5(a)). It is proposed that 15 minutes plasma interaction with the surface at 973 K (700 °C) was long enough to heat up the sample and accelerate the diffusion or grain growth. Overall, it seems that no new phases were created and only some porosity added to the compound layer. The hardness value for the substrate was 98.5 HV0.5 and the hardness of the compound layer diminished to 960 HV0.5. The decrease in the microhardness values can be related to some kind of annealing or stress relieving in the substrate and the compound layer due to the longer heating of the sample.
The point EDX analysis of the compound layer showed 28 at. pct iron and 72 at. pct aluminum which was correlated to Fe2Al5 compound similar to that of the sample treated for 10 minutes.
The line EDX analysis of 15-minute-treated sample (Figure 9) was similar to that of the sample treated for 10 minutes (along the arrow shown in Figure 8(b)). In fact, a sharp increase in the aluminum content happened at the interface of the compound layer and substrate. This indicated the sudden changes in the composition from iron based alloy to the intermetallic compound of Fe2Al5. This result clarified the reason for the sharp interface between the substrate and the compound layer in Figure 8(a).
The XRD analysis of the 15-minute-treated sample (Figure 10) confirmed the existence of Fe2Al5 similar to that of the sample treated for 10 minutes. However, the intensity of Fe2Al5 (002) peak diminished, and those of other peaks increased. This observation may be correlated to some type of homogenization in the compound layer which occurs during the longer heat treatments and can be investigated in the future works by TEM studies.
3.2 Oxidation Observations
From the above observations, it may be concluded that the 10-minute treatment was long enough for achieving a suitable compound layer on the surface of low carbon steel. Therefore, the sample treated for 10 minutes was selected for oxidation investigations. The oxidation was carried out at 973 K (700 °C) and 15 mbar in synthetic air (21 pct oxygen and 79 pct nitrogen).
Plasma air oxidation for 60 minutes resulted in severe oxidation of untreated low carbon steel and led to a very rough surface on the material. The oxide layers spalled and separated easily (Figure 11(a)). It is estimated that approximately half of the 2-mm thickness of the untreated sample converted to oxide products. Plasma air oxidation of the 10-minute-aluminized sample only changed the surface color, and neither deep oxidation nor spallation was observed on the surface (Figure 11(b)). It is worth mentioning that only the initial size of the oxidized untreated sample was different from other samples.
Figure 12 shows the XRD pattern of plasma air-oxidized sample of the untreated low carbon steel. This XRD analysis was performed from the remaining piece of the oxidized untreated sample after separation of a few oxide layers. XRD spectra of this sample showed peaks of Fe3O4 and Fe2O3 oxides which form naturally on iron in hot air.[8]
Plasma air oxidation was carried out on the 10-minute-aluminized sample. Cross section of the oxidized sample showed oxide layers with the sizes ranging from 10 to 150 μm on the surface. As can be observed in Figure 13(a), the oxide thickness in some portion of the sample was different from that in another portion (Figure 13(b)) of the sample.
(a) and (b) Optical micrographs show the cross section of the plasma pack-aluminized sample at 973 K (700 °C) after 10 min and that oxidized in plasma air for approximately 60 min, and Vickers indentations’ impressions and hardness values obtained under 500 gf. (c) BSE micrograph shows the position of EDX line and point analyses and the values for the relevant phases in the sample
Also the hardness values of the phases were different in these two portions. The hardness of intermetallic phases was as low as 558 HV0.5 in some portions (Figure 13(a)), whereas it was up to 930 HV0.5 in some other small areas (Figure 13(b)). It is concluded that heating during oxidation converted the phases or structures of the compound layers under the oxide products.
When the thicknesses of the oxides on the untreated and the treated samples are compared, it can be concluded that plasma pack aluminizing can improve the oxidation resistance of low carbon steel.
EDX analysis (Figure 14) across a thick portion of the oxide layer (Figure 13(c)) showed a high variation of aluminum and detected remarkable amounts of oxygen and iron. This indicated the existence of aluminum and iron oxides. The aluminum increased gradually from very low amounts to nearly 50 pct in the analysis distance ranging approximately from 200 to 400 μm. This revealed the existence of Fe-Al compounds with the composition of 50 pct iron or less at the interface of the oxide layer and substrate. Also considerable amounts of oxygen and aluminum for the analysis distance ranging from 400 to 750 μm could show the existence of aluminum and iron oxides on the surface of the sample.
XRD analysis (Figure 15) confirmed the existence Al2O3 oxide, and no ferrous oxide appeared in the pattern. This may be due to the very low amount of ferrous oxides in this sample.
From the phase analysis point of view, it can be suggested that both FeAl and Fe3Al phases appeared in the XRD analysis. This is correlated to the overlap of all peaks of FeAl (B2 structure) with those of Fe3Al (DO3 structure). B2 structure transforms to DO3 structure due to ordering of the compound, and only (111) peak from DO3 structure does not overlap with the B2 peaks.[12] Therefore, it is difficult to determine whether the intermetallic compound at the interface between the oxide layers and substrate has DO3 or B2 structure. However, the result of EDX line analysis shows that the aluminum content at the interface varies from approximately 15 to 60 pct. Therefore, the compound composition could be both FeAl and Fe3Al. The aluminum content in the transformed layer, determined by EDX analysis (Figure 13(c)), is approximately 42 pct which is close to that of FeAl. Therefore, it can be stated that the compound in that layer is identified mainly as FeAl. As the ordering transformation of Fe3Al from B2 structure to DO3 structure is slow (100 hours, at 823 K (550 °C)[12]), the composition of the compound layer has been concluded to be FeAl. It is suggested that during oxidation, some amount of aluminum in the intermetallic compound of Fe2Al5 has diffused out, combined with oxygen, to result in Al2O3 oxide, and thus created the phases of FeAl and Fe3Al compounds.[13] In fact, the 60-minute plasma heating (ion bombardment) of the samples has played the role of heating at high temperatures for longer times or several hours in atmospheric pressure without plasma.[14] Moreover, aluminum’s affinity to oxidation is higher than iron and Al2O3 oxide is stabler than ferrous oxide. Therefore, the aluminum oxide formed first and protected iron from massive oxidation and remained on the surface as the major oxidation product, which appeared in the XRD spectrum (Figure 15).
4 Conclusions
-
1.
Protective aluminized layers were synthesized on the surface of low carbon steel using the technique of plasma pack aluminizing.
-
2.
The surface layers were composed of aluminum, FeAl3, and Fe2Al5 compound after 5-minute treatment of the samples. After 10 minutes, the surface layers were completely converted to Fe2Al5 compound.
-
3.
The morphology of the compound layers was irregular and finger like at the interface of the compound layers and the substrates for all samples.
-
4.
The hardness of Fe2Al5 compound ranged from 950 to 980 HV0.5, while that of the substrate ranged from 90 to 110 HV0.5.
-
5.
The surface layers of the aluminized samples were oxidized to mainly Al2O3 after 60-minute plasma air oxidation, whereas the oxide layers on the untreated low carbon steel were Fe3O4 and Fe2O3. Also the intermetallic compounds at the interface of the compound layer and the substrate varied from Fe2Al5 to FeAl and probably to Fe3Al.
-
6.
Oxidation of the untreated low carbon steel resulted in thick and nonadherent oxide layers under low-pressure plasma air heating, and approximately half of the 2-mm-thick samples were oxidized, whereas the adherent oxide layers on plasma pack-aluminized samples were of thicknesses ranging from nearly 10 to 150 μm. Therefore, it could be suggested that plasma pack aluminizing can better protect low carbon steel from air oxidation at temperatures around 973 K (700 °C).
References
M.B. Isiko: PhD Thesis, Norwegian University of Science and Technology, 2012.
K.H. Kim, V.D. Benny, V.T. Gustaaf and J.K. Yoon: Mater. Sci. Forum, 2006, vol. 519-521, pp. 1871-1875.
I.I. Danzo: PhD Thesis, Gent University, 2012.
M. Zhe, O.B. DezellusGardiola, M. Braccini and J.C. Viala: J. Phase. Equilib. Diff., 2011, vol. 32 (6), pp. 486-497.
A.R. Rastkar: Appl. Surf. Sci., 2013, vol. 276, pp. 112–119.
M. Nastasi, J.W. Mayer and J.K. Hirvonen: Ion-Solid Interactions: Fundamentals and Applications, pp. 295–330 Cambridge University Press, Cambridge, 1996.
F.C. Yin, M.X. Zhao, Y.X. Liu, W. Han and Z. Li: Trans. Nonferrous Met. Soc. China, 2013, vol. 23, pp. 556–561.
M. Badaruddin: Makara Teknologi, 2011, vol. 15(2), pp. 137-141.
Y. Tanaka and M. Kajihara: Mater. Trans., 2009, vol. 50(9), pp. 2212 – 2220.
X. Si, B. Lu and Z. Wang: J. Mater. Sci. Technol., 2009, vol. 25(4), pp. 433-436.
Z.C. Zhou, F.S. Han, and Z.Y. Gao: Acta Materialia,2004, vol. 52, pp. 4049–4054.
X.L. Zhu, Z.J. Yao, X.D. Gu, W. Cong and P.Z. Zhang: Trans. Nonferrous Met. Soc. China, 2009, vol. 19, pp. 143-148.
M. Boufenghour, A. Hayoune, N.Y.A. Rokhmanov, M. Boucher and D. Hamana: Functional Materials, 2004, vol. 11 (2), pp. 397-401.
Y Xia, SX Yu, M Yao, TF Li (2001). Trans. Nonferrous Met. Soc. China, 11(6): 817-821.
Acknowledgments
The authors are grateful to Shahid Beheshti University of Iran for the financial support to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 12, 2014.
Rights and permissions
About this article
Cite this article
Rastkar, A.R., Rezvani, N. The Effects of Processing Time on the Microstructure and Composition of Plasma Pack-Aluminized and -Oxidized Surface Layers on Low Carbon Steel. Metall Mater Trans A 46, 4132–4142 (2015). https://doi.org/10.1007/s11661-015-3034-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3034-x