Abstract
Generally, Laves phase and M23C6 are regarded as undesirable phases in creep-resistant steels due to their very high-coarsening rates and the resulting depletion of beneficial alloying elements from the matrix. In this study, a computational alloy design approach is presented to develop martensitic steels strengthened by Laves phase and/or M23C6, for which the coarsening rates are tailored such that they are at least one order of magnitude lower than those in existing alloys. Their volume fractions are optimized by tuning the chemical composition in parallel. The composition domain covering 10 alloying elements at realistic levels is searched by a genetic algorithm to explore the full potential of simultaneous maximization of the volume fraction and minimization of the precipitates coarsening rate. The calculations show that Co and W can drastically reduce the coarsening rate of Laves and M23C6 and yield high-volume fractions of precipitates. Mo on the other hand was shown to have a minimal effect on coarsening. The strengthening effects of Laves phase and M23C6 in the newly designed alloys are compared to existing counterparts, showing substantially higher precipitation-strengthening contributions especially after a long service time. New alloys were designed in which both Laves phase and M23C6 precipitates act as strengthening precipitates. Successfully combining MX and M23C6 was found to be impossible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Martensitic creep-resistant steels have been widely used in high-temperature components in power plant applications. To increase the operation temperature so as to improve the efficiency, new high-strength steels are required since existing martensitic steels are not suitable for higher temperatures. Generally, alloy designers are inclined to add desirable components to the alloy system and to get rid of undesirable ones. MX carbonitrides, mainly containing Ti, Nb, V, C, and N, are examples of such desirable components. They generally have very small initial sizes and are very stable, and hence they are very good precipitation-hardening sources even during very long time service.[1–3] On the other hand, some precipitates have large initial sizes and are unstable during service at elevated temperatures, and hence are regarded as undesirable components. Laves phase and M23C6 are two such examples. They have a very high-coarsening rate, which is 10 to 100 times faster than that of MX carbonitrides.[2,4,5] Their growth and coarsening not only lead to a loss of strengthening but also lead to depletion of useful elements, such as Mo and Cr, from the matrix. A common solution to prevent the formation of such undesirable phases is to decrease the Mo and Cr levels, to a level at which such precipitates do not form. However, these two elements themselves are crucial for a desired creep performance: Mo increases the solid solution strengthening and stabilizes the microstructure, while a high amount of Cr is necessary to obtain a material with a good corrosion and oxidation resistance. An alternative approach is to tailor the characteristics of such potentially detrimental phases by adding some other alloying elements in order to slow down the coarsening. In the literature, it has been reported that the coarsening rate of M23C6 can be decreased moderately by adding B, Co, or W.[6–10] Abe[11,12] reported that the coarsening rate of Laves phase can also be effectively reduced by substituting W for Mo. The shape of W-enriched Laves phases is also near spherical, which is more coherent and stable than needle-shaped precipitates.[13] In addition to the more stable feature of Laves phase in W-containing alloys, a high Laves phase volume fraction, which is beneficial in order to reach a high-precipitation-strengthening level, can be achieved by increasing the W level. As a consequence, several researchers now use Laves phase as the principal strengthening precipitate in the design of new creep-resistant alloys.[14,15] Recently, a high W (6 wt pct) ferritic creep-resistant steel has been designed which has an extraordinary creep strength[16,17] well beyond that of conventional alloys. This alloy is mainly strengthened by W-enriched Laves phase (Fe2W), which is very stable even after 10,000 hours service at 923 K (650 °C).
However, the design of alloys cannot simply be done by adding new alloying elements to the composition of known steel grades since the addition of new alloying elements will change the thermodynamic and kinetic property of the alloy as a whole, and may also lead to the formation of undesirable phases in addition to the intended strengthening precipitates. Moreover, the full potential of the newly selected alloying element is also difficult (and costly) to be determined by the traditional trial and error method. In order to reach the target and to avoid the obstacles, a computational design approach coupling thermodynamic and kinetic data is developed in combination with a genetic algorithm to search the multi-element search domain for optimal stability and high-volume fraction of Laves phase and M23C6 precipitates. In this work, the search space covers 10 regular alloying elements given our intention to explore the full potential of Laves phase and M23C6 for new creep and corrosion-resistant martensitic steels with an intended use time of 105 hours and a fixed operating temperature of 923 K (650 °C).
2 Model Descriptions
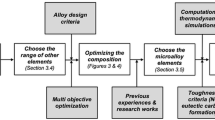
As mentioned in the introduction, either Laves phase or M23C6 precipitates are selected as the phase to be optimized as the main, long time—high use temperature-strengthening phase in martensitic creep steels. Martensite is chosen as the (initial) microstructure of the matrix because of its good creep strength and its high resistance to thermal and creep fatigue during service in power plants.[18] Thus, the target microstructure is (1) a fully martensitic matrix, (2) a high-volume fraction of Laves phase or M23C6 with a tuned low-coarsening rate throughout its intended life time, and (3) a limited volume fraction of undesirable phases. In order to achieve the microstructural features listed above, a computational alloy design model involving a Genetic Algorithm (GA) optimization routine has been developed.[19–21] More computational details of the genetic algorithm can be found elsewhere.[22]
The main concept of the model is as follows: Firstly, random compositions and heat treatment parameters are generated by the GA from the predefined parameter ranges listed in Table I. Eleven variables are taken into account (10 alloying elements and the austenization temperature) each uniformly spaced at 32 levels between predefined minimal and maximal values. As mentioned in the introduction, W and Mo are expected to have similar effects on the strengthening phase, but may have different overall effects. To illustrate this difference as clearly as possible, the search domains were specified either for Mo or for W. Alloy compositions involving both W and Mo were evaluated but did not yield interesting results and they are not presented here. The intended service temperature in all simulations is fixed at 923 K (650 °C) and the intended service time for continuous operation is set to a high value of 105 hours.
Then, to check if a candidate combination of composition and heat treatment conditions can lead to the desirable microstructure, various go/nogo criteria are defined and applied in the sequence of the thermal treatments to which such steels intended for long life-high load applications are subjected. In precipitation-hardened martensitic creep-resistant steels, the typical heat treatment includes an austenization/solution treatment to dissolve undesirable primary precipitates and to achieve compositional homogeneity, followed by quenching and a tempering treatment to form desirable precipitates in the martensitic matrix. The present study deals with Laves phase or M23C6-strengthened steels for very long-term applications and hence the final precipitate state after long-term service will be close to the equilibrium condition at the service temperature rather that at the tempering temperature. Therefore, in the present study, the tempering temperature is set equal to the service temperature [923 K (650 °C)]. In the order of the actual heat treatment sequence, thermodynamic calculations are performed at the (variable) austenization temperature and three go/nogo criteria are defined: (1) the equilibrium volume fraction of austenite should be larger than 99 pct, (2) the amount of primary carbides should be less than 0.5 pct in volume. (3) liquid and delta phase should be absent. After austenization, such steels are quenched to room temperature, and the austenite should completely transform to martensite. Therefore, a fourth go/nogo criterion is imposed: (4) the Martensite start (T Ms) temperature should be higher than 523 K (250 °C). The T Ms is obtained by calculating the chemical composition using the formula proposed by Ishida.[23] After quenching to room temperature, the alloy is used at its service/aging temperature. Hence a second set of themodynamic calculations at the service temperature is performed. Two additional go/nogo criteria at this use temperature are enforced: (5) the amount of precipitates other than Laves phase/M23C6 should be less than 1 vol pct as Laves phase or M23C6 are defined as the principal and property determining strengthening phases. (6) The Cr concentration in the matrix upon completion of the precipitation reactions should at least be 11 wt pct to assure adequate corrosion and oxidation resistance (other residual Cr concentrations could have been chose, but the current level is selected on the basis of discussions with industrial experts. The appropriate minimal Cr level obviously depends on the operating conditions in the installation).
Finally, the aim of this study is to tailor the undesirable Laves phase or M23C6 to a desirable phase by tuning the chemical composition, so as to drastically reduce their coarsening rates. Therefore, the time-dependent precipitation-hardening (PH) effect of Laves phase or M23C6 is considered to be the sole optimization criteria to rank all qualified solutions fulfilling all above 6 go/nogo criteria. The PH is determined by the average precipitate particle size, its volume fraction, and its spatial distribution.[24] The actual values for the three parameters depend on their initial condition and evolution during service. Given the extremely long service time, Laves phase and M23C6 will grow and coarsen considerably during the exposure at high temperatures, and in doing so change the interparticle spacing. The precipitation-hardening contribution σ p is inverse proportional to interparticle spacing, which is estimated by considering the coarsening kinetics only,[25–27]
in which
and
where L is the average interparticle spacing, f p is the equilibrium volume fraction of the strengthening precipitate at the service temperature, r 0 is the critical precipitate nucleus size, γ is matrix-precipitate interfacial energy, ΔG v is volume thermodynamic driving force for the precipitation, K is the coarsening rate, t is the exposure time at the high temperature, x is the equilibrium interfacial concentrations (in mole fraction) of the relevant chemical element on both the matrix (m) and the precipitate (p) sides, \( V_{\text{m}}^{\text{p}} \) is the molar volume of precipitate, T is the service temperature, and D is corresponding diffusion coefficient. The necessary thermodynamic values including \( x_{i}^{\text{p}} \), \( x_{i}^{\text{mp}} \), D i ,and \( V_{\text{m}}^{\text{p}} \) are obtained via Thermo-Calc® using the TCFE6 and Mob2 databases. For lack of information on the actual change in surface tension with chemical composition, the value of interfacial energy γ is kept constant and set to a value of 1 J/m2.
The coarsening rate of precipitates is generally very well described by Eq. [3],[5,28] and is used in the simulations performed to calculate the coarsening rate for the Laves phase and M23C6 precipitates at a fixed temperature of 923 K (650 °C). Prior to the design study optimization it was verified for a number of existing 9 to 12 pct Cr steels that the coarsening rate of Laves phase and M23C as predicted by Eq. [3] indeed drops with increasing W level. The result is shown in Figure 1. The coarsening rate of Laves phase is much lower than that of M23C6. Moreover, the coarsening rate of Laves phase or M23C6 both decreases when the level of W is increased. A similar shift, not shown, occurs with increasing Co level. The calculated behavior agrees well with the practical experience.
At very long times, the strengthening contributions of the dislocations accompanying the formation of Laves phases will have dropped to a low level due to recovery. Furthermore, there are no indications that this final dislocation level varies with type of Laves phase or matrix composition. Moreover, the strengthening contribution of lath boundaries is also negligible owing to their significant growth after long-term service. Hence, the dislocation and lath boundary contributions in the strength model for the steels analyzed in the present work were ignored. Precipitate hardening of Laves phase and/or M23C6 is taken as the principle strengthening source for long-term creep strength in this study. Effectively, the time and temperature-dependent interparticle spacing of Laves phase or M23C6 is taken as the PH optimization parameter. In this study, the service time is fixed at 105 hours. The factor 1/L (Eq. [1]) is called the ‘PH factor’ and is to be maximized by tuning the chemical composition and heat treatment parameters.
3 Model Predictions
The GA-based search covering about 106 of unacceptable or less well-performing variants finally led to four alloys with the highest long-term precipitation-strengthening effect. Their composition, austenization temperatures, volume percent, coarsening rate, and Laves phase or M23C6 determined PH factor are listed in Table II. The alloys strengthened by Laves phase with either W or Mo as the modifying element, and those strengthened by M23C6 with either W or Mo as the modifying element are labeled as Alloy LavesW, Alloy LavesMo, Alloy M23C6W, and Alloy M23C6Mo, respectively. These alloys have a high Cr level and a maximal Co level. The high Co level is required so as to keep a high Cr level in the matrix and hence stabilize the austenite and suppress the ferrite. This is necessary to obtain a fully austenitic matrix at a reasonable austenization temperature within the search range. For the LavesW alloy, the W level equals the imposed maximum level. In contrast, for the LavesMo alloy, the Mo level is well below the maximum possible level. In both M23C6 alloys, the C and Cr levels are equal to the maximal values, but the Mo and W levels are substantially lower than the maximum allowed level. The lower recommended concentration levels are the result of strict go/nogo constraint boundaries being met in the optimization cycles. The concentration of Ni in Laves phase-strengthened alloys LavesW and LavesMo is much higher (3 to 5 wt pct) than that in M23C6-hardened steels to further stabilize austenite at the austenization temperature, since the content of ferrite stabilizer elements W in alloy LavesW or Mo, Al, and Nb in alloy LavesMo is much higher than those in M23C6-hardened steels.
It is interesting to note that the coarsening rate of Laves phase or M23C6 in W-containing alloys is much slower than that in Mo-containing alloys. Clearly, W has a higher capacity to stabilize the precipitates. To further visualize its effect on coarsening rates, the PH factor evolution with time for the optimal alloys defined is shown in Figure 2. The PH factor invariably decreases with time but with different kinetics. In Laves phase-strengthened alloy, the degradation kinetics of alloy LavesW is much slower, leading to a much higher PH factor at the long intended service time. The W effect is also observed in M23C6-strengthened alloy but the effect is less pronounced. Moreover, the coarsening rate of Lave phases in alloy LavesW is close to that of the well accepted very stable MX carbonitride,[4,28] proving that indeed undesirable Laves phase can be turned into desirable precipitates. While being equally stable the volume percent of Laves phase (11.3 vol pct) is much higher than the maximum volume percent of MX carbonitride (0.5 to 1 vol pct) which increases the net strengthening contribution of the Laves precipitates significantly.
4 Discussion
4.1 Effects of Alloying Elements on the Precipitate Configuration
As demonstrated in the previous section, the type of alloying element can significantly influence the precipitate characteristics. To further explore this effect, the alloy compositions of LavesW and LavesMo are taken as baselines and the concentrations of Co, W/Mo in each alloy are varied, while keeping the levels of all other elements unchanged by adjusting the Fe level. The results are shown in Figure 3. The two vertical dashed lines represent the predefined ranges of alloying elements (as Table I). The green box defines the concentrations that meet all the go/nogo criteria. The black arrows indicate the original composition of alloys LavesW or LavesMo, which, as expected, always shows the highest PH factor in the predefined ranges, confirming the robustness of the GA optimization model. As demonstrated in Figures 3(a) and (c), the addition of Co in both alloys can significantly decrease the coarsening rate (by ~10 times in Alloy LavesW and by ~5 times in Alloy LavesMo) as well as increase the volume fraction of Laves phase, leading to a substantial increase in PH factor (Eq. [1]). The difference between Figure 3(a) and (c) is that the volume fraction of Laves phase in Alloy LavesMo reaches a maximum value at 10 wt pct Co and then decreases with further addition of Co. The effect of W is similar to that of Co, as shown in Figure 3(b). When the concentration of W is low (<0.3 wt pct), there is no Laves phase since W is the principal Laves forming element. At a further increase in W concentration, Laves phase begins to form and its concentration increases almost linearly and reaches maximum PH at the upper limit of 10 wt pct. At the same time, the coarsening rate of Laves phase is significantly decreased. On the contrary, addition of Mo in alloy LavesMo increases the coarsening rate when Mo content is lower than 5 wt pct as shown in Figure 3(d). A further increase in Mo level will only decrease the coarsening rate slightly. This trend explains why the Mo-containing alloy LavesMo has a much higher coarsening rate than that of W-added alloy LavesW. The effects of both Co and W on the coarsening rate of Laves phase agree qualitatively very well with existing experimental results. By analyzing the coarsening rate as defined in Eq. [3], it is found that the coarsening rate mainly depends on the diffusion coefficients and concentration gradients of alloying elements at the interface. The diffusion coefficient of W at the set temperature of 923 K (650 °C) is very low, close to 10−20 m2/s, while those of other elements (including Mo) are higher than 10−19 m2/s. As shown in Figure 3(b), the coarsening rate decreases rapidly with the addition of W when its content is lower than 2.5 wt pct. The coarsening rate as calculated by Eq. [3] depends on the sum of the contributions from each element which is determined by the concentration gradient at the interface and diffusion coefficient. The small addition of W introduces a large concentration gradient of W at the interface of Laves phase and matrix. Considering the very small diffusion coefficient of W, the coarsening rate is significantly reduced by the addition of W (Eq. [3]). In addition, W atom is bigger than Fe atom and can cause lattice misfit, which can possible decrease the diffusion rate of other Laves phase forming elements. Further addition of W only decreases the coarsening rate slightly, suggesting there is no significant change in interface concentration gradient. This phenomenon is not so pronounced in Co, since increase the Co level also decreases the diffusion coefficient of other alloying elements.[7]
Effect of concentration variation of alloying element on the volume percent (VP), coarsening rate, and PH factor of Laves phase. (a) Co, (b) W in alloy LavesW and (c) Co and (d) Mo in alloy LavesMo. The two vertical dashed lines mark the predefined range of alloying elements. The green box defines the concentration that meets all the go/nogo criteria, and the concentration in other areas do not fulfill one or multiple go/nogo criteria. The black arrows indicate the original composition in alloy LavesW or LavesM
4.2 Comparison of Designed Alloys and Existing Alloys
The coarsening rates, volume percent, and PH factors after 105 hours exposure at 923 K (650 °C) for Laves phase, M23C6, and MX carbonitride in existing alloys are calculated using their compositions and compared to those of designed alloys. The results are listed in Table III. In the two designed Mo-enriched alloys, LavesMo and M23C6Mo, the volume percent of Laves phase and M23C6 is slightly higher and their coarsening rates are similar to those in existing alloys. Hence the two newly designed alloys have a PH factor at 105 hours similar to that of existing alloys (Eq. [2]). In contrast in the two W-containing alloys LavesW and M23C6W, not only the volume fraction of Laves phase and M23C6 is higher, but also their coarsening rates are much slower than those in existing alloys, leading to a much higher PH factor at 105 hours (Eq. [2]). The much reduced coarsening rates in the two W-containing alloys are mainly attributed to the high levels of W and Co (see Table II), which are much higher than those in existing alloys. Although the coarsening rate of Laves phase is still faster than that of MX carbonitride in existing and previous designed alloys, the volume percent of Laves phase (11.3 vol pct) can be much higher than that of MX carbonitride (~0.3 vol pct). As a consequence, the PH factor of Laves phase at 105 hours in alloy LavesW is even greater than the predicted PH of existing stable MX carbonitride reinforced (1.3 to 1.8E+06) alloys.
Similar to the optimization of M23C6 or Laves in W-contained system, the composition using MX carbonitride as the main strengthening precipitate is optimized and labeled as MXW. The composition of alloy MXW is listed in Table IV, and the coarsening rate and volume percent of MX carbonitride are listed in Table III. The coarsening rate of MX carbonitride in alloy MXW is slower than that of MX carbonitrides in known alloys, which is in agreement with the prediction. However, the volume fraction of MX carbonitride in alloy MXW is still similar to that of existing alloys. Combining the effects of coarsening rate and volume fraction, the PH factor of alloy LavesW based on conventionally undesirable Laves phase remains higher than that of alloy MXW which is based on optimizing the conventionally precipitate MX carbonitride. Therefore, in the newly designed alloy LavesW, the Laves phase has become a truly desirable component.
4.3 Combining M23C6 and Laves Phase in One Alloy
Generally, M23C6 precipitates are located at lath boundaries and have a positive effect by pinning the lath boundaries during creep,[29] while the W-enriched Laves phase can be found both in the grain interior and boundaries[12,14,30] and restricts the movement of dislocations. Both effects decrease the creep rate, especially if the coarsening rates of both types of precipitates can be kept at a low value during the service. It will be very interesting to combine both types of precipitates in one alloy to further optimize the creep resistance. However, the optimization procedure as sketched above can only deal with one PH factor per optimization cycle. In order to optimize both PH factors simultaneously in one alloy, two optimizations are performed respectively: either only for Laves, or only for of M23C6 at 105 hours, while the PH factors of both precipitates are recorded for all eligible solutions that fulfill all the go/nogo criteria in both optimizations. Such a dual precipitate optimization search was only performed for the search space containing W. Putting all the qualified solutions together, the property map Figure 4 can be constructed. The best combination of two precipitates can be found as indicated by the circle on the upper right corner. The new alloy, which has only slightly lower PH factors than either maximum from dedicated single optimization, is labeled as Alloy LavM. Its composition, austenization temperature, and PH factor are listed in Table V.
To benchmark the newly designed alloy against existing alloys, the PH factor after 105 hours of exposure to 923 K (650 °C) was calculated for a number of commercial 9 to 12Cr pct martensitic creep-resistant steels (other precipitates than Laves phase and M23C6 were not considered in their analysis). Their results are shown in Figure 4, and are indicated by stars. The PH values for all existing alloys are located in the lower left corner, indicating that in existing alloys Laves phase and M23C6 indeed have no significant strengthening effects for long time exposure due to the high-coarsening rate. By the simultaneous optimization of the volume fraction and the coarsening rate, not only alloy LavM itself but also many other solutions with different combinations of PH factors of Laves phase and M23C6, as shown in Figure 4, are identified which are predicted to significantly outperform existing alloys. This clearly demonstrates that the undesirable phase can be tailored to desirable phases by tuning the composition and austenization temperature. While the current analysis and optimization route only take into account the precipitation-strengthening factor and present the idealized solution, the procedure employed here can be extended to take into account other criteria such as cost, weldability, processibility, etc.,[31] provided the new design parameters can be captured in quantitative parameter values. Such additional considerations are likely to eliminate some solutions included in the figure, but even then it is clear that there is a substantial potential to improve the long-term creep strength of current martensitic creep-resistant stainless steels by employing this new alloy design concept.
Finally, a similar analysis of the possibility to combine the contributions of two different sets of precipitates in a single alloy has been performed for the W-containing steel grades. As shown in Figure 5, a significant difference can be found between on the one hand the MX/M23C6 and on the other hand the Laves/M23C6 systems. Most notably, there is no best combination point in MX/M23C6 system. The main reason for this phenomenon is that these two precipitates are competing for the same alloying elements. For MX and M23C6 strengthening, a high amount of C (~0.15 wt pct) ensures a high-volume fraction of MX and M23C6. For M23C6, the coarsening rate does not change significantly with the C level, and thus a high initial PH factor is obtained if the C level is high. However, a high level of C (>0.08 wt pct) will dramatically increase the coarsening rate of MX carbonitride since the C gradient at the interface of MX and Matrix (Eq. [3]) will be changed, which decrease the PH factor. Therefore, if the PH factor of M23C6 is high (a high C level), that of MX will be low, and vice versa. There is no such competing effect in the Laves/M23C6 system. Moreover, the highest PH factor for MX is still lower than that of the best combination alloy LavM. Therefore, the Laves/M23C6 system offers a higher probability of success in obtaining new martensitic steel grades with very long life times at very high temperatures.
5 Conclusions
A computational alloy design approach has been developed to design W or Mo-containing martensitic creep-resistant steels strengthened by Laves phase and/or M23C6 precipitates by tuning the coarsening rate of the strengthening phase to a minimum value and by simultaneously tuning their volume fraction to a maximum level. The coarsening kinetics of both precipitates can be significantly lowered by changes in the overall alloy composition and the precipitation-hardening contributions are hence substantially increased by tuning the composition. Addition of Co can significantly reduce the coarsening rate of laves phase. The main Laves phase forming elements W and Mo have different effects on the coarsening rate of Laves phase. Addition of W significantly decreases the coarsening rate and the effect increases with W level. In contrast, Mo shows an optimum low-coarsening level at 4 to 5 wt pct Mo. For W-based creep steels, the combination of Laves phase and M23C6-strengthening precipitates shows great potential for creating new alloys outperforming existing martensitic creep-resistant steel grades. Successfully combining MX and M23C6 as strengthening precipitates was found to be impossible.
References
M. Taneike, F. Abe and K. Sawada: Nature 2003, vol. 424, pp. 294–96.
D. Rojas, J. Garcia, O. Prat, G. Sauthoff and A. R. Kaysser-Pyzalla: Mater. Sci. Eng. A 2011, vol. 528, pp. 5164–76.
F. S. Yin and W. S. Jung: Metall. Mater. Trans. A 2008, vol. 40A, pp. 302-309.
O. Prat, J. Garcia, D. Rojas, C. Carrasco and A. R. Kaysser-Pyzalla: Mater. Sci. Eng., A 2010, vol. 527, pp. 5976–83.
Å. Gustafson and M. Hättestrand: Mater. Sci. Eng., A 2002, vol. 333, pp. 279–86.
F. Abe, T. Horiuchi, M. Taneike and K. Sawada: Mater. Sci. Eng. A 2004, vol. 378, pp. 299–303.
Å. Gustafson and J. Ågren: ISIJ Int. 2001, vol. 41, pp. 356–60.
A. Kipelova, M. Odnobokova, A. Belyakov and R. Kaibyshev: Metall. Mater. Trans. A 2013, vol. 44A, pp. 577–83.
F. Abe: Mater. Sci. Eng. A 2004, vol. 387–389, pp. 565–69.
F. Liu, D. H. R. Fors, A. Golpayegani, H. O. Andrén and G. Wahnström: Metall. Mater. Trans. A 2012, vol. 43A, pp. 4053–62.
F. Abe: Mater. Sci. Eng. A 2001, vol. 319-321, pp. 770–73.
F. Abe (2005) Metall. Mater. Trans. A 36:321–32.
Y. Yamamoto, M. Takeyama, Z. P. Lu, C. T. Liu, N. D. Evans, P. J. Maziasz and M. P. Brady: Intermetallics 2008, vol. 16, pp. 453–62.
B. Kuhn, M. Talik, L. Niewolak, J. Zurek, H. Hattendorf, P. J. Ennis, W. J. Quadakkers, T. Beck and L. Singheiser: Mater. Sci. Eng. A 2014, vol. 594, pp. 372–80.
I. Tarigan, K. Kurata, N. Takata, T. Matsuo and M. Takeyama: Mater. Res. Soc. Symp. Proc., pp. 317–22.
K. Kimura, K. Seki, Y. Toda and F. Abe: ISIJ Int. 2001, vol. 41, pp. S121–25.
Y. Toda, H. Tohyama, H. Kushima, K. Kimura and F. Abe: JSME Int. J. Ser. A, vol. 48, pp. 125–31.
F. Masuyama: ISIJ Int. 2001, vol. 41, pp. 612–25.
Q. Lu, W. Xu, and S. van der Zwaag: Comput. Mater. Sci. 2014, vol. 84, 198–205.
Q. Lu, W. Xu, and S. van der Zwaag: Acta Mater. 2014, vol. 64, pp. 133–43.
W. Xu, P. E. J. Rivera-Díaz-del-Castillo, W. Wang, K. Yang, V. Bliznuk, L. A. I. Kestens and S. van der Zwaag: Acta Mater. 2010, vol. 58, pp. 3582–93.
W. Xu, P. E. J. Rivera-Díaz-del-Castillo and S. van der Zwaag: Comput. Mater. Sci. 2008, vol. 44, pp. 678–89.
K. Ishida: J. Alloys Compd. 1995, vol. 220, pp. 126–31.
E. Orowan: Theory of dislocation bowing. (Institute of Metals Symposium on Internal Stresses in Metals and Alloys, London, 1948), pp. 451.
A. Kelly: Philos. Mag. 1958, vol. 3, pp. 1472–74.
J. Ågren, M. T. Clavaguera-Mora, J. Golczewski, G. Inden, H. Kumar and C. Sigli: Calphad-Comput. Coupling Phase Diagr. Thermochem. 2000, vol. 24, pp. 41-54.
R. W. Cahn, P. Haasen: Physical Metallurgy. (Elsevier, Amsterdam, North-Holland, 1996).
J. Hald and L. Korcakova: ISIJ Int. 2003, vol. 43, pp. 420-427.
A. Kostka, K. G. Tak, R. J. Hellmig, Y. Estrin and G. Eggeler: Acta Mater. 2007, vol. 55, pp. 539–50.
M. Shibuya, Y. Toda, K. Sawada, H. Kushima and K. Kimura: Mater. Sci. Eng. A 2011, vol. 528, pp. 5387–93.
W. Xu and S. van der Zwaag: ISIJ Int. 2011, vol. 51, pp. 1005–10.
Acknowledgments
This work was carried out with financial support from the Chinese Scholarship Council (CSC) as well as TU Delft internal funding. The authors thank Dr. Toda from the National Institute of Materials Science (NIMS) for helpful discussions during the final stages of the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 3, 2014.
Rights and permissions
About this article
Cite this article
Lu, Q., Xu, W. & van der Zwaag, S. The Computational Design of W and Co-Containing Creep-Resistant Steels with Barely Coarsening Laves Phase and M23C6 as the Strengthening Precipitates. Metall Mater Trans A 45, 6067–6074 (2014). https://doi.org/10.1007/s11661-014-2557-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-014-2557-x