Abstract
In the current investigation, hydroxyapatite (HA) powder was mixed with titania (TiO2) in 50:50 wt pct for depositing composite coatings on a Ti-alloy substrate using a thermal-spray coating technique. The coatings were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM)/energy-dispersive X-ray spectroscopy (EDS) analyses. The corrosion behavior of the coatings was studied by electrochemical corrosion testing in simulated human body fluid. After the corrosion testing, the samples were analyzed by XRD and SEM/EDS analyses. HA and TiO2 (rutile) were the main phases observed in the developed coatings. Bulk HA coating was amorphous; however, the addition of TiO2 effectively improved the crystallinity of HA in HA-TiO2 coating. The SEM analysis confirmed the formation of a well-formed HA-TiO2 composite coating. HA coating exhibited higher bond strength (67.8 MPa) compared with HA-TiO2 composite coating (37.6 MPa). The electrochemical study showed a significant improvement in the corrosion resistance of the Ti alloy after the deposition of the coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metals with excellent mechanical properties and reasonable biocompatibility are widely used as biomedical implants. The ultimate goal of using biomaterials in medicine is to restore the function of natural living tissues and organs in the human body.[1] Pure titanium (Ti) and its alloy Ti-6Al-4V (wt pct) [Ti-6-4] are the most widely used materials for biomedical applications because of their good fatigue strength, excellent biocompatibility, and corrosion resistance.[2] Besides the formation of a highly stable TiO2 layer in Ti-6-4, the oxides of Al and V form an additional passive layer, causing subsequent resistance against corrosion.[3] The stability of the surface oxide layer is one of the most important features of a biomaterial.[4] The composition of the surface oxide layer changes because of reactions between the implant surface and living tissues.[5] The two major issues regarding the long-term use of metallic implants in highly aggressive human body environment are corrosion of metallic implants and the lack of integration of metallic implants with human tissues.[6]
Surface coating or modification is one alternative that has been widely considered for improving the mechanical, chemical, and biological properties of Ti and its alloys for biomedical applications.[7] The coating of biomaterials with calcium phosphate (CaP)-based hydroxyapatite (HA) (Ca10(PO4)6(OH)2) has attracted wide interest from both the orthopedic and dental fields in clinical applications because of its excellent biocompatibility and tissue bioactivity properties.[8] Osteoconductive CaP coatings promote bone healing leading to the rapid biological fixation of implants.[9]
Currently, the plasma spraying process is used for depositing HA on bioimplants. Although it improves the bone strength and initial osseointegration, still optimum coating properties with maximum bone response are yet to be achieved.[10] It has been reported that elevated temperatures in plasma spraying result in the alteration of structural properties, peeling, and subsequent dis-solution of the coating, which further reduce the bond strength between the coating and substrate. Moreover, because of the number of process parameters, plasma spray is a very complex process.[10,11] Intensive work is being conducted to overcome the problems of existing plasma spray process and to create more stable HA coatings by alternative coating techniques. This study is a step towards this direction, in which a proprietary thermal-spray equipment has been used to deposit the coatings on the Ti alloy.
An alternative approach for improving the mechanical, chemical and biological properties of Ti and its alloys, is the use of bond or composite coatings. Titania (TiO2), zirconia (ZrO2), and alumina (Al2O3) are used as bond coats or as composite coats with HA in different ratios to enhance the mechanical and biological properties of HA coatings.[12–14] TiO2 has attracted much attention as a composite or bond coat material because of its biological and corrosion-resistance effects.[15] The melting point of TiO2 [2116 K (1843 °C)] is much lower than that of Al2O3 and ZrO2, which are above 2273 K (2000 °C). The relatively lower melting point of TiO2 is helpful in the formation of a homogenous HA-TiO2 composite coating. Moreover, HA has lower thermal diffusivity (α) and conductivity (k) values than those of TiO2. Therefore, TiO2 particles need lower thermal energy to reach a given temperature compared with that of HA. To improve the thermal mismatch between HA coating and Ti substrate, the addition of TiO2 is favorable.[16]
Several earlier studies[17–23] have reported an increase in the mechanical and biological properties of the HA-TiO2 composite coatings than the HA coatings. In high-velocity oxygen fuel (HVOF) sprayed HA-TiO2 composite coatings, the addition of TiO2 is found to improve the Young’s modulus, fracture toughness, and shear strength of HVOF-sprayed HA-based coatings. It was suggested that addition of less than 20 vol pct TiO2 is suitable for satisfactory toughening of HA coatings.[12,24] Ramires et al.[18] reported that the sol-gel fabricated HA-TiO2 coatings were not cytotoxic and allowed the proliferation of human osteoblast like cells. Kim et al.[25] suggested that the sol-gel deposited HA-TiO2 composite coatings possess excellent properties for hard tissue applications from the mechanical and biological perspective.
In the current work, a proprietary flame-spray system has been used to deposit HA and HA-TiO2 coatings on a Ti alloy. Subsequently, the corrosion behavior of uncoated, HA-coated, and HA-TiO2–coated alloy has been investigated. The corrosion tests were conducted by an electrochemical potentiodynamic method in a simulated body fluid (Ringer’s solution).
2 Experimental Details
2.1 Deposition of Coatings
A commercially available biomedical material titanium-aluminum-vanadium alloy (Ti-6-4, ASTM F-136) having chemical composition in wt pct — N: 0.02; C: 0.05; H: 0.01; Fe: 0.20; O: 0.16, Al: 6.10; V: 3.95 and Ti: balance, was used as the substrate material in this study. Disc shaped Ti-6-4 specimens each measuring 12 mm × 5 mm were prepared for the studies. The specimens were polished by silicon carbide papers down to 180 grit followed by cloth wheel polishing with alumina paste on a polishing machine. These specimens were washed with deionized water followed by acetone rinsing. The substrates were then grit blasted using grit blasting equipment (Pressure Blaster 9182; Surface International, Jodhpur, India). Al2O3 grits of 20 mesh size were used during the grit-blasting operation at a pressure of 5 bars for 2 minutes. The substrates were subsequently air blasted to remove any residual grit. The HA powder (Captal S; Plasma Biotal, Derbyshire, U.K.) of 10 μm average particle size and TiO2 powder (Amperit 782.0; H.C. Starck GmbH, Goslar, Germany) with a particle size distribution from 5 to 22 μm were used in this study. Both the powders (HA and TiO2) were mixed mechanically in the ratio of 50:50 (wt pct). The Ti-6-4 specimens were thermal sprayed using flame-spray system (CERAJET) at Metalizing Equipment Company Private Limited (MECPL; Jodhpur, India). CERAJET is proprietary product of MECPL, specially designed for ceramic coatings. The particle velocity of conventional flame-spraying systems is less than 100 m/s, whereas the particle velocity of the current flame-spray system is ≈300 m/s. The temperature of oxyacetylene flames in CERAJET system is ≈2973 K (2700 °C), which is much lower than the plasma spraying technique. The spraying parameters used for both HA and HA-TiO2 coatings are given in Table I.
The thermal spraying of composite powder was carried out immediately after grit blasting of substrates. The coating thickness to be achieved was targeted in a range of 150 to 200 μm.
2.2 Characterization of the Coatings
The phase composition of the starting powder as well as the as-sprayed coatings was analyzed using an X’pert-PRO (PANalytical B.V., Almelo, the Netherlands) X-ray diffractometer (XRD) with Cu K α radiation, operating at 40 KV/30 mA. The coated samples were scanned over 2θ range of 20 deg to 60 deg. The morphology of feedstock powders and as-sprayed specimens was examined by using QUANTA 200 field-emission gun (FEI Corporation, Hillsboro, OR, scanning electron microscope (SEM) coupled with an energy-dispersive spectroscope (EDS). After surface characterization, the samples were sectioned with a low-speed precision saw at 75 rpm speed and mounted in an epoxy resin using a specimen mounting press. The mounted samples were polished with emery papers of 220, 400, 600, and 800 grade and subsequently 1/0, 2/0, 3/0, and 4/0 grades. Then samples were mirror polished with slurry of alumina on a napped cloth to highlight the surface of the coated substrates. The samples were gold plated to achieve the desired conductivity before observation in SEM. A surface as well as a cross-sectional SEM/EDS analysis of the coated samples was done to study their elemental compositions along with the microstructural features. EDS elemental maps were obtained to display the distribution of elements in the coating on JEOL JSM-6610LV SEM (JEOL Ltd., attached with OXFORD Instruments EDS (Oxfordshire, U.K.).
2.3 Surface Roughness
The surface roughness of Al2O3 blasted, HA-coated, and HA-TiO2 coated Ti-6-4 samples was measured by a roughness tester (SJ-400; MITUTOYO America Corporation, Aurora, IL), using a filter of Gaussian type and roughness standard JIS1994 for a cut-off length (λc) of 0.8 mm. Each reported value of roughness is the average of five measurements at various positions on the surface of the coated samples. The measured roughness parameters are Ra (the arithmetic mean of departures of the roughness profile from the mean line), Rq (root mean square [RMS] of average roughness), and Rz (average of the highest peaks and the lowest valleys on five measurement lengths). Among these parameters, Ra is the most commonly used roughness parameter.
2.4 Bond Strength
The bond strength of both the coatings on stainless steel (SS) specimens is determined as per ASTM-C633 standard for thermal-spray coatings. For this test, identical cylindrical SS rod (25.4 mm diameter) specimens were prepared. HA coatings of thickness ≈300 μm and HA-TiO2 coating of thickness ≈350 μm were deposited on SS rods by the flame-spray system (CERAJET). The flat surface of the uncoated rod was grit blasted and bonded to the coated rod by a special adhesive glue. The assembly was given a heat treatment to cure and harden the bonding glue and tested on a universal testing machine. Each reported value of bond strength is the average of three measurements.
2.5 Electrochemical Measurements
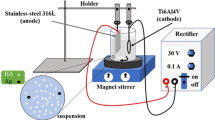
Electrochemical techniques are preferred to measure the corrosion rate of implant materials as these techniques are quick and sensitive to measure even a low corrosion rate. To investigate the electrochemical corrosion behavior of the uncoated, HA-coated, and HA-TiO2–coated Ti-6-4 specimens, a potentiodynamic polarization test was conducted using a Potentiostat/Galvanostat (Series G-750; Gamry Instruments, Warminster, PA), interfaced to a computer and supported with Gamry electrochemical software DC105. The electrolyte used for simulating human body fluid conditions was Ringer’s solution with chemical composition (in g/L) as 9-NaCl, 0.24-CaCl2, 0.43-KCl, and 0.2-NaHCO3 at pH 7.2. Before conducting the corrosion studies, each specimen was immersed in Ringer’s solution for 24 hours for stabilization. In the electrochemical cell, each specimen was kept such that it remained just below the surface of Ringer’s solution for in vitro potentiodynamic corrosion tests. All the experiments were conducted in Ringer’s solution at a temperature of 310 K ± 1 K (37 °C ± 1 °C), which is the normal temperature of human body. The temperature was maintained using a heating mantle. The exposed area of the samples in the Ringer’s solution was 1 cm2. The Ti-6-4 specimen formed the working electrode. All potentials were measured with respect to a saturated calomel electrode (SCE) as the reference electrode. A graphite rod served as the counterelectrode. All tests were performed at a scan rate of 1 mV/sec and fresh solution was used for each experiment. Polarization curves were initiated at –250 mV below the open circuit potential and the tests were started after a steady open-circuit potential was achieved (not more than ±5 mV drift in 5 minutes). The corrosion rate was determined using the Tafel extrapolation method and all the tests were carried out on three fresh samples to verify repeatibilty of test results. In Tafel methods, tangents were drawn on graphical plot of E vs log I, which on extrapolation to corrosion potential (E Corr) intersected at a point, which on X axis gives the corrosion current (I Corr) value. After corrosion testing, the specimens were analyzed by SEM/EDS and XRD techniques to analyze the microstructure/composition and phase formation, respectively.
3 Results and Discussion
3.1 XRD Analysis
The XRD scans of HA and TiO2 powders are shown in Figure 1. The scans indicate that both the HA and TiO2 powders consist of crystalline phases in accordance with JCPDS cards 9-432 and 21-1276, respectively. The analysis shows that all the major peaks belong to HA in Figure 1(a) and TiO2 rutile phase in Figure 1(b). TiO2 crystallizes in three major structures: rutile (tetragonal), anatase (tetragonal), and brookite (rhombohedrical).[15] The XRD analysis of the HA-TiO2 composite powder is shown in Figure 2.
The XRD analysis for the flame-sprayed HA coating on Ti-6-4 substrate is shown in Figure 3(a). It is found that the XRD pattern of HA coatings is extremely different from that of the feedstock HA powder. A large hump is observed around 30 deg (2θ) showing amorphous structure without the appearance of any crystalline peak. The formation of the amorphous phase may be attributed to the fine particle size of HA powder, which might have melted completely during the thermal spraying. Previous literature reports that amorphous phases are more soluble than crystalline HA, which leads to rapid disintegration of HA coating from implant surface.[26,27]
Interestingly, in HA-TiO2 composite coatings as shown in Figure 3(b), the addition of TiO2 effectively improved the crystallinity of HA in HA-TiO2 coating; otherwise, the HA coating was amorphous. In HA-TiO2 composite coatings, the coating structure mainly comprises HA and TiO2 (rutile) phases. Besides the crystalline HA and TiO2 phases, the as-sprayed coating consists of minor β-tri-calcium phosphate (β-TCP) phase, but no chemical products such as CaTiO3 were detected from the XRD scans. The formation of CaTiO3 is suggested to reduce adhesive strength and destroy the HA structure.[12] In HVOF-sprayed HA-TiO2 (anatase) composite coatings, the following chemical reaction at ~1683 K (1410 °C) temperature indicates the formation of CaTiO3.[12]
Of three polymorphic forms of titania, rutile is the only stable phase, whereas anatase and brookite are metastable and are transformed to rutile irreversibly by heating.[4] A previous study found that HA prefers to react chemically with anatase than rutile TiO2.[28]
3.2 SEM/EDS Analysis
3.2.1 Surface analysis
The morphology of the individual and composite feedstock powders is shown in Figure 4. The SEM micrographs show that HA powder particles are spherical, whereas TiO2 powder particles are of angular and irregular shape. The size distribution range seems to be wider in both powders. It is worthwhile to mention that the particle shape, size, and morphology influence the final microstructure of the thermal-sprayed coatings.
The SEM micrograph along with EDS point analysis of an HA-coated specimen is shown in Figure 5. The microstructure in general consists of well-formed interconnected splats with uneven surface. There is a significant presence of unmelted particles, which have retained their spherical morphology. Some voids/pores can also be observed but no microcracks were observed on the coating surface. The EDS analysis as shown in Figure 5 confirms the presence of calcium (Ca), phosphorous (P), carbon (C), and oxygen (O) elements with their atomic pct. The Ca/P ratio of feedstock HA powder is 1.67, which may change after thermal spraying. The Ca/P ratio of 1.64 (at point 1 in Figure 5) is characteristic for hydroxyapatite, which is close to the Ca/P ratio of 1.67. The observed different Ca/P ratios confirm the presence of phases other than HA in the as-sprayed coatings.
The HA-TiO2 composite coating (Figure 6) consists of both nonmelted and semimelted powder particles. The coating microstructure seems to be uneven and heterogeneous. There is significant presence of some spherical particles, which may be of HA powder. The micrograph indicates that both the HA and TiO2 powders have melted enough to form a dense composite coating. The difference in thermal conductivity of the TiO2 (7.4 W/mK) and HA (1.25 W/mK) bulk materials shows that TiO2 is a better heat conductor.[29] Therefore, an HA particle needs more thermal energy to reach a given temperature level than needed for a TiO2 particle.[4] No microcrack was observed at the surface of the as-sprayed samples. The TiO2 in the coating may have assisted in matching the thermal properties between the Ti-6-4 substrate and HA-TiO2 composite coating. It seems that TiO2 with a higher melting point ~2123 K (1850 °C) than that of HA ~1823 K (1550 °C) is melted enough to form a HA-TiO2 composite coating.
EDS point analysis of HA-TiO2 coating at different points indicates the presence of Ca, P, O, Ti, and C elements with their relative atomic pct values. The Ca/P ratio of 1.5 (at points 1 and 3 in Figure 6) indicates the formation of α-TCP and β-TCP. A point analysis of HA-TiO2 coatings confirms that the spherical particles consist of HA. Both HA and TiO2 seem to be well distributed throughout the HA-TiO2 coating microstructure. A point analysis shows that Ca, P, and Ti elements coexist in different proportions in some melted particles.
3.2.2 Cross-sectional analysis
The cross-sectional EDS elemental map of flame-sprayed HA coating on the Ti-6-4 substrate is shown in Figure 7. Although because of the brittle nature of HA coatings there is a possibility of some HA coating particles getting removed during grinding and polishing process, these maps provide significant information about the distribution of various elements in the coatings. The mappings of Ca and P elements clearly demonstrated that these elements are distributed uniformly in the coating and are found to coexist in the coating region. O is present throughout the coating. The maps indicate that no diffusion of Ti and V elements from the substrate to the coating occurs; however, Al is present in the coating region in a scattered manner. This may be because of the diffusion of Al from the substrate or artifacts of alumina polishing. The latter looks to be the more probable reason, as surface EDS measurements have not indicated the presence of Al, Ti, and V (Figure 5). The average value of the HA coating thickness measured from a cross-sectional micrograph is 120 μm. To understand the formation of cross-sectional microstructures in the composite coatings, EDS elemental maps (Figure 8) were used to differentiate between the HA and TiO2 in the HA-TiO2 coating. The average coating thickness measured from the SEM micrograph is found to be 140 μm. The coating contains mainly Ca, P, and Ti elements. The presence of Ti in both the coating as well as the substrate is obvious. Ca and P elements are found to coexist in the coating region. Wherever these elements are present, Ti is found to be absent and vice versa. These maps indicate that TiO2 is dispersed as a particulate phase in the HA matrix, which means that a composite-like microstructure exists. Small diffusion of Al and V is also evident into the coating region.
3.3 Surface Roughness
Surface topography plays a significant role in the improvement of coating adhesion.[30] Rough-surfaced implants favor both bone anchoring and biomechanical stability.[9] The surface roughness parameters (Ra, Rq, and Rz) for alumina-blasted, HA-coated, and HA-TiO2–coated substrates Ti-6-4 are shown in Figure 9. The average Ra value for blasted Ti-6-4 substrates was 4.00 μm, whereas in HA-coated Ti-6-4 it was 3.87 μm. In other words, the roughness values (Ra) of bare and HA-coated substrates do not differ significantly. Ra for HA-TiO2 coated substrates was 8.04 μm, which is greater than roughness of HA-coated Ti-6-4 (Ra = 3.87 μm). The increase in roughness can be attributed to the presence of TiO2 in the composite coating.
3.4 Bond Strength
The average bond strength of the current flame-sprayed HA coating was measured 67.8 MPa, which is much greater than the bond strength reported for other thermal-spray HA-coating techniques, as reported in Table II.
It is obvious that the technique used in the study could give relatively higher bond strengths compared with the reported techniques. The bond strength of the flame-sprayed HA-TiO2 coating was found to be 37.6 MPa. The addition of 50 wt pct TiO2 in the HA has drastically reduced the bond strength of the pure HA coating from 67.8 MPa to 37.6 MPa for HA-TiO2 composite coating. Similar observations have been reported by Li et al.[12] for HVOF-sprayed HA coatings on the Ti-6-4 substrate. They found that with the addition of 10 pct vol TiO2, the adhesive strength decreased from 27.9 (±1.5) MPa for pure HA coating to 26.8 (±2.3) MPa for the HA-TiO2 composite coating. The bond strength was found to decrease further to 17.2 (±1.2) MPa as the TiO2 addition was increased to 20 vol pct. Furthermore, the bond strength of the investigated HA-TiO2 coating is found to be higher than that reported by Li et al.[12] This result further shows that the investigated flame-spraying technique can give better results compared with the reported HVOF spraying technique.
3.5 Corrosion Behavior
The potentiodynamic curves of uncoated, HA-coated and HA-TiO2–coated Ti-6-4 in Ringer’s solution at 310 K ± 1 K (37 °C ± 1 °C) temperature are shown in Figure 10. These curves are selected because their data is nearest to the mean values of current densities of the corresponding group of specimens. The corrosion parameters determined from these curves by tafel extrapolation method are summarized in Table III.
The various corrosion parameters are anodic tafel slope (β a), cathodic tafel slope (β c), corrosion potential (E Corr), corrosion current density (I Corr), and corrosion rate (C R). According to Table III, the uncoated Ti-6-4 possesses a higher corrosion current density (I Corr = 906 nAcm–2) and, thus, lower corrosion resistance than HA- and HA-TiO2–coated Ti-6-4. The polarization curve, for the HA coating (Figure 10), was shifted to the left when compared with uncoated and HA-TiO2–coated Ti-6-4 curves. It shows that HA-coated specimens are more corrosion-resistant compared with both uncoated and HA-TiO2–coated Ti-6-4 specimens. The HA-TiO2 coating decreased the corrosion current density of Ti-6-4 (I Corr = 150 nAcm–2) and the corrosion current density of the HA-coated Ti-6-4 decreased further (I Corr = 120 nAcm–2). When I Corr is low, passivation is more easily obtained and corrosion resistance is excellent.[35] Even though all I Corr values reflect excellent corrosion resistance including an uncoated Ti-6-4 specimen, it can be deduced through this study that both HA and HA-TiO2 coatings have shown improvement in corrosion resistance compared with uncoated Ti-6-4. In another study,[36] it has been observed that an electrolytic HA/ZrO2 coating showed higher corrosion resistance than the HA-coated Ti-6-4 implant material. Similarly, sol-gel–fabricated HA coatings showed improvement in the corrosion resistance of titanium porous scaffolds.[37] Fathi et al.[38] reported that plasma-sprayed, HA-coated commercially pure titanium (cp Ti) and 316L SS showed a significant effect on corrosion resistance compared with uncoated cp Ti and 316L SS substrates.
Corrosion in HA coatings is associated with the incursion of chloride ion and water into the coating and then subsequent electrochemical reactions at the interface of HA and Ti-6-4.[39] Each micropore acts as a active site for entrapping surrounding corrosive impurities. Zhang et al.[40] reported the corrosion mechanism of HA coatings with pores and cracks in two steps:
Hydrogen ions (H+) are produced at the interface area where corrosion occurs as evident from the following equation
It is then followed by the dissolution of HA in the high concentration area:
After electrochemical corrosion testing in Ringer’s solution, the XRD scans of HA-coated (Figure 11) and HA-TiO2–coated Ti-6-4 (Figure 12) showed an interesting behavior. The HA coating remained amorphous, but some XRD peaks appeared after immersion in Ringer’s solution. The HA-TiO2 coating appeared more crystalline, and it was found that the intensity of the XRD peaks increased after corrosion testing in Ringer’s solution. Previously, it was reported that in electrophoretic deposited HA coatings on Ti-6-4 specimens, immersed in Hank’s solution at 310 K (37 °C) for 4 weeks, the XRD analysis showed a significant increase in intensity of apatite peaks. The apatite formation reveals the bone bioactive behavior of HA coatings.[39]
The exposed specimens were examined by SEM/EDS for the microstructural analysis of their scales. Few studies report the microstructure of thermal-sprayed HA coatings after their corrosion testing in Ringer’s solution. The microstructure of the corroded HA coating (Figure 13) consists of well-flattened splats with the presence of some spherical-shaped particles. The splats seem to be fused to give a well-bounded appearance. A comparison of the SEM micrographs of the as-sprayed and exposed HA-TiO2 coating (Figures 6 and 14) showed that the coating has retained its morphology even after exposure to the corrosion testing. An EDS point analysis confirms the presence of Ca, P, O, and C elements in both HA- and HA-TiO2–coated Ti-6-4 specimens. Except these basic elements, the HA-coated specimen also shows the presence of sodium (Na) and chlorine (Cl). The presence of Na and Cl in EDS spectra (Figure 13) can be attributed to the Ringer’s solution as the specimens were immersed in the solution for their corrosion testing. No cracks are found on the surface of both HA and HA-TiO2–coated specimens, but some pores are clearly visible on the surface of HA-TiO2–coated Ti-6-4 specimens after immersion in Ringer’s solution. The presence of Ti was also confirmed along with other basic elements in HA-TiO2–coated specimens (Figure 14). It was also found that after immersion in Ringer’s solution, the percentage of O increased in both HA and HA-TiO2 coatings, which indicates the onset of oxidation. The comparison of both HA and HA-TiO2 coatings, shows that the quantity of Ca and P decreased after their immersion in Ringer’s solution. The Ca/P ratios (in atomic pct) of flame-sprayed HA and HA-TiO2 coatings before and after corrosion testing are tabulated in Table IV.
The results show the decrease of the Ca/P ratio after immersion in Ringer’s solution. The observed different Ca/P ratios confirm the presence of different calcium phosphate compounds. The Ca/P ratio of 1.37 in both the HA (at point 1 in Figure 13) and HA-TiO2 coatings (at point 1 in Figure 14) is very close to the Ca/P ratio of 1.33, which confirms the formation of octocalcium phosphate. The Ca/P ratio of 2.00 and 1.67 indicates the presence of TCP and HA, respectively.
The cross-sectional microscopic view of uncoated Ti-6-4 substrate (Figure 15) reveals the presence of a dense and uniform oxide scale over the Ti-6-4 substrate after corrosion testing in Ringer’s solution. The corrosion behavior of an implant material during its exposure to the human body environment highly depends on the stability of the protective oxide layer. EDS maps confirm the presence of Ti, Al, and V elements in the oxide scale, along with a significant presence of O in outer layers of the scale. This indicates the formation of oxides of Ti, Al, and V. There is an inner band in the scale, which is completely depleted of Al. This result indicates that Al may have preferentially oxidized to form an Al-rich oxide scale at the top of the alloy, leaving an Al-depleted inner band. Some Ti is present, as well as V-depleted zones in the oxide scale; however, the extent is marginal compared with Al. It is pertinent to mention that such depletions are not favorable for any alloy as they may degrade the material properties. The cross section of HA coating (Figure 16) shows a lamellar structure. The coating–substrate interface seems to be defect free. EDS maps show that Ti, Al, and V elements are present in the substrate only without showing any diffusion into the coating area, which is a desirable characteristic. The presence of Ti is apparent in both the coating and substrate in HA-TiO2 coating (Figure 17). The O is present throughout in both the coatings. The presence of a few Al-rich spot elements in both the coatings may be caused by Al2O3 polishing of coated samples during the sample preparation. The comparison of EDS maps of the as-sprayed (Figures 7 and 8) and exposed (Figures 16 and 17) specimens of both HA and HA-TiO2 coatings has not shown any significant differences. These observations endorse that both the coatings were successful to retain their adherence and identity even after the corrosion testing. Moreover, the coatings by and large were successful to avert depletion of various base alloy elements during corrosion testing. These observations show that these coatings can be useful to protect the base alloy.
4 Conclusions
HA-TiO2 composite coatings (50:50 wt pct) were developed by thermal-spray technique on Ti alloy substrate. The following conclusions have been drawn from the study:
-
In HA-TiO2 composite coatings, the coating structure is mainly composed of HA and TiO2 (rutile) phases. Besides the crystalline HA and TiO2 phases, as-sprayed coating consists of minor β-TCP phase, but no chemical compounds such as CaTiO3 were detected from the XRD scans. The addition of TiO2 effectively improved the crystallinity of HA in HA-TiO2 coating; otherwise, the HA coating was amorphous.
-
The SEM/EDS analysis confirmed that both powders HA and TiO2 melted enough to form a dense and homogeneously distributed HA-TiO2 composite coating without any crack formation.
-
The average value of the HA coating thickness is 120 μm, whereas in HA-TiO2 coatings, the observed coating thickness is 140 μm. The HA-TiO2 coatings exhibit a higher roughness (Ra = 8.04 μm) than HA-coated Ti-6-4 (Ra = 3.87 μm). The increase in roughness can be attributed to TiO2 in composite coating.
-
The flame-sprayed HA coating exhibited higher bond strength (67.8 MPa) compared with the investigated HA-TiO2 coating (37.6 MPa) and other thermal-spray HA-coating techniques reported in literature.
-
The electrochemical study showed an improvement in the corrosion resistance of the Ti-alloy after the deposition of flame-sprayed HA and HA-TiO2 coatings in simulated human body fluid conditions. After corrosion testing in Ringer’s solution, the intensity of XRD peaks increased and HA-TiO2 coating appeared more crystalline.
-
The cell culture studies of these coatings are in progress, which would provide useful information regarding their in vitro biological behavior and possible applications in the biomedical field.
References
S. Yilmaz, M. Ipek, G.F. Celebi, and C. Bindal: Vacuum, 2005, vol. 77, no. 3, pp. 315–21.
M. Niinomi: Sci. Techno. Adv. Mater., 2003, vol. 4, no. 5, pp. 445–54.
M. Songür, H. Celikkan, F. Gökmeşe, S.A. Şimşek, N.S. Altun, and M.L. Aksu: J. Appl. Electrochem., 2009, vol. 39, no. 3, pp. 1259–65.
M. Gaona, R.S. Lima, and B.R. Marple: Mater. Sci. Eng., 2007, vol. 458, nos. 1–2, pp. 141–49.
G. Manivasagam, D. Dhinasekaran, and A. Rajamanickam: Recent. Pat. Corr. Sci., 2010, vol. 2, pp. 40–54.
M.H. Fathi: J. Mater. Process. Technol., 2009, vol. 209, no. 3, pp. 1385–91.
X. Liu, P.K. Chu, and C. Ding: Mater. Sci. Eng., 2004, vol. 47, pp. 49–121.
U. Vijayalakshmi, A. Balamurugan, and S. Rajeshwari: Trends Biomater. Artif. Organs, 2005, vol. 18, no. 2, pp. 101–05.
L. Le Guehennec, A. Soueidan, P. Layrolle, and Y. Amouriq: Dent. Mater., 2007, vol. 23, no. 7, pp. 844–54.
J.L. Ong, M. Appleford, S. Oh, Y. Yang, W.H. Chen, J.D. Bumhardner, and W.O. Haggard: J. Miner., Met. Mater. Soc., 2006, vol. 58, no. 7, pp. 67–69.
R.R. Kumar and M. Wang: Mater. Lett., 2002, vol. 55, no. 3, pp. 133–37.
H. Li, K.A. Khor, and P. Cheang: Biomaterials, 2002, vol. 23, no. 1, pp. 85–91.
H. Li, K.A. Khor, and P. Cheang: Biomaterials, 2003, vol. 24, no. 6, pp. 949–57.
X. Nie, A. Leyland, and A. Matthews: Surf. Coat. Technol., 2000, vol. 125 (1–3), pp. 407–14.
F.X. Ye, A. Ohmori, T. Tsumura, K. Nakata, and C.J. Li: J. Therm. Spray Technol., 2007, vol. 16 (5–6), pp. 776–82.
J. Breme and V. Biehl: Handbook of Biomaterial Properties: Part II, J. Black and G. Hastings, eds., Chapman & Hall, London, U.K., 1998, pp. 135–213.
A. Ferrer, I. García, J. Fernández, and J.M. Guilemany: Mater. Sci. Forum, 2010, vols. 636–637, pp. 82–88.
P.A. Ramires, A. Romito, F. Cosentino, and E. Milella: Biomaterials, 2001, vol. 22, no. 12, pp. 1467–74.
E. Milella, F. Cosentino, A. Licciulli, and C. Massaro: Biomaterials, 2001, vol. 22, no. 11, pp. 1425–31.
K.A. Khor, H. Li, and P. Cheang: Biomaterials, 2003, vol. 24, no. 13, pp. 2233–43.
T.A. Vu and R.B. Heimann: J. Mater. Sci. Lett., 1997, vol. 16, no. 20, pp. 1680–82.
L.D. Piveteau, B. Gasser, and L. Schlapbach: Biomaterials, 2000, vol. 21, no. 21, pp. 2193–2201.
P. Li, K. Groot, and T. Kokubo: J. Sol-Gel Sci. Techno., 1996, vol. 7, pp. 27–34.
H. Li, K.A. Khor, and P. Cheang: Surf. Coat. Technol., 2002, vol. 155, pp. 21–32.
H.W. Kim, H.E. Kim, V. Salih, and J.C. Knowles: J. Biomed. Mater. Res. B, Appl. Biomater., 2005, vol. 72 (1), pp. 1–8.
F.J. Gil, A. Padros, J.M. Manero, C. Aparício, M. Nilsson, and J.A. Planell: Mater. Sci. Eng., 2002, vol. C22, pp. 53–60.
K.A. Gross, C.C. Berndt, D.D. Goldschlag, and V.J. Iacono: Int. J. Oral Maxillofac. Implants, 1997, vol. 12, no. 5, pp. 589–97.
J. Weng, X. Liu, X. Zhang, and X. Ji: J. Mater. Sci. Lett., 1994, vol. 13, no. 3, pp. 159–61.
M. Miyayama, K. Koumoto, and H. Yanagida: Engineered Materials Handbook, Ceramic and Glasses, vol. 4, S.J. Schneider, ed., ASM International, Materials Park, OH, 1991, pp. 748–57.
Z. Mohammadi, A.A. Ziaei-Moayyed, and A. Sheikh-Mehdi Mesgar: Mater. Proc. Technol., 2007, vol. 194, pp. 15–23.
M. Vilotijević, P. Marković, S. Zec, J.S. Marinkovic, and V. Jokanovic: Mater. Process. Technol., 2011, vol. 211, no. 6, pp. 996–1004.
Y.C. Yang and E. Chang: Biomaterials, 2001, vol. 22, no. 13, pp. 1827–36.
S.W.K. Kweh, K.A. Khor, and P. Cheang: Biomaterials, 2002, vol. 23, no. 3, pp. 775–85.
R.S. Lima, K.A. Khor, H. Li, P. Cheang, and B.R. Marple: Mater. Sci. Eng., 2005, vol. 396, nos. 1–2, pp. 181–87.
A.M. Al-Mayouf, A.A. Al-Swayih, N.A Al-Mobarak, and A.S. Al-Jabab: Mater. Chem. Phys., 2002, vol. 14, nos. 2–3, pp. 118–25.
S.K. Yen, S.H. Chiou, S.J. Wu, C.C. Chang, S.P. Lin, and C.M. Lin: Mater. Sci. Eng., 2006, vol. 26, no. 1, pp. 65–77.
S.C.P. Cachinho and R.N. Correia: J. Mater. Sci. Mater. Med., 2008, vol. 19, no. 1, pp. 451–57.
M.H. Fathi, M. Salehi, A. Saatchi, V. Mortazavi, and S.B. Moosavi: Dent. Mater., 2003, vol. 19, no. 3, pp. 188–98.
C.T. Kwok, P.K. Wong, F.T. Cheng, and H.C. Man: Appl. Surf. Sci., 2009, vol. 255, nos. 13–14, pp. 6736–44.
Z. Zhang, M.F. Dunn, T.D. Xiao, A.P. Tomsia, and E. Saiz: Nanotech. Biotech. Convergence, Stamford, CT, 2002, pp. 291–96.
Acknowledgments
The work presented in this article has been supported by ISIRD Research Grant from Indian Institute of Technology Ropar, Rupnagar, India. The authors express their gratitude to Metalizing Equipment Company Private Limited (MECPL), Jodhpur, India for providing a flame-spraying (CERAJET) facility to deposit the coatings.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 25, 2011.
Rights and permissions
About this article
Cite this article
Sarao, T.P.S., Sidhu, H.S. & Singh, H. Characterization and In Vitro Corrosion Investigations of Thermal Sprayed Hydroxyapatite and Hydroxyapatite-Titania Coatings on Ti Alloy. Metall Mater Trans A 43, 4365–4376 (2012). https://doi.org/10.1007/s11661-012-1175-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-012-1175-8