Abstract
Brazing Inconel 625 (IN-625) using the copper foil has been investigated in this research. The brazed joint is composed of nanosized CrNi3 precipitates and Cr/Mo/Nb/Ni quaternary compound in the Cu/Ni-rich matrix. The copper filler 50 μm in thickness is enough for the joint filling. However, the application of Cu foil 100 μm in thickness has little effect on the shear strength of the brazed joint. The specimen brazed at 1433 K (1160 °C) for 1800 seconds demonstrates the best shear strength of 470 MPa, and its fractograph is dominated by ductile dimple fracture with sliding marks. Decreasing the brazing temperature slightly decreases the shear strength of the brazed joint due to the presence of a few isolated solidification shrinkage voids smaller than 15 μm. Increasing the brazing temperature, especially for the specimen brazed at 1473 K (1200 °C), significantly deteriorates the shear strength of the joint below 260 MPa because of coalescence of isothermal solidification shrinkage voids in the joint. The Cu foil demonstrates potential in brazing IN-625 for industrial application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The chemical composition of Inconel 625 (IN-625) alloy is 61Ni-21.5Cr-2.5Fe-9Mo-3.6Nb in wt pct, and it is featured with excellent corrosion resistance, especially for the presence of chloride ions (e.g., sea water).[1–4] The IN-625 alloyed with Mo and Nb belongs to a solid-solution strengthened alloy, and it possesses excellent formability and weldability compared with other Ni-based alloys such as Inconel 738.[5–9] Therefore, IN-625 has the potential to be applied in production of corrosion-resistant plate heat exchangers. The plate heat exchanger is a crucial component in the heat exchanger industry.[10,11] The brazing-type plate heat exchanger has advantages of compact volume, high reliability and high heat exchange rate. Furnace brazing is one of the most common approaches to manufacture a plate heat exchanger.
Traditional copper brazing used to produce a 316 stainless steel (316SS) plate heat exchanger is well developed in the past two decades.[12,13] However, the copper-brazed 316SS plate heat exchanger has failed to satisfy all requirements in modern industry because of its poor corrosion resistance.[14,15] Because the Cu is almost insoluble with Cr and Fe, the chemical composition of the Cu-brazed 316SS joint is almost pure Cu.[16] The combination of Cu and 316SS easily results in galvanic corrosion of the Cu-brazed joint. Accordingly, the application of Cu-brazed 316SS plate heat exchanger is significantly confined by the type of coolant and environment. Only selected noncorrosive refrigerants are suitable for the Cu-brazed 316SS plate heat exchanger.
The Ni-based IN-625 alloy can be brazed using the copper filler foil because nickel and copper are completely miscible.[16] It is well known that the corrosion resistance of pure copper is significantly improved by alloying with Ni.[17,18] It is expected that the Cu-brazed IN-625 joint mainly consists of Cu-Ni alloy. When Ni is added to Cu, a series of solid-solution alloys forms of approximately 10 pct, 20 pct, and 30 pct Ni called cupronickels.[2] The Ni additions increase the strength, oxidation, and corrosion resistance of Cu. The cupronickels are used for marine condensers and tubing for conducting sea water because of their moderate high strength and resistance to the corrosive and erosive effects of high-velocity sea water.[2] Therefore, pure copper foil has the potential to be used as the filler metal in brazing IN-625 substrate for plate heat exchanger application. Microstructural evolution and the shear strengths of IN-625/Cu/IN-625 joints with different brazing conditions are performed in the study.
2 Material and Experimental Procedures
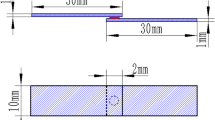
IN-625 alloy is used as the base metal with the dimensions of 15 mm in length, 6 mm in width, and 3 mm in thickness. All IN-625 plates were ground by SiC papers up to grit 600 and subsequently cleaned in an ultrasonic bath using acetone solution prior to brazing. The thickness of Cu filler foil is preferred to be 50 μm. However, the Cu foil of 100 μm thickness is used in brazing a huge plate heat exchanger. The thickness effect of Cu filler is also evaluated in the experiment. Pure Cu foils with the thicknesses of 50 and 100 μm were selected as brazing fillers. The Cu foil with the dimensions of 15 mm in length and 6 mm in width was sandwiched between IN-625 base metals using a graphite fixture. The load applied in sandwiching the material is approximately 0.2 MPa. The melting point of Cu is 1357.7 K (1084.7 °C).[16] Because Cu has a high vapor pressure if it is heated above 1273 K (1000 °C), furnace brazing was performed under the vacuum of 5 × 10−2 mbar. All brazed samples were evacuated at least 1 hour to reduce residual oxygen and water vapor in the furnace. The heating rate was set at 0.33 K/s throughout the brazing process. The liquidus and solidus temperatures of IN-625 are 1623 K and 1563 K (1350 °C and 1290 °C), respectively.[17] The liquidus temperature of cupronickel alloyed with 30 pct Cu is 1513 K (1240 °C).[18] All specimens were preheated at 1273 K (1000 °C) for 1800 seconds prior to brazing for the purpose of equilibrating the temperature gradient in the sample. Furnace brazing was performed at 1393 K, 1413 K, 1433 K, 1453 K, and 1473 K (1120 °C, 1140 °C, 1160 °C, 1180 °C, and 1200 °C) for 600 seconds, 1800 seconds, and 3600 seconds, respectively.
Cross-sections of brazed joints were cut by a low-speed diamond saw and examined by using a JEOL JSM 6510 (JEOL Ltd., Tokyo, Japan) scanning electron microscope (SEM). A JEOL 8600SX electron-probe microanalyzer (EPMA) equipped with the wavelength-dispersive spectroscope (WDS) was used for quantitative chemical analyses of various phases in the brazed joint. Its operation voltage was 15 kV, and the minimum spot size was set at 1 μm. A few nanoscale precipitates were examined using a Philips TECNAI G2 (Philips, Amsterdam, The Netherlands) transmission electron microscope (TEM) equipped with an energy-dispersive spectroscope (EDS) for a chemical analysis of the specific location in the joint. The specimens were sectioned into thin slices within brazed zones of the joint. Thin foils were prepared by a standard twin jet polisher using an electrolyte of 25 pct HNO3 and 75 pct CH3OH at 233 K (−40 °C). The operation voltage of twin jet polisher was kept at 30 V, and its current was 25 mA to 30 mA.
Shear tests were performed to evaluate the bonding strength of the brazed joint.[19] The specimen was compressed by a Shimadzu AG-10 (Shimadzu Corporation, Kyoto, Japan) universal testing machine with a constant speed of 0.0167 mm/s. At least three specimens were measured for each brazing condition. Failure analyses of fractured surfaces after shear tests were conducted using the SEM secondary electron images (SEIs). Cross-sections of brazed joints after shear tests were inspected using SEM backscattered electron images (BEIs) to locate fracture locations of the joints.
3 Results and Discussion
3.1 Microstructural Characterization of Cu-Brazed IN-625 Joint
Figure 1 illustrates SEM BEI and WDS chemical analysis results in atomic percent of IN-625/Cu (50 μm)/IN-625 joints brazed at 1393 K, 1433 K, and 1473 K (1120 °C, 1160 °C, and 1200 °C) for 1800 seconds, respectively. There are at least two phases observed from the joint including Cu/Ni-rich matrix as marked by B, E, H, and Cr/Mo/Nb/Ni quaternary compound as marked by F and I. According to the Cu-Ni binary alloy phase diagram, copper and nickel are completely miscible.[16] The nickel-based IN-625 substrate is readily dissolved into the copper melt during brazing. The IN-625 particle alloyed with copper are observed from the joint as marked by D in Figure 1. Meanwhile, the copper is diffused into the IN-625 substrate as demonstrated by A, C, and G in Figure 1. Dissolution of IN-625 substrate results in introducing Cr, Mo, Ni, and Nb into the Cu-rich melt. Because Cr, Mo, and Nb are not miscible with Cu, white Cr/Mo/Nb/Ni quaternary compounds are widely observed from the brazed joint. There are many gray, insoluble, Cr-rich precipitates with the size of nanoscale spreading in the Cu/Ni-rich matrix, and they will be subsequently dissected by TEM.
The dissolution rate of IN-625 substrate into the Cu-rich melt is greatly decreased with decreasing the brazing temperature from 1433 K to 1393 K (1160 °C to 1120 °C). White Cr/Mo/Nb/Ni compounds are primarily found at the interface between IN-625 substrate and Cu-rich braze for the specimen brazed at 1393 K (1120 °C). Different from the joint brazed at 1433 K (1160 °C), there is no IN-625 particles alloyed with copper observed from the joint brazed at 1393 K (1120 °C). Increasing the brazing temperature results in an enhanced dissolution rate of IN-625 substrate, which is evidenced by increasing the Ni content in the Cu/Ni-rich matrix as compared among B, E, and H points. The amount of Cr/Mo/Nb/Ni quaternary compound is also greatly decreased with increasing the brazing temperature to 1473 K (1200 °C).
Figure 2 illustrates microstructural evolution of IN-625/Cu (50 μm)/IN-625 joints using SEM BEIs under various brazing conditions. Fine, interfacial white Cr/Mo/Nb/Ni compounds are widely observed from the joints brazed below 1433 K (1160 °C) as illustrated in Figures 2(a) through (f). Cr/Mo/Nb/Ni compounds are coarsened with increasing the brazing temperature to 1433 K (1160 °C) for 1800 seconds and 3600 seconds (Figures 2(e) and (f)). Further increasing the brazing temperature and/or time causes a dissolution of Cr/Mo/Nb/Ni compounds into the IN-625 substrate as shown in Figures 2(g) through (i). The widths of brazed joints are sensitive to brazing conditions, especially for joints brazed above 1433 K (1160 °C); the Cu/Ni-rich matrix is gradually shrunk with increasing the brazing temperature and/or time. For the specimens brazed at 1473 K (1200 °C) for 1800 seconds and 3600 seconds, most of the Cu/Ni-rich matrix is eliminated from the joint as displayed in Figures 2(h) and (i).
Figure 3 shows a microstructural evolution of IN-625/Cu (100 μm)/IN-625 joints brazed at various brazing conditions. The microstructural evolution displayed in Figure 3 using 100 μm Cu filler is very similar to that shown in Figure 2 using 50 μm Cu foil. Similar phases are observed from the brazed joints. Widths of brazed joints using 100 μm Cu foil are approximately 10 μm larger than those using 50 μm Cu foil. Because the Cu braze melt is highly fluid, it overflows out of the joint for specimen using the 100 μm Cu foil. Similar microstructure of the joint may result in similar bonding strength of the joint, and it will be discussed in the following section.
Figure 4 shows EPMA chemical analysis results in atomic percent across IN-625/Cu (50 μm)/IN-625 joint brazed at 1393 K, 1433 K, and 1473 K (1120 °C, 1160 °C, and 1200 °C), respectively. WDS quantitative chemical analyses of Cu and Ni were performed from center of Cu/Ni-rich matrix into IN-625 substrate with an interval of 8 μm. The Ni concentration in the Cu/Ni-rich matrix of joint is as low as 17 at. pct for the specimen brazed at 1393 K (1120 °C) for 1800 seconds. The Ni additions increase the strength, oxidation, and corrosion resistance of Cu. It is expected that chemical composition of the Cu-brazed joint is significantly improved by alloying Ni into the Cu-rich braze.
Figure 5 shows TEM/EDS analysis results of an IN-625/Cu (50 μm)/IN-625 joint brazed at 1433 K (1160 °C) for 1800 seconds. Figure 5(a) displays a dark-field (DF) image of Ni/Cr-rich matrix, and its selected-area diffraction pattern (SADP) with the zone axis of \( \left[ {\bar{1} \bar{1} 0} \right] \) is illustrated in Figure 5(b). Chemical composition of the Cu/Ni-rich matrix is 1.9Cr-79.1Cu-19.0Ni in at pct as marked by J in the figure. The crystal structure of Cu/Ni-rich matrix belongs to face-centered cubic with the lattice constant of 3.615 Å. There are many nanosized precipitates in the Cu/Ni-rich matrix. The chemical composition of the nanosized precipitate analyzed by EDS is 20.9Cr-6.5Cu-4.3Fe-4.0Mo-64.2Ni in at. pct. Based on the Cr-Ni binary alloy phase diagram, the tiny Cr/Ni-rich precipitate is identified as CrNi3.[20–22] It has a cubic crystal structure with the lattice constant of 3.54 Å. Figure 5(c) displays a DF image of CrNi3 precipitate using the (020) spot, and SADP of CrNi3 precipitate with the zone axis of \( \left[ {\bar{1} 0 0} \right] \) is illustrated in Figure 5(d). The Cu is completely soluble with Ni but is almost insoluble with the Cr.[16] The Cr cannot be dissolved into the Cu-rich melt, and it reacts with Ni to form the CrNi3 precipitates upon cooling cycle of brazing.
TEM/EDS analyses of IN-625/Cu (50 μm)/IN-625 joint brazed at 1433 K (1160 °C) for 1800 s: (a, b) DF image of Cu/Ni-rich matrix using the (002) spot with zone axis \( \left[ {\bar{1} \bar{1} 0} \right] \), (c, d) DF image of CrNi3 precipitate using the (020) spot with the zone axis of \( \left[ {\bar{1} 0 0} \right] \)
The brazed IN-625/Cu/IN-625 joint is primarily composed of Cu/Ni-rich matrix. Nanosized CrNi3 precipitates and Cr/Mo/Nb/Ni quaternary compound particles are dispersed in the Cu/Ni-rich matrix. Increasing the brazing temperature and/or time results in the dissolution of Cr/Mo/Nb/Ni compounds into the IN-625 substrate. Additionally, the widths of the brazed joints are sensitive to brazing conditions, especially for joints brazed above 1433 K (1160 °C). Because the dissolution of IN-625 substrate into the braze melt during brazing is enhanced with increasing the brazing temperature and/or time, the Cu/Ni-rich matrix of the joint is gradually shrunk with increasing the brazing temperature and/or time. The minimum Ni content in Cu/Ni-rich matrix is increased up to 23 at. pct with increasing the brazing temperature to 1473 K (1200 °C).
3.2 Mechanical Property of Cu-Brazed IN-625 Joint
Figure 6 displays the average shear strengths of IN-625/Cu/IN-625 joints with various brazing conditions using the copper foils of 50 and 100 μm, respectively. For specimens brazed below 1433 K (1160 °C), the average shear strengths of brazed joints exceed 400 MPa. It has been reported that the tensile strength of brazed 304SS and pure Cu using rapidly solidified 50Cu-40Mn-10Ni filler is 260 MPa.[23] The average tensile strength of brazed 304SS joint using amorphous Ni/Fe-based filler foil is up to 433 MPa.[24,25] The average tensile strengths of brazed IN-625 joints using amorphous Ni/(Fe)-based filler foils are between 293 MPa and 443 MPa.[24] It is obvious that the Cu-brazed IN-625 joint demonstrates good bonding strength compared with previous studies.
Based on the aforementioned result, the microstructural evolution of brazing joints using 50 μm and 100 μm Cu foils is similar under the same brazing condition, and the widths of brazed joints using 100 μm Cu foil are approximately only 10 μm larger than those using 50 μm Cu foil. The thickness of the Cu filler foil has little effect on the shear strengths of brazed joints as illustrated in Figure 6. It indicates that the copper filler 50 μm in thickness is enough for the joint filling. However, the application of Cu foil with 100 μm in thickness will not deteriorate the shear strength of the brazed joint. This is beneficial for industrial application due to its wide processing window. The specimen brazed at 1433 K (1160 °C) for 1800 seconds demonstrates the best shear strength up to 470 MPa. Decreasing the brazing temperature slightly decreases the shear strength of the brazed joint. In contrast, increasing the brazing temperature, especially for the specimen brazed at 1473 K (1200 °C), greatly deteriorates the shear strength of the joint below 260 MPa.
Figure 7 shows SEM BEI cross sections of IN-625/IN-625 joints brazed at 1433 K (1160 °C) for 1800 seconds after shear tests. All the cracks are located in the Cu/Ni-rich matrix. Both CrNi3 precipitates and Cr/Mo/Nb/Ni quaternary compounds show little effect on the joint strength. A lower brazing temperature, e.g., 1393 K (1120 °C), causes higher viscosity of Cu-rich melt. On the other hand, the joint brazed at higher temperature enhances dissolution of IN-625 substrate into the Cu-rich melt, and it may result in isothermal solidification of the joint during brazing. Isothermal solidification of the braze melt may result in solidification shrinkage voids that deteriorate bonding strength of the joint.
Figure 8 shows SEM SEI fractographs of IN-625/Cu/IN-625 joints after a shear test under different brazing conditions. Ductile dominated fracture with sliding marks is widely observed from fractographs for specimens brazed at 1433 K (1160 °C) as illustrated in Figures 8(a) and (c). Therefore, similar average shear strengths of joints are observed for specimens brazed below 1433 K (1160 °C). In contrast, solidification shrinkage voids are found from the fractographs of specimens brazed at 1473 K (1200 °C) as marked by arrows in Figures 8(b) and (d). The average shear strength of brazed joint is as low as 233 MPa.
The location shrinkage voids cannot be clearly demonstrated in Figures 1 through 3 because SEM BEIs are insensitive to shrinkage voids. Two representative images using SEM SEIs are displayed in Figure 9. It shows solidification shrinkage voids of IN-625/Cu/IN-625 joints brazed with a 50-μm Cu at 1393 K and 1473 K (1120 °C and 1200 °C) for 1800 seconds, respectively. For the specimen brazed at 1393 K (1120 °C), there are only a few isolated solidification shrinkage voids with the size below 15 μm observed from a selected location of the joint as shown in Figure 9(a). The shear strength of the brazed joint is slightly deteriorated due to the presence of these minor cracks. The cross section of the joint brazed at 1433 K (1160 °C) is free of void and the joint shows the best shear strength up to 470 MPa. In contrast, coalescence of isothermal solidification shrinkage voids (Figure 9(b)) significantly deteriorates the shear strength of the joint brazed at 1473 K (1200 °C) due to enhanced dissolution of IN-625 substrate during brazing.
4 Conclusions
Microstructural evolution and the shear strengths of IN-625/Cu/IN-625 joints with different brazing conditions have been evaluated from the experiment. Important conclusions are summarized as follows:
-
1.
The brazed joint is comprised of nanosized CrNi3 precipitates and Cr/Mo/Nb/Ni quaternary compound in the Cu/Ni-rich matrix. The widths of brazed joints are sensitive to brazing conditions, especially for joints brazed above 1433 K (1160 °C). The Cu/Ni-rich matrix is gradually shrunk with increasing the brazing temperature and/or time.
-
2.
The thickness of the Cu filler foil has little effect on the shear strengths of brazed joints. The copper filler 50 μm in thickness is enough for the joint filling. However, the application of Cu foil 100 μm in thickness will not deteriorate the shear strength of the brazed joint.
-
3.
For the specimen brazed at 1433 K (1160 °C) for 1800 seconds demonstrates the best shear strength of 470 MPa. The fractograph is dominated by dimple fracture with sliding marks. Decreasing the brazing temperature slightly decreases the shear strength of the brazed joint due to the presence of a few isolated solidification shrinkage voids with the size below 15 μm. In contrast, increasing the brazing temperature, especially for the specimen brazed at 1473 K (1200 °C), significantly impairs the shear strength of the joint below 260 MPa. Coalescence of isothermal solidification shrinkage voids resulting from dissolution of IN-625 substrate into the Cu-rich melt greatly deteriorates the shear strength of the joint.
References
J.R. Davis: ASM Handbook, vol. 2. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, ASM International, Materials Park, OH, 1990, pp. 428–45.
W.F. Smith: Structure and Properties of Engineering Alloys, 2nd ed., McGraw-Hill, New York, NY, 1993, pp. 279, 494–98.
K.P. Cooper, P. Slebodnick, and E.D. Thomas: Mater. Sci. Eng. A Struct., 1996, vol. 206, no. 1, pp. 138-49.
K.-H. Chang, J.-H. Huang, C.-B. Yan, T.-K. Yeh, F.-R. Chen, and J.-J. Kai: Prog. Nucl. Energy, 2012, vol. 57, pp. 20-31.
M. Montazeri and F.M. Ghaini: Mater. Charact., 2012, 67, pp. 65-73.
M. Zhong, H. Sun, W. Liu, X. Zhu, and J. He: Scripta Mater., 2005, vol. 53, no. 2, pp. 159-64.
S.M. Mousavizade, F.M. Ghaini, M.J. Torkamany, J. Sabbaghzadeh, and A. Abdollah-zadeh: Scripta Mater., 2009, vol. 60, no. 4, pp. 244-47.
A.T. Egbewande, R.A. Buckson, and O.A. Ojo: Mater. Charact., 2010, vol. 61, no. 5, pp. 569-74.
M.A. Arafin, M. Medraj, D.P. Turner, and P. Bocher: Mater. Sci. Eng. A-Struct., 2007, vol. 447 (1–2), pp. 125–33.
M. Reppich: Int. J. Therm. Sci., 1999, vol. 38, no. 11, pp. 999-1008.
M.M. Abu-Khader: Renew. Sust. Energ. Rev., 2012, vol. 16, no. 4, pp. 1883-91.
A. Munitz: J. Mater. Sci., 1995, vol. 30, no. 11, pp. 2901-910.
L.H. Chiu, W.C. Hsieh, and C.H. Wu: Mater. Sci. Eng. A-Struct., 2003, vol. 354 (1–2), pp. 82–91.
K.M. Deen, M.A. Virk, C.I. Haque, R. Ahmad, and I.H. Khan: Eng. Fail. Anal., 2010, vol. 17, no. 4, pp. 886-93.
O. Narivs’kyi: Mater. Sci., 2007, vol. 43 (1), pp. 124–32.
W.G. Moffatt: The Handbook of Binary Phase Diagrams, Genium Pub. Corp., Schenectady, NY, 1976, pp. 1266–68, 1442–46.
J.R. Davis: Nickel, Cobalt, and Their Alloys, ASM International, New York, NY, 2000, pp. 29-30.
T.B. Massalski: Binary Alloy Phase Diagrams, ASM International, New York, NY, 1990, pp. 1442-46.
T. Hodgkiess and D. Mantzavinos: Desalination, 1999, vol. 126 (1–3), pp. 129–37.
K.Z. Al-Subaie and T. Hodgkiess: Desalination, 2003, vol. 158 (1–3), pp. 43–50.
R.K. Shiue, S.K. Wu, and S.Y. Chen: Acta Mater., 2003, vol. 51, no. 7, pp. 1991-2004.
P. Villars: Pearson’s Handbook: Crystallographic Data for Intermetallic Phases, ASM International, Materials Park, OH, 1997, pp. 2706-07.
R.K. Roy, S. Singh, M.K. Gunjan, A.K. Panda, and A. Mitra: Fusion Eng. Des., 2011, vol. 86, pp. 452-55.
W.S. Chen and R.K. Shiue: ISIJ Int., 2012, vol. 52, no. 5, pp. 939-41.
W.S. Chen and R.K. Shiue: Metall. Mater. Trans. A, 2012, vol. 43A, pp. 2177-82.
Acknowledgments
The authors gratefully acknowledge the financial support of this research by National Science Council, Taiwan, Republic of China (contract numbers NSC 99-2221-E-002-120-MY3 and NSC 102-2221-E-002-148).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 30, 2013.
Rights and permissions
About this article
Cite this article
Chen, WS., Wang, CY. & Shiue, RK. Brazing Inconel 625 Using the Copper Foil. Metall Mater Trans A 44, 5724–5731 (2013). https://doi.org/10.1007/s11661-013-1954-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-013-1954-x