Abstract
In the present work, mechanisms are proposed for solidification crack initiation and growth in aluminum alloy 6060 arc welds. Calculations for an interdendritic liquid pressure drop, made using the Rappaz–Drezet–Gremaud (RDG) model, demonstrate that cavitation as a liquid fracture mechanism is not likely to occur except at elevated levels of hydrogen content. Instead, a porosity-based crack initiation model has been developed based upon pore stability criteria, assuming that gas pores expand from pre-existing nuclei. Crack initiation is taken to occur when stable pores form within the coherent dendrite region, depending upon hydrogen content. Following initiation, crack growth is modeled using a mass balance approach, controlled by local strain rate conditions. The critical grain boundary liquid deformation rate needed for solidification crack growth has been determined for a weld made with a 16 pct 4043 filler addition, based upon the local strain rate measurement and a simplified strain rate partitioning model. Combined models show that hydrogen and strain rate control crack initiation and growth, respectively. A hypothetical hydrogen strain rate map is presented, defining conceptually the combined conditions needed for cracking and porosity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although most aluminum alloys are considered to have good weldability, there are certain alloys that are particularly susceptible to solidification cracking. Significant effort has been devoted to characterizing the relative weldability of different alloys, using a variety of weldability tests.[1–3] However, the mechanisms involved in crack formation remain ill defined and are not well understood.
Solidification cracking is complex in nature and involves interplay between thermal, mechanical, and metallurgical components. Cracking is generally believed to result from the uniaxial tensile fracture of liquid films at grain boundaries within the two-phase mushy zone.[4] Stresses and strains at the trailing edge of the weld pool can be either compressive or tensile and arise from an interaction between the weld thermal experience (i.e., heating and cooling cycles), restraining forces, and solidification shrinkage.[5–7]
The great diversity of ways in which solidification cracking has been perceived is indicated in recent reviews on the subject.[8–11] The occurrence of solidification cracking in welds, for example, has been defined in terms of critical stresses, strains, and strain rates.[12] A majority of these models suffer from one common shortfall in that they fail to address how the liquid is fractured. One notable exception to this is the recent model of Rappaz, Drezet, and Gremaud (RDG),[13] in which liquid fracture is specifically related to an interdendritic pressure drop and cavitation.
In the present work, three models are proposed that, when combined, help to define the underlying mechanisms involved in solidification crack initiation and growth in aluminum weld metal. A crack initiation model considers a stable interdendritic micropore as a crack source, and a crack growth model is assumed to be controlled by mass balance requirements. Important to both models is the knowledge of the transverse strain rate at grain boundaries within the mushy zone. Accordingly, a strain rate partitioning model was developed to provide input to both initiation and growth models and thus to provide a link with experimental strain rate measurements.
To provide experimental input to these models, the Controlled Tensile Weldability (CTW) test was used to determine the critical strain rate for cracking in aluminum 6060 arc welds made with 4043 filler metal. For purposes of calculation in the models, a particular filler dilution of 16 pct 4043 was selected. The local strain rate was measured across the weld and then related to mushy-zone grain boundaries using the strain rate partitioning model.
2 Background
2.1 Crack Initiation Models
While some cracking models consider the maximum stress a liquid film can sustain,[14–16] most assume that fracture in the mushy zone is strain limited.[17–19] Arguing that a large solidification range permits a large buildup of strain and a greater likelihood to crack,[20,21] it has been demonstrated that cracking will occur if the accumulated strain exceeds a ductility limit represented by characteristic ductility curves established for specific alloys. Ductility-based models have recognized that strain rate is also an important factor, but only insofar as it serves to determine how much strain can be accumulated during the time of solidification.[18,19] However, recent developments suggest that the strain rate may actually play a more direct role in the liquid fracture mechanism.
The RDG model for solidification cracking[13] has put into mathematical form the shrinkage-feeding concepts of Feurer[22] and Campbell[4] while additionally accounting for transverse deformation within the mushy zone by incorporating a strain rate term. The RDG model estimates the interdendritic liquid pressure drop at the dendrite base due to insufficient liquid feeding, to compensate solidification shrinkage and thermal contraction:
where
in which P L and P 0 represent the liquid pressure at dendrite tip and root, respectively, μ is the liquid viscosity, β is the shrinkage factor, ν T is the isotherm velocity, G is the thermal gradient, T S and T L are the solidus and liquidus temperatures, respectively, K is the dendrite permeability, and f S (T) is the solid fraction at temperature T. The first term on the right side of Eq. [1] is the contribution of thermal strain to the liquid pressure drop (ΔP ε ), whereas the second term is the contribution from solidification shrinkage (ΔP sh ). Higher pressure drops are associated with higher solidification cracking susceptibility. The interdendritic liquid is assumed to fracture due to cavitation when the liquid pressure falls below some critical value.
Classical nucleation theory applied to vapor pore nucleation[23] defines cavitation as occurring when the liquid pressure becomes sufficiently negative (i.e., hydrostatic tension) to overcome the barriers to homogeneous nucleation, involving the creation of a vapor-liquid interface. Liquid metals are predicted to withstand negative pressures of considerable magnitude (≈104 atm), which may be reduced by an order of magnitude when nucleating on a nonwettable foreign substrate.[23] Even so, it has been questioned how such large pressure drops can be achieved under normal casting conditions.[4]
2.2 Crack Growth Models
Mechanisms for solidification crack growth have received only limited attention in the literature. Stress-based models have been used to characterize crack growth by applying solid-state fracture mechanisms to the liquid film rupture, taking into account surface energy effects[24] and a modified Griffith criterion.[25] In one pressure-based model, a gas pore has been assumed to grow as a crack if the sum of the pressures contributing to its growth (e.g., liquid pressure drop and dissolved gas pressure) exceeds the pressures contributing to its shrinkage (metallostatic pressure, capillary pressure, and atmospheric pressure).[26] Another approach considers growth in terms of a simplified mass balance,[27,28] in which the crack opening due to transverse deformation is compensated by advancement of the crack and feeding of the liquid. Of particular interest to welding is the boundary condition that crack growth is fixed at the welding speed, where the crack tip must remain in the mushy zone.[29]
3 Experimental Input To Model
Specifically, there were two experimental inputs utilized in the model development, as follows: (1) the critical local strain rate based upon extensometer measurement and (2) the weld metal solidification path based upon thermocouple measurement. Information associated with these experiments was likewise incorporated, including the weld metal grain size, weld travel speed, temperature gradient, etc. Details regarding experimental procedures are discussed below.
3.1 Critical Strain Rate Measurement
The weldability of aluminum 6060 extruded bars welded with 4043 filler metal was investigated using the CTW test.[30,31] The basic concept regarding the CTW test is reviewed here, with additional details provided in Reference 30. The CTW test consists of a horizontal tensile machine, with tensile strain applied transverse to the weld direction at a fixed crosshead speed during welding. An extensometer is attached underneath the weld coupon in the path of the weld at midlength (Figure 1), to measure the local transverse strain across the mushy zone as it passes directly over the extensometer.
By fixing the crosshead speed at a controlled value, one can influence the local strain rate across the mushy zone, with a high (outward) strain rate contributing to crack formation. Accordingly, by running multiple CTW tests at incremented crosshead speeds and observing crack/no-crack conditions, one can determine the critical local strain rate needed for crack formation.
Full penetration, bead-on-plate, gas-tungsten arc welds were made on 4-mm-thick 6060-T6 extrusions with variable 4043 filler dilution, using the weld parameters given in Table I. Compositions for both 6060 and 4043 alloys are given in Table II, showing that silicon is the primary alloying element affected by filler dilution. The results from the CTW testing are summarized in Figure 2, in which conditions of crack/no crack are plotted in terms of the local strain rate and 4043 dilution. The strain rate is, by convention, negative when the material is moving toward the weld centerline and positive when it is moving away.
Dilution strain rate map showing critical conditions needed for solidification cracking in 6060 weld metal with variable 4043 filler dilution (10.5-mm gage)[30]
Under conditions suitable to CTW crack formation, a single through-thickness centerline crack is formed at the instant the crosshead speed is applied and follows behind the weld pool until the crosshead speed is terminated. When close to critical conditions, however, intermittent cracking can be observed at the weld surface, as shown in Figure 3. These intermittent cracks are intergranular, forming near the weld centerline and growing toward the centerline. However, it is not clear whether they initiate above or below the surface, and there is no evidence as to the source of their origin.
A boundary can be established (dashed line in Figure 2) indicating critical cracking conditions where intermittent cracking was observed. It is noted that higher local strain rates require higher 4043 dilution to avoid cracking. The reason for this improvement in weldability with filler dilution is not completely understood, but it is likely a combination of grain refinement, bead shape, and the ability to feed shrinkage.[30] For purposes of the model calculations and development in this study, a particular alloy dilution was selected (i.e., 16 pct 4043) that had a critical local strain rate of +0.35 pct/s.
3.2 Solidification Path
The solidification path was characterized for 6060 + 16 pct 4043 by welding over 0.5-mm-outer-diameter type-K thermocouples pre-embedded in the path of the weld. Welding parameters were the same as those used in the CTW testing reported in Table II. Two different thermal analysis techniques were used, including the cooling rate method[32] and single-sensor differential thermal analysis.[33] Phase transformations associated with this solidification have been summarized elsewhere.[31,34,35] The temperature at dendrite coherency was 624 °C and the associated solidification start and stop temperatures were 660 °C and 491 °C, respectively.
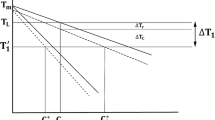
Important to the RDG pressure drop evaluation is the solid-fraction–temperature curve, determined from cooling curve data for a 6060 + 16 pct 4043 casting using a method outlined by Bäckerud.[32] In this method, the amount of latent heat evolved during solidification is partitioned according to the solid fraction, measured in terms of a difference between the observed cooling rate and a hypothetical baseline, representing the cooling rate for zero latent heat (approximated). Details regarding the application of this analysis to 6060/4043 castings are provided in Reference 34. The resulting curve for a 6060 + 16 pct 4043 casting, shown in Figure 4, is used as a basis for establishing the curve corresponding to a weld, corrected for differences in solidus T s and coherency T c temperatures, and assuming the same solid fraction at coherency.
Solid fraction-vs-temperature curves for 6060+16 pct 4043: (a) measured for casting[34] and (b) approximated for weld metal
4 Modeling of Cracking Mechanisms
4.1 Strain Partitioning in Mushy Zone
Similar to what is observed in practice, solidification cracking in CTW testing occurs along grain boundaries located near the weld centerline. Thus, defining how the local strain rate is partitioned between grain boundaries in the mushy zone is of particular importance to modeling. Because experimental input to the model consists of local displacement measurements made across the mushy zone with an extensometer, it is convenient to consider this measured local displacement rate (\( \dot{\delta } \)) as equally divided between the N adjacent grains of equal size separated by liquid films of equal thickness.[28] Likewise, if a distinction is made between the grain boundary liquid (δ L ) and grain (δ G ) displacement, the displacement rate per grain from Figure 5 can be given as
The number of grains N is found by dividing the weld pool width (8.0 mm) by the average grain size (51 μm) to get 157 grains. The local deformation rate (\( \dot{\delta } \)) can be found by multiplying the critical strain rate (0.35 pct/s) by the weld width (8 mm) to get +28.0 μm/s. The deformation rates \( \dot{\delta }_{L} \) and \( \dot{\delta }_{G} \) are values to be determined using the partition model.
Schematic showing deformation rate across mushy zone (\( \dot{\delta } \)), grain (\( \dot{\delta }_{G} \)), and grain boundary liquid (\( \dot{\delta }_{L} \)), as considered by strain partition model in Eq. [2]
The deformation rate of grains in the mushy zone (\( \dot{\delta }_{G} \)) can be further expanded as the sum of the solidification shrinkage (\( \dot{\delta }_{G/sh} \)) and deformation originating from the liquid pressure drop (\( \dot{\delta }_{G/\sigma } \)):
The grain solidification shrinkage (\( \dot{\delta }_{G/sh} \)) contributes to tension at grain boundaries, where a volume solidification shrinkage of 6 pct is assumed.[36] This gives a linear shrinkage of \( \root {3} \of {6} = 1.82\,{\text{pct}} \), applied between the coherency and solidus temperatures in proportion to the solid fraction.
A relationship for \( \dot{\delta }_{G/\sigma } \) follows from work of Braccini et al.,[28] in which the stress (σ) was related to the strain rate (\( \dot{\varepsilon }_{G/\sigma } \)) from the established rheological behavior of semisolids:
where Q is the activation energy, T is the absolute temperature, m is the strain rate sensitivity coefficient, α and σ 0 are material constants, and R is the gas constant. For purposes of calculation, values for an Al-Cu alloy were used from Reference 28: m = 0.26, Q = 160 kJ·mol−1, α = 10.2, and σ 0 = 4.5 Pa. The stress (σ) was assumed to be equal to the RDG pressure drop. Equal pressures were assumed within the grain and at grain boundaries. The grain deformation rate \( \dot{\delta }_{G/\sigma } \) in Eq. [3] can be calculated from \( \dot{\varepsilon }_{G/\sigma } \), multiplying by grain size.
The RDG liquid pressure drop analysis (Eq. [1]) was applied to 6060 + 16 pct 4043 welds using the physical parameters listed in Table III. The weld temperature gradient G was determined from the thermocouple data (25 °C/mm); the isotherm velocity \( \nu_{T} \) was taken to be the welding speed (4 mm/s); the primary dendrite arm spacing λ 1 was taken to be 10 μm; and the solid fraction temperature relationship for welds in Figure 4 was used. Pressure drop values were calculated up to a 0.98 solid fraction, at which strain resistance from interdendritic bridging is assumed sufficient to resist cracking.[13] Calculations show a pressure drop near the dendrite base (0.98 solid fraction) of 0.55 atm (0.055 MPa).
The strain rate partitioning model was implemented by requiring that the deformation rate values (\( \dot{\delta }_{L} \) and \( \dot{\delta }_{G} \)) comply with Eqs. [1] through [4] and by defining σ as the RDG pressure drop. A trial-and-error iterative process was applied to these four equations until the imposed boundary condition (i.e., measured local deformation rate) was satisfied. The results from this analysis, summarized in Table IV, demonstrate that the deformation rate is concentrated at grain boundaries. The grain deformation rate \( \dot{\delta }_{G/\sigma } \) can be neglected, which follows from assumptions implicit to the model. Dendrites within individual grains are assumed to be coherent (i.e., from semisolid rheology) and resist deformation, whereas grain boundaries are assumed void of coherency. In reality, there may be some coherency at grain boundaries, depending upon grain orientation and dendrite structure,[40] but this is expected to always be less than within the grains.
Accordingly, this concentration of the strain rate at grain boundaries instead of at grains helps to explain why solidification cracks are typically always associated with grain boundaries. This also helps to explain the beneficial role of grain refinement (i.e., large N) in reducing cracking susceptibility,[41–43] by lowering the strain rate experienced at any one grain boundary. The refined grain structure at high silicon contents, observed in both aluminum castings[44] and welds,[30] may contribute in large part to the weldability improvement at high 4043 filler dilutions.
4.2 Crack Initiation Mechanism
By applying the classical nucleation theory of Fisher,[23] Campbell[4] showed that a tensile hydrostatic fracture pressure (P f ) of 30,500 atm (3050 MPa) is needed for homogeneous vapor pore nucleation (i.e., cavitation) in pure molten aluminum at 660 °C. A lesser value of 1760 atm (176 MPa) is required for heterogeneous vapor pore nucleation on Al2O3 oxides poorly wet by liquid aluminum (wetting angle 160 deg). Still, these values far exceed the actual pressure drop associated with the conditions in aluminum welding (≈0.5 atm (Table IV)). From this, it appears that vapor pore nucleation is not possible under these assumed conditions.
Also of interest is the possible beneficial effect of dissolved gas contributing to the internal pressure needed for liquid fracture, with hydrogen serving as the sole source of gas porosity in aluminum.[4,39] Hydrogen is partitioned heavily to the liquid during solidification (k H = 0.05), with concentrations increasing in the interdendritic liquid. With limited data regarding hydrogen equilibrium in aluminum alloys, values for pure aluminum have been used for the partition coefficient, surface tension, and hydrogen solubility, as listed in Table III.
From Campbell,[45] the liquid fracture pressure (P f ) is taken to be the condition in which the hydrogen partial pressure (P H2) exceeds all external pressures (P e ), to allow pore nucleation:
The external pressure (P e ) is the combined sum of the hydrostatic pressure (P h ), atmospheric pressure (P a ), and pressure drop due to solidification shrinkage (ΔP sh ) and external strains (ΔP ε ). For welds, the P h is negligible, the P a equals 1 atm, and the sum ΔP sh + ΔP ε is approximately 0.5 atm (Table IV). Because the fracture pressure P f of liquid pure aluminum is at least 1760 atm,[4] the external pressure (P e ≈ 1 atm) can easily be neglected. According to Sievert’s law, P H2 (atm) is directly proportional to the square of the dissolved hydrogen content in the liquid [H] L (mL/100 g), expressed as
where K S is Sievert’s constant represented by the Van’t Hoff equation for pure aluminum:[39]
where T is the absolute temperature (K). In alloy 6060 welds, values for K S vary over a solidification temperature range of 170 °C, from 0.84 (dendrite tip) to 0.44 (dendrite root). Assuming equilibrium at the solid-liquid interface and complete mixing in both solid and liquid, the hydrogen content [H] L can be calculated using the equilibrium Lever law:
where f S is the solid fraction, k H is the hydrogen partition ratio, and [H]0 is the initial hydrogen content in the weld pool. Taking the estimated solid fraction temperature curve for a 6060 + 16 pct 4043 weld (Figure 4) and applying it to Eqs. [5] through [8] reveals that weld pool hydrogen contents of 1.25 and 5.20 mL/100 g are required to achieve gas pressures of 1760 and 30,500 atm, respectively, for heterogeneous and homogeneous gas pore nucleation at the dendrite base. The value of 1.25 mL/100 g is close to the reported threshold value of 1.00 mL/100 g needed to get macroporosity in 6061/4043 gas-metal arc welds (Table V), determined by adding controlled hydrogen contamination to the weld shielding gas.[46,47] Also from Table V, it is clear that the alloy content plays an important role in determining the threshold hydrogen values. In comparison, wrought aluminum alloys typically contain less than 1 mL/100 g hydrogen, although much higher values over 3 mL/100 g can sometimes be encountered.[48]
From this analysis, it appears that heterogeneous gas pore nucleation is possible, but only at abnormally high weld pool hydrogen contents, e.g., higher than 1.25 mL/100 g for the 6060/4043 system. Experience shows, however, that pores can also form at much lower hydrogen concentrations. For example, only 0.15 mL/100 g H2 is reported as necessary to get porosity in aluminum castings.[39,49] One possible explanation for this behavior is that metastable pore nuclei may already exist in the liquid. Liquid metals are believed to contain micron-sized pores that cannot escape during processing because of their limited buoyancy.[4] Synchrotron microtomography has revealed that as-cast aluminum alloys contain a high density of spherical pores (101/mm3 to 102/mm3) that are only a few microns in size.[50] It has been proposed that such micropores may originate from high-energy α-particle radiation, causing small atom clusters in the liquid to vaporize.[45] Metastable pore nuclei may also take the form of double-sided oxide films[51] or gas trapped at the apex of oxides.[52]
Double-sided oxides may originate as pores in cast ingots that are then collapsed and compressed by subsequent forming operations.[51] These oxides may then become entrained in the weld pool when fusing the base plate, and later become deposited at the solidification interface. Such metastable pore nuclei must survive for a long enough period of time until favorable conditions are met to activate and expand them into stable micropores. Their stability is not likely controlled simply by inertial and surface-tension forces or by the volume diffusion of gas, but also by the mechanical rigidity of the oxide skin.[51] Should these micropores form in the coherent region of the mushy zone (i.e., the region of tensile as opposed to hydrostatic stress), this could conceivably provide a source for crack initiation.
4.3 Activation of Pre-Existing Pore Nuclei
Conditions needed to expand a pre-existing pore nuclei are assumed as follows: (1) the hydrogen content in the liquid must exceed its solubility[4,39] and (2) the pores must satisfy a stability criterion. Grandfield et al.[26] combined the stability criterion of Campbell[4] with the RDG pressure drop calculation,[13] in which internal pressures that contribute to expansion (i.e., hydrogen partial pressure P H2, solidification shrinkage ΔP sh , and thermal contraction ΔP ε ) must exceed external pressures that act to collapse the pore (atmospheric pressure P a and surface tension pressure P γ ):
This pore stability criterion will be applied in Section D to establish the stable pore size for a given weld pool hydrogen content, using the physical property values listed in Table III. The P H2 was defined by Eqs. [5] through [8], the P a taken as 1 atm, and the sum (ΔP sh + ΔP ε ) assumed to be 0.5 atm. Pores were assumed to be spherical at the moment of their expansion, with the surface tension pressure P γ defined by
where r is the pore radius and γ the liquid-vapor surface tension.
4.4 Formation of Crack from Micropore
The concept for a porosity-based crack initiation model is illustrated in Figure 6. Three possibilities exist, assuming that the hydrogen concentration exceeds its solubility limit and that pre-existing nuclei are present. When formed ahead of the coherency region, a stable pore can either be expelled in front of the advancing dendrites as a macropore or become entrapped between the dendrites as a micropore. This distinction is determined by whether the stable pore diameter (2r) exceeds the interdendritic spacing at the point of formation:
where λ 1 is the primary dendrite arm spacing. However, if a stable micropore forms within the coherency region, it then becomes a potential crack initiation site. The surface tension pressure P γ (Eq. [10]) increases at higher solid fractions as the liquid film thickness decreases (i.e., smaller radius), reaching a value of 163 atm at a 0.98 solid fraction. Accordingly, this must be counterbalanced by high hydrogen content, if a stable micropore is to form at the dendrite base. In order to get macroporosity, a minimum of 0.10 mL/100 g H2 is required in the weld pool to exceed the hydrogen solubility (0.88 mL/100 g H2)[37] at the coherency point (f S = 0.94), at which the corresponding stable pore diameter (2r = 24 μm) exceeds the interdendritic space of 0.6 μm. This is close to the minimum value reportedly needed to generate porosity in aluminum castings (0.15 mL/100 g H2).[39]
Rearranging Eq. [9], a stable micropore can form when the term (P H2 − P γ ) exceeds the term (P a − ΔP sh − ΔP ε ), which equals approximately 1 atm. Plotting (P H2 − P γ ) as a function of the solid fraction for the initial hydrogen contents in the weld pool, between 0.1 and 0.8 mL/100 g in Figure 7, it is observed that the formation of a stable micropore beyond coherency (i.e., crack initiation) requires an initial weld pool hydrogen content of approximately 0.5 mL/100 g H2 (more precisely, 0.44 mL/100 g), in order for P H2 − P γ to exceed 1 atm. Because the term (P H2 − P γ ) can vary over 100 atmospheres, the term ΔP sh + ΔP ε (≈0.5 atm) can be neglected. This demonstrates that the initiation of pore-related solidification cracking in aluminum welds does not depend on local strain rate conditions but rather on the dissolved hydrogen content.
4.5 Model for Crack Growth
Both pressure balance- and mass balance-based models were initially considered here for crack growth. Because the pressure balance model considers crack growth defined by Eq. [9] through the term ΔP ε ,[26] this concept was deemed inapplicable to alloy 6060 welds, because the term ΔP ε is negligible compared to P H2 and P γ . In comparison, the mass balance crack growth model[28] is particularly well suited to the boundary conditions of welding, at which a crack must grow in a continuous manner (i.e., steady state) behind the weld pool.
Considering grains in the mushy zone separated by a liquid film of thickness h as depicted in Figure 8, the mass balance model relates the transverse deformation rate \( \dot{\delta }_{L} \) needed to grow a solidification crack at a rate \( \dot{x} \) equal to the weld travel speed, 4 mm/s. The (L − x) is taken as the length of liquid film exposed to transverse strain in the region of dendrite coherency. The transverse deformation rate \( \dot{\delta }_{L} \) is compensated by both the advancement of the crack and the liquid feeding (flow rate v L ) in the form of a mass balance:
where h 1 and h 2 are the liquid film thickness at the crack tip and coherency point positions. At a solid fraction f S , the liquid film thickness h equals (1 − f S )·λ 1, where λ 1 is the primary dendrite arm spacing.
Applying this model to 6060 + 16 pct 4043 welds, it was assumed that the crack tip is located at the 0.98 solid fraction (x = 0.98), at which the corresponding temperature is 507 °C. At higher solid fractions, the strain resistance from interdendritic bridging is believed sufficient to resist cracking.[13] From thermal analysis,[30,31] the coherency point is located at the 0.82 solid fraction and the liquid film distance (L − x) equals 4.77 mm. The liquid flow v L was calculated from Darcy’s law:
where f L is the liquid fraction at the point of initial coherency, with K and μ values listed in Table III. The pressure gradient was estimated to be 104 Pa/mm within the coherent zone, based upon RDG pressure drop calculations (Eq. [1]). Combining Eqs. [12] and [13], the crack growth model predicts a critical deformation rate \( \dot{\delta }_{L} \) = 0.33 μm/s, which is close to the value estimated for strain rate partitioning in the mushy zone (Table V). This demonstrates a crack growth dependence on the deformation rate that could explain the strain rate dependence observed in many weldability tests.[30,53–56]
For crack velocities slightly less than the welding speed (i.e., less than 4 mm/s), crack growth will terminate if the crack tip lags behind the solidus. However, if conditions for crack initiation are still satisfied, a new crack will again form, resulting in a succession of several small cracks instead of one long, continuous crack. For intermittent cracking, the crack growth model predicts a critical deformation rate \( \dot{\delta }_{L} \) = 0.11 μm/s. Experimentally, intermittent cracking was observed when close to the critical conditions required for weld solidification crack formation (Figure 3). At the other extreme, if the crack grows faster than the welding speed, it will advance toward the weld pool and encounter conditions for rapid liquid feeding, thus promoting slower crack growth.
From Eq. [12], it is found that by reducing the extent of the coherency region, i.e., lowering the distance (L − x) by either advancing the crack tip to lower solid fractions or reducing the coherency temperature, there is an increase in the critical deformation rate \( \dot{\delta }_{L} \) needed for crack growth. This follows from the ease of feeding liquid over shorter distances. This also agrees with welding practice, in which alloys with a small solidification range typically demonstrate lower cracking susceptibility.[1,57]
The strain rate partition model was used to convert the grain boundary liquid deformation rate into the local strain rate, comparable to what was measured experimentally using the weld width as a gage length. The resulting critical local strain rate needed to grow a crack is +0.41 pct/s. Compared with the experimental value (+0.35 pct/s), the predicted value was within 17 pct.
5 Discussion
The three models outlined earlier represent a comprehensive approach for relating observed behavior with mechanisms for crack initiation and growth in aluminum 6060/4043 weld metal. Combining the experiment with mechanistic models has allowed the quantification of the grain boundary conditions needed for cracking to occur. By their simplified nature, however, the assumptions made in these models also limit the accuracy of their predictions in addition to uncertainties in material constants.
Partitioning of the thermal strain in the mushy zone, for example, must involve a complex interaction between grain morphology, coherency between grains, and grain boundary orientation relative to strain. Grains located along the weld centerline, where cracking is most often observed, are typically the only grains oriented normal to transverse strain, and should experience a proportionately higher strain rate. The strain rate partitioning model as it now stands is too simple to effectively account for differences in grain structure involving curvature. For example, the model was unable to correctly predict weldability differences between 6060 and 6060 + 16 pct 4043 involving large differences in grain structure (Figure 9).[31] The large stray grains along the center section in the 6060 weld contribute significantly to its poor weldability but are not accounted for in the current model.
While the proposed strain rate partition model has its limitations, it has been useful in identifying and approximating the critical condition needed to get solidification cracking in 6060 + 16 pct 4043 weld metal (i.e., \( \dot{\delta }_{L} \approx 0.3\mu {\text{m/s}} \)). This suggests that crack growth, and not crack initiation, is the critical factor defining weldability. Crack initiation, shown to be more a function of hydrogen concentration than of strain rate, appears not to be a limiting factor, at least not at the hydrogen levels encountered in the 6060 material (≈0.6 mL/100 g diffusible H, reported in Reference 31). It is possible that cracks may initiate from pore formation in the coherent region between dendrites, either from heterogeneous pore nucleation (at a high hydrogen concentration) or from expansion of pre-existing pore nuclei (at a lower hydrogen concentration). While it is conceded that there may exist other crack initiation mechanisms, the pore mechanism has been shown to be feasible.
Assuming that hydrogen concentration determines crack initiation and that strain rate determines crack growth, it follows that both mechanisms (initiation and growth) must occur simultaneously in order to get cracking, as suggested in the hypothetical schematic of Figure 10. At very low levels of hydrogen (region I), it is not possible to get cracking because pores cannot form. At higher levels of hydrogen (region II), it is possible to form macropores but not cracks. At still higher levels of hydrogen (region III), micropores can form and cracking is possible, provided that the critical strain rate has been exceeded in tension. At a subcritical strain rate, intermittent cracking may occur. For sufficiently high hydrogen (region IV), it may be possible to initiate cracking by means of cavitation, eliminating the need for pre-existing pore nuclei.
That gas pores should be associated with weld solidification cracking is not a new concept[4,58] and is not surprising considering that both porosity and solidification cracking involve the formation of a liquid-vapor interface. Experience with cast aluminum alloys suggests that solidification cracking is not possible below 0.10 mL/100 g H2.[59] Weldability tests on aluminum 6060 have demonstrated that small hydrogen additions made to the welding gas can reduce weldability, but large additions can improve it.[31,53] The latter observation may be related to excessive porosity helping to feed shrinkage. While hydrogen is the only gas former in aluminum, other interstitial elements may be associated with pore formation in other metals (e.g., H2, N2, or CO in steel). Real-time radiographic observations have provided evidence that weld solidification cracking in steel may be connected to porosity.[60]
When evaluating crack growth, the mass balance approach was deemed particularly applicable to the steady-state conditions of continuous crack advancement behind the weld pool. However, it is appreciated that in the early transient stage of crack formation and growth, gas pressure may also serve to advance the pore in the direction of cracking and, hence, may be partially dependent upon hydrogen concentration. The exit of cracks to the surface at the weld crown or root, however, would clearly eliminate any pressure effect. Even so, at high enough levels of hydrogen (e.g., region IV), cavitation may support crack growth, as suggested by the dashed lines in Figure 10. Likewise, the presence of through-thickness cracks will also influence local straining conditions, with strain partitioned directly to the crack, leading to the lower strain rates needed to terminate as opposed to initiate crack growth.
From a contamination standpoint, the lowering of hydrogen in the plate, filler wire, and welding process (e.g., shielding gas and joint preparation) could conceivably provide an opportunity to improve the weldability of aluminum alloys. There is typically a large variation in hydrogen content from one heat of material to another[46] that may explain inconsistencies in weldability behavior. More efficient removal of oxide films with filters during ingot production could likewise improve weldability and consistency. This contamination issue could likewise apply to other ferrous or nonferrous alloys and involve other dissolved interstitial elements.
From a mechanical standpoint, local strain rates are difficult to measure and control, being related to specific thermal, material, and restraining conditions. The measurement of the strain rate, nevertheless, represents a unique means to monitor a material’s weldability and demands more attention in future weldability testing. The accurate prediction of the critical strain rate through modeling will permit the useful assessment of weldability, but this will require a more detailed understanding of grain boundary coherency and segregation and strain rate partitioning.
6 Conclusions
Three comprehensive models have been developed that characterize the underlying mechanisms involved in solidification cracking of aluminum weldments. A porosity-based crack initiation model has been proposed that links cracking to the formation of a micropore within the coherent interdendritic region. Pores are assumed to form from pre-existing pore nuclei, controlled primarily by hydrogen content and surface tension. Following crack initiation, a mass balance-controlled crack growth model has been proposed that gives the critical transverse strain rate needed to sustain growth at the welding speed. With the aid of a strain partition model, the critical local strain rate measured in the CTW test has been related to the critical grain boundary liquid deformation rate needed to support crack growth. Liquid fracture by cavitation may be possible, but only at elevated levels of hydrogen contamination.
References
J.C. Lippold: Hot Cracking Phenomena in Welds, Springer-Verlag, Berlin Heidelberg, Germany, 2005, pp. 271–90.
J.C.M. Farrar: Hot Cracking Phenomena in Welds, Springer, New York, NY, 2005, pp. 291–04.
H. Heuser: Hot Cracking Phenomena in Welds, Springer, New York, NY, 2005, pp. 305–27.
J. Campbell: Castings, Butterworth-Heinemann, Oxford, Great Britain, 1991.
R.A. Chihoski: Weld. J., 1972, vol. 51 (1), pp. 9s–18s.
T. Zacharia: Weld. J., 1994, vol. 73 (7), pp. 164s–172s.
Z. Feng: Weld. World, 1994, vol. 33, pp. 340–47.
D.G. Eskin, W.H. Suyitno, and L. Katgerman: Progr. Mater. Sci., 2004, vol. 49, pp. 629–711.
D.G. Eskin and L. Katgerman: Metall. Mater. Trans. A, 2007, vol. 38A, pp. 1511–19.
Suyitno, W.H. Kool, and L. Katgerman: Metall. Mater. Trans. A, 2005, vol. 36A, pp. 1537–46.
C.E Cross: Hot Cracking Phenomena in Welds, Springer, New York, NY, 2005, pp. 3–18.
C.E. Cross and N. Coniglio: Hot Cracking Phenomena in Welds II, Springer, New York, NY, 2008, pp. 39–58.
M. Rappaz, J.-M. Drezet, and M. Gremaud: Metall. Mater. Trans. A, 1999, vol. 30A, pp. 449–55.
V.N. Saveiko: Russ. Cast. Prod., 1961, (11), pp. 453–56.
C.H. Dickhaus, L. Ohm, and S. Engler: AFS Trans., 1994, vol. 101, pp. 677–84.
D.J. Lahaie and M. Bouchard: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 697–705.
W.S. Pellini: Foundry, 1952, vol. 80, pp. 125–99.
N.N. Prokhorov: Svar. Proiz., 1956, vol. 6, pp. 5–11.
T. Senda, F. Matsuda, G. Takano, K. Watanabe, T. Kobayashi, and T. Matsuzaka: Trans. JWS, 1971, vol. 2 (2), pp. 141–62.
W.I. Pumphrey and P.H. Jennings: J. Inst. Met., 1948, vol. 75, pp. 235–56.
J.C. Borland: Brit. Weld. J., 1961, vol. 8, pp. 526–40.
U. Feurer: Proc. Int. Symp. Eng. Alloys, Delft University of Technology, Delft, The Netherlands, 1977, pp. 131–45.
J.C. Fisher: J. Appl. Phys., 1948, vol. 19, pp. 1062–67.
H. Murakawa, H. Serizawa, and M. Shibahara: Mathematical Modelling of Weld Phenomena 7, TU Graz, Graz, Austria, 2005, pp. 539–54.
J.A. Williams and A.R.E. Singer: J. Inst. Met., 1968, vol. 96, pp. 5–12.
J.F. Grandfield, C.J. Davidson, and J.A. Taylor: Light Metals 2001, TMS, Warrendale, PA, 2001, pp. 895–901.
M. Braccini, C.L. Martin, M. Suéry, and Y. Bréchet: Modeling of Casting, Welding, and Advanced Solidification Processes IX, Shaker Verlag, Aachen, Germany, 2000, pp. 19–24.
M. Braccini, C.L. Martin, M. Suéry, and Y. Bréchet: Mater. Techniq., 2000, vols. 5–6, pp. 19–24.
C.V. Robino, M. Reece, G.A. Knorovsky, J.N. DuPont, and Z. Feng: Proc. 7th Int. Conf. Trends in Welding Research, ASM INTERNATIONAL, Materials Park, OH, 2005, pp. 313–18.
N. Coniglio, C.E. Cross, T. Michael, and M. Lammers: Weld. J., 2008, vol. 87 (8), pp. 237s–247s.
N. Coniglio: Doctoral Thesis, Otto-von-Guericke University, Magdeburg, Germany, BAM, Berlin, Germany, 2008.
L. Bäckerud, E. Krol, and J. Tamminen: Solidification Characteristics of Aluminum Alloys, Skanaluminum, Oslo, Norway, vol. 1, 1986, pp. 63–74.
B.T. Alexandrov and J.C. Lippold: IIW Doc. IX-2163-05, International Institute of Welding, Paris, France, 2005.
N. Coniglio and C.E. Cross: Weld. World, 2006, vol. 50 (11–12), Doc. IIW-1755-06, pp. 14–23.
N. Coniglio, C.E. Cross, I. Dörfel, and W. Österle: Mater. Sci. Eng., A, 2009, vol. 517, pp. 321–27.
L.F. Mondolfo: Aluminum Alloys—Structure & Properties, Butterworth and Co., London, 1976, p. 63.
Aluminum, Vol. 1: Properties, Physical Metallurgy, and Phase Diagrams, K.R. Van Horn, ed., ASM, Metals Park, OH, 1967.
S. Ganesan, C.L. Chan, and D.R. Poirier: Mater. Sci. Eng., A, 1992, vol. A151, pp. 97–105.
D.E.J. Talbot: The Effects of Hydrogen in Aluminum and Its Alloys, Maney Publishing, London, 2004.
M. Rappaz, A. Jacot, and W.J. Boettinger: Metall. Mater. Trans. A, 2003, vol. 34A, pp. 467–79.
D. Warrington and D.G. McCartney: Cast Met., 1989, vol. 2 (3), pp. 134–43.
M.G. Mousavi, C.E. Cross, and Ø. Grong: Sci. Technol. Weld. Joining, 1999, vol. 4 (6), pp. 381–88.
M.J. Dvornak, R.H. Frost, and D.L. Olson: Weld. J., 1989, vol. 68 (8), pp. 327s–337s.
L.A. Tarshis, J.L. Walker, and J.W. Rutter: Metall. Trans., 1971, vol. 2, pp. 2589–97.
J. Campbell: The Solidification of Metals, Iron and Steel Institute, London, 1968, pp. 18–26.
R.A. Woods: Weld. J., 1974, vol. 53 (3), pp. 97s–108s.
R.P. Martukanitz and P.R. Michnuk: Trends in Welding Research, ASM, Metals Park, OH, 1982, pp. 315–30.
G. Huismann, F. Wittemann, T. Behrendt, and H. Krüger: IIW Doc. Commission IX, International Institute of Welding, Paris, France, 2004.
D.E.J. Talbot and D.A. Granger: J. Inst. Met., 1963–1964, vol. 92, pp. 290–97.
H. Toda, T. Hidaka, K. Minami, M. Kobayashi, K. Uesugi, Y. Suzuki, and T. Kobayashi: Proc. 11th Int. Conf. on Aluminum Alloys, Wiley-VCH, Weinheim, Germany, 2008, pp. 575–581.
J. Campbell: Brit. J. Appl. Phys., 1968, vol. 1 (2), pp. 1085–88.
S.N. Tiwari and J. Beech: Met. Sci., 1978, pp. 356–62.
N. Coniglio and C.E. Cross: Hot Cracking Phenomena in Welds II, Springer, New York, NY, 2008, pp. 277–310.
H. Tamura, N. Kato, S. Ochiai, and Y. Katagiri: Trans. JWS, 1997, vol. 8 (2), pp. 16–22.
Y. Arata, F. Matsuda, K. Nakata, and K. Shinozaki: Trans. JWRI, 1977, vol. 6, pp. 91–104.
F. Matsuda, H. Nakagawa, K. Nakata, and H. Okada: Trans. JWRI, 1979, vol. 8, pp. 85–95.
C.E. Cross and D.L. Olson: Proc. Int. Conf. on Aluminum Alloys: Physical and Mechanical Properties, University of Virginia, Charlottesville, Virginia, 1986, vol. III, pp. 1869–75.
C.E. Cross, D.L. Olson, and G.R. Edwards: Int. Conf. Proc. on Modeling and Control of Joining Processes, American Welding Society, Miami, FL, 1993, pp. 549–57.
R. Otsuka: Handbook of Aluminum, Vol. 1: Physical Metallurgy and Processes, Marcel Dekker, New York, NY, 2003, p. 661.
B. Dixon: IIW Asian Pacific Regional Welding Congress, Hobart, TAS, Australia, 1988, pp. 731–51.
Acknowledgment
The authors are grateful to BAM for providing internal funding for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 14, 2008.
Rights and permissions
About this article
Cite this article
Coniglio, N., Cross, C. Mechanisms for Solidification Crack Initiation and Growth in Aluminum Welding. Metall Mater Trans A 40, 2718–2728 (2009). https://doi.org/10.1007/s11661-009-9964-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-009-9964-4