Abstract
The lap joint of a commercially pure titanium plate (CP Ti) to a low-carbon steel plate was produced with a vacuum-brazed furnace using a silver-based filler alloy at different temperatures and lap widths in order to investigate the effects of such brazing parameters on the joint strength and structure. It was found that the shear strength of brazed joints depends strongly on the lap width. Furthermore, we have discovered that the shear strength of the joints increases as the lap width decreases. The maximum shear strength of the joints, 54 MPa, was obtained when they were brazed at 850 °C for 15 minutes. Our observations indicate that intermetallic compound layers of CuTi and Cu2Ti were formed at the interface of the brazed area. An increase in the brazing temperature led to increased growth of these layers. Shear tests show that the fracture path occurred mainly at the CuTi layer, because this layer is hard and brittle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Steel is the most widely employed structural material in modern industry. However, in many applications, the characteristics of steel (i.e., corrosion resistance, high-temperature mechanical properties, and low density for power saving) do not satisfy the requirements for use in certain industries. In such cases, there was considerable interest in joining steel to high corrosion-resistance materials such as titanium alloys. The high strength to weight ratio and excellent corrosion resistance of titanium and titanium alloys[1–3] have led to considerable interest in joining titanium to steel, because there is a wide range of applications for this joint in the aerospace, petrochemical, power generation, and transportation industries.[4,5]
As a result of their poor miscibility, titanium and steel exhibit poor metallurgical compatibility, which promotes the formation of brittle intermetallic phases.[6] Such joints, if successfully made, will possess all the requirements for high-quality joints and also reduce the amount of materials needed, thus making them economically attractive and viable. There have been various reports that demonstrate that diffusion bonding has been used successfully in joining titanium to steel alloys. However, the great care required in the surface preparation stage and the impracticality of this method for mass production have limited the usage of this process.[7–11] Explosive welding has also been used to clad titanium to steel alloys in many applications.[12–15] Although brazing has the potential to join dissimilar metals, because it involves melting of the filler material only which eliminates the problems that occur when dissimilar metals are fused,[16,17] to the best of our knowledge, only data on microstructure investigations on brazing titanium to stainless steel have been reported.[18,19] Of the various brazing filler materials that could be employed, silver and its alloys have the potential to braze this joint, because they are widely used to braze most titanium alloys.[19–21] However, silver-based braze alloys with Zn or Cd additions cannot be applied in vacuum brazing due to the high vapor pressures of these elements. Near-eutectic silver-based alloy with a few percent of Ti (Ag-Cu45.5-Ti 3.5, at. pct) has been selected as the brazing alloy. Normally, a braze alloy with eutectic composition has excellent fluidity upon melting, and Ti has been selected to promote the wettability to parent metals by the formation of reaction products.
The present study employed a vacuum brazing process for joining a CP Ti plate to a low-carbon steel plate. The effect of the brazing temperatures on the joint microstructure and strength was investigated. In addition, the effect of the lap width on the joint strength was studied.
2 Experimental Procedures
The base metals used in this work are 2-mm-thick CP Ti (ASTM grade 2) strengthened by small additions of oxygen, nitrogen, carbon, hydrogen, and iron and 2-mm-thick low-carbon steel. The nominal chemical compositions of the base metals are given in Table I. The plates were cut into 125 × 28 × 2 mm chips for shear strength testing and 10 × 10 × 2 mm chips for microstructure analysis. These specimens were then prepared using several stages of grinding papers up to 1000 grit and were subsequently ultrasonically cleaned in acetone before brazing. The filler alloy was a 100-μm Ag-Cu45.5-Ti3.5 (at. pct) foil, which has a melting range of 780 °C to 850 °C. The brazing foil was cleaned in acetone before brazing and then sandwiched between the overlapped areas of base metal.
The overlap width was changed from three times (3t) the thickness of parent metal to 6t in order to investigate its effect on the joint strength. The joints were fixed with a stainless steel clamp and then carefully placed into a vacuum furnace (Schmetz GmbH, Menden, Germany). Brazing experiments were carried out between 850 °C to 930 °C for 15 minutes at a pressure of 2 × 10–5 Pa. The heating and cooling rate was adjusted at 15 °C/min.
The cross sections of the bonded titanium/steel joints were prepared for metallographic analysis by standard polishing techniques and subsequently etched with a solution comprised of 5 pct HF, 20 pct HNO3, and 75 pct glycerol for 60 seconds on the titanium side and a 3 pct Nital solution on the steel side. The microstructures were investigated using a Zeiss-Axiophot (Oberkochen, Germany), and the fracture surfaces of the brazed joints were examined using a JEOLFootnote 1 JXA-840 scanning electron microscope equipped with an energy dispersive spectrometer (EDS) and an electron probe X-ray microanalysis (EPMA). Moreover, X-ray diffraction (XRD) analyses were carried out using a Druker D8 Advance diffractometer (Stoe GmbH, Darmstadt, Germany) to identify the phases formed on joint fracture surfaces after performing the shear test. The Cu K α and Ni were chosen as the X-ray source and filter, respectively. The X-ray scan rate was set at 4 deg/min in the range of 20 and 150 deg.
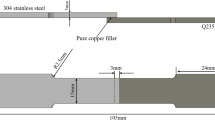
The hardness measurement was performed with a Vickers hardness testing machine (LECOFootnote 2 M-400) with different loads and a 25-second impressing time. Shear specimens were machined, as shown in Figure 1 in accordance to AWS C3.1-63 (Standard Test for Brazed Joints). The test was performed at room temperature and the displacement speed was 0.5 mm/s.
3 Results and Discussion
3.1 Microstructure of Joint
The characteristic microstructures of the base metals are shown in Figure 2. The microstructure of the steel base metal is a normal ferritic structure due to its low-carbon content, while the CP Ti possesses equiaxed α-phase grains.
Microstructure of the titanium-steel joint brazed at 850 °C for 15 minutes is shown in Figure 3. There are obvious reaction layers close to the titanium side in contrast to the steel side, which showed no reaction layer with the silver-based braze alloy, although a coarse grain structure at the steel boundary to the silver-brazed foil was formed. This coarse grain structure results from diffusion growth accompanied by recrystallization of the steel substrate at high temperature.
Observations with SEM revealed that different regions were formed in the brazed area with the various chemical analyses, as indicated in Figure 4. The titanium substrate showed no evidence of diffusion by elements of the braze alloy, because the analysis of region A, as shown in Table II, showed only Ti. A large amount of Ti was dissolved from the titanium side to the brazed area close to the titanium substrate, as shown in layers B and C (Table II). The ratios between Ti and Cu in layer B are close to the CuTi phase, which formed as a thin layer, as shown in Figure 4. In layer C, the ratio between Ti and Cu was closer to Cu2Ti phase. Region D represents the eutectic Ag-Cu phase, while the consumption of Cu in both the CuTi and Cu2Ti phases resulted in a silver-rich solid solution phase, as indicated in region E. These results are in agreement with those previously reported.[22,23]
The microstructure of the brazed joints changed significantly with increased brazing temperature. Figure 5 shows the joint brazed for 15 minutes at 880 °C and 930 °C. It is clear that the coarse grain structure, which formed at the steel interface at 850 °C, changed to a columnar structure when brazed at 930 °C, as shown in Figure 5(c). This is most likely attributed to the generation of a ferrite phase in the vicinity of the interface by the diffusion of the ferrite-stabilizing Ti element into the steel, which is located in the austenite state at the holding temperatures. In general, the formation of ferrite in an austenite matrix is important in the case of steels that exhibited columnar grains.[24] Therefore, the diffusion of Ti into the steel represents the main reason for the formation of the columnar ferrite structure in the brazed joint.
The titanium substrate did not show recrystallization and phase transformation at the brazing temperature of 880 °C, as shown in Figure 5(a). However, at 930 °C, the grains of titanium showed excessive grain growth after recrystallization, as indicated in Figure 5(c).
Based on EPMA for the brazed joint, CuTi and Cu2Ti, which formed at a brazing temperature of 850 °C, continued to spread in the brazing area upon increasing the temperature to 880 °C and reached the ultimate content at 930 °C. Layer A in Figure 5(d) represents a thick diffusion layer between the brazed foil and titanium substrate. This layer contained mainly Ti with a high percent of Cu and small amount of Ag, which corresponds to β-Ti. Layer E represents a thin layer of Ti2Cu because the percentage of Ti is almost twice that of Cu. Chemical analyses of all the regions are shown in Table III.
At a brazing temperature of 930 °C, most of the Cu in the eutectic Ag-Cu is consumed to form CuTi and Cu2Ti represented by regions B and C, respectively, and no eutectic structure remained at such high temperature. Some islands of silver-based solid solution are isolated in the brazed region, as represented by area D in Figure 5(d). Based on EPMA for layer F, it is expected to contain FeTi and Cu. Van Beek et al. reported that nearly 38 at. pct Cu could be dissolved in FeTi.[25]
Because all phases in the brazed joint consist primarily of Ti and Cu, a Cu-Ti binary alloy phase diagram (Figure 6) is cited here for convenience.[26] This system exhibits six intermetallic compounds, which are Ti2Cu, TiCu, Ti3Cu4, Ti2Cu3, TiCu2, and TiCu4 for which the structure and homogeneity ranges are fairly well established. Based on the Cu-Ti binary alloy phase diagram, there are no other phases besides Ti2Cu between Ti and TiCu. It is deduced that the Ti2Cu phase with the smooth interface is probably caused by solid-state interdiffusion between TiCu and the titanium substrate.
The Cu-Ti binary alloy phase diagram.[26]
Because the melting point of the brazed silver-based alloy is as low as 780 °C, it is obvious that the molten braze can dissolve more Ti from the titanium substrate at the brazing temperature of 850 °C. Figure 7 shows the liquidus projection of the Ag-Cu-Ti ternary alloy phase diagram.[27] According to Figure 7, the equilibrium solubility of Ti in a molten braze is enhanced by increasing the brazing temperature above 850 °C. Because Ti reacts with Cu in the molten braze alloy to form CuTi and Cu2Ti intermetallics, the Cu content in the molten braze is consumed and the islands of silver-based solid solution were formed at the brazing temperature of 930 °C. Owing to the high percentages of titanium dissolved in the molten braze, particularly at the high brazing temperature, titanium reacted with the steel substrate and formed a FeTi intermetallic phase, as represented by region G in Figure 5(d).
Liquids projection of the Ag-Cu-Ti ternary alloy phase diagram.[27]
3.2 Mechanical Properties of Joints
The hardness value of the primary eutectic structure measured in the joint brazed at 850 °C is 72 HV, as shown in Figure 8(a). The interaction layers at the titanium/silver-based brazing alloy presented high hardness values compared to silver solid solution and the eutectic structure owing to the formation of CuTi and Cu2Ti at the interface. Hardness values at the joint brazed at 930 °C are shown in Figure 8(b). All values were higher than the primary eutectic structure due to the presence of the different reaction layers, which contain intermetallic compounds. Cu2Ti, which formed at low brazing temperature and grew at high temperature, presented the highest hardness value in the joint, probably because of its high content of Fe, as presented in Table III.
The fracture shear strength was calculated as the failure load divided by the overlap area. The average shear strength of the joint showed a general tendency to decrease with increasing lap width, as clearly shown in Figure 9. This phenomenon has been discussed previously.[28,29] It has been shown that Von Mises stress was successfully used to demonstrate the decrease of the average shear stress in the lap joint with an increase of the overlap width.[30] As the overlap width of joint increases, the Von Mises stress distribution becomes increasingly less uniform. The middle portion of the overlap contributes less to the overall load-carrying capacity of the joint, whereas the ends of the joint become overloaded. The nonuniform stress distribution in the lap joint causes the decrease in the shear strength.
On the other hand, Figure 9 also indicates the effect of temperature on the average shear strength. Increasing the temperature also led to a decrease in the shear strength of the joints, because the microstructure contained thick, intermetallic compounds and the hardness was much higher in joints brazed 930 °C than the joints brazed at 850 °C. This caused an increase in the brittleness and weakened the joints brazed at high temperatures compared to the joints brazed at low temperatures. The maximum shear strength of the joints was 54 MPa for the specimen brazed at 850 °C for 15 minutes at 3t.
The fracture path after performing the shear test is shown in Figure 10. It is notable that, at all brazing temperatures, the fracture took place at the interfacial region between the titanium substrate and the brazed foil. This implies that the CuTi intermetallic compound is the weakest structure at the low brazing temperature, and Ti2Cu and CuTi are the weakest at high temperature. The brittleness of these intermetallics and the resulting harmful effect on the shear strength of the joints are clearly confirmed from the initiation of several cracks during the shear test, as shown in Figure 10.
Figure 11 shows microscope fractography of the middle areas in the steel side of the specimens brazed at temperatures of 850 °C and 930 °C. At 850 °C, the fracture pattern produced is a tearing topography surface (TTS), which is derived from a tearing process that operates on every small submicron scale.[31] The EPMA for the fracture area revealed nearly equal atomic percentages (48.45 pct Ti, 47.45 pct Cu), which support the notion that the fracture path follows the CuTi intermetallic layer formed at the interface. On the other hand, at a brazing temperature of 930 °C, the fracture pattern shows a notably flat area of a quasi-cleavage type with 49.10 pct Ti and 45.30 pct Cu. This also suggests that the fracture took place at the CuTi layer and, due to the very thin layer of Ti2Cu, does not reflect much Ti in the fracture surface.
In order to identify the intermetallic compounds formed at the fracture surfaces of the joints brazed at 850 °C and 930 °C, X-ray diffraction patterns from fractured surfaces of the steel and titanium sides were analyzed, as shown in Figure 12. It can be seen that Ag and Ti are widely observed in all specimens in contrast to Fe, which produces no peaks because the fracture path in all specimens was not near the steel side. The specimen brazed at 850 °C produced peaks for CuTi, Cu2Ti, and TiAg. On the other hand, the specimen brazed at 930 °C showed two more intermetallic compounds, FeTi and Ti2Cu.
X-ray diffraction patterns of fracture surfaces of (a) steel and (b) titanium for joint brazed at 850 °C for 15 min; (c) steel and (d) titanium for joint brazed at 930 °C for 15 min, where diffraction lines from Ag, Ti, CuTi, Cu2Ti, AgTi, Ti2Cu, and FeTi were indicated by ○, □, •, ■, ▴, ◣, and

The XRD analyses confirm the presence of intermetallic compounds in the brazed joint. Those phases are detrimental to the strength of the joint, particularly at high temperature, since thicker and variable types of intermetallics were formed.
4 Conclusions
-
1.
Interaction layers containing CuTi and Cu2Ti were formed at the titanium/silver-brazed alloy, while no interaction layers were formed at the steel/silver-brazed alloy interface in the case of the joint brazed at 850 °C with 15 minutes of soaking time.
-
2.
Increasing the brazing temperature led to an increase in thickness of both CuTi and Cu2Ti, which consumed much of the copper from the eutectic Ag-Cu brazed alloy. At a brazing temperature of 930 °C, there was no eutectic structure remaining in the brazed area and silver-based solid solution islands were formed. Moreover, a reaction layer consisting of FeTi is formed at the steel/silver-brazed alloy interface.
-
3.
Microhardness measurements revealed that Cu2Ti, which formed at low brazing temperature and increased at high temperature, presented the highest hardness value in all of the joints. Meanwhile, the silver-based solid solution produced the lowest hardness value.
-
4.
In the shear test, the average fracture load of the joint showed a general tendency to decrease with increasing lap width and temperature.
-
5.
At all brazing temperatures, the fracture took place at the interfacial region between the titanium substrate and the silver-brazed alloy as a result of the formation of intermetallic compounds of CuTi at low temperature and CuTi with Ti2Cu at high temperature.
-
6.
The XRD analyses confirmed the presence of intermetallic compounds of CuTi, Cu2Ti, and TiAg when brazed at 850 °C for 15 minutes. On the other hand, the specimen brazed at 930 °C produced two more intermetallic compounds, namely, FeTi and Ti2Cu.
Notes
JEOL is a trademark of Japan Electron Optics Ltd., Tokyo.
LECO is a trademark of LECO Corporation, St. Joseph, MI.
References
L.S. Smith, P. Threadgill, M. Gittos: Welding Titanium–A Designer and User Handbook, TWI, Abington, United Kingdom, 1999, pp. 1999–33.
R.R. Wang, G.E. Welsch: J. Prosthetic Dentistry, 1995, vol. 74, pp. 521–30.
F.J. Lancaster: Metallurgy of Welding, 6th ed., Abington Publishing, Cambridge, United Kingdom, 1999, pp. 25–50
J.R. Davis: Metals Handbook: Properties and Selection: Nonferrous Alloys and Special Purpose Materials, 10th ed., ASM International, Materials Park, OH, 1990, pp. 586–91
S. Kundu, M. Ghosh, A. Laik, K. Bhanumurthy, G.B. Kale, S. Chatterjee: Mater. Sci. Eng., 2006, vol. A428, pp. 18–23
K. Nishio, M. Kato, T. Yamaguchi, T. Tokunaga, A. Matsumoto: Welding Int., 2004, vol. 18, pp. 771–76
B. Kurt, N. Orhan, E. Evin, A. Calik: Mater. Lett., 2007, vol. 61, pp. 1747–50
V.A. Sidyakin, D.K. Pechenkin, V.M. Arbuzov, V.S. Khaustov: Welding Int., 2004, vol. 18, pp. 977–81
M. Ghosh, S. Chatterjee, B. Mishra: Mater. Sci. Eng., 2003, vol. A363, pp. 268–74
B. Qin, G.M. Sheng, J.W. Huang, B. Zhou, S.Y. Qiu, C. Li: Mater. Charact., 2006, vol. 56, pp. 32–38
N. Orhan, T.I. Khan, M. Eroglu: Scripta Mater., 2001, vol. 45, pp. 441–46.
N. Kahraman, B. Gülenç, F. Findik: J. Mater. Proc. Technol., 2005, vol. 169, pp. 127–33.
G. Arthur: Mater. Des., 1985, vol. 6, pp. 37–41
A. Nobili, T. Masri, and M.C. Lafont: Proc. Reactive Metals in Corrosive Applications Conf., Wah Chang Corporation, Albany, OR, Sept. 1999, Wah Chang, ed., pp. 89–98
H. Akbulut, O.T. Inal, C.A. Zimmerly: J. Mater. Sci., 1999, vol. 34, pp. 1641–52.
D.L. Olson, T.A. Siewert, S. Liu, G.R. Edwards: ASM Handbook: Welding, Brazing and Soldering, 10th ed., ASM International, Materials Park, 1993, p. 271
G. Humpston and D.M. Jacobson: Principles of Soldering and Brazing, ASM International, Materials Park, OH, 1993, pp. 19–28
Y. Miyazawa, C.S. Chang, H. Sato, J. Suda, T. Hiraoka, K. Kanda, T. Ariga: Mater. Sci. Forum, 2007, vols. 539–543, pp. 4031–35
H.Y. Chan, D.W. Liaw, R.K. Shiue: Int. J. Refractory Met. Hard Mater., 2004, vol. 22, pp. 27–33
R. Boyer, E.W. Collings, G. Welsch: Materials Properties Handbook: Titanium Alloys, ASM International, Materials Park, OH, 1994, pp. 1159–67
C.C. Liu, C.L. Ou, R.K. Shiue: J. Mater. Sci., 2002, vol. 37, pp. 2225–35
R.K. Shiue, S.K. Wu, S.Y. Chen: Acta Mater., 2003, vol. 51, pp. 1991–04
P. He, J.C. Feng, W. Xu: Mater. Sci. Eng., 2006, vol. A418, pp. 53–60
Y. Morizono1, M. Nishida, A. Chiba, T. Yamamuro: Mater. Sci. Forum, 2004, vols. 465–466, pp. 373–78
J.A. van Beek, A.A. Kodentsov, F.J.J. van Loo: J. Alloys Compd., 1995, vol. 217, pp. 97–03
H. Baker: ASM Handbook: Alloy Phase Diagrams, 10th ed., ASM International, Materials Park, OH, 1992, p. 2.181.
G. Petzow, G. Effenberg: Ternary Alloys, VCH Publishers, New York, NY, 1988, p. 57
I.T. Hong, C.H. Koo: Mater. Chem. Phys., 2005, vol. 94, pp. 131–40
Y. Flom, L. Wang: Welding J., 2004, vol. 7, pp. 32–38
E. Lugscheider, H. Reimann, O. Knotek: Welding J., 1977, vol. 6, pp. 189–92
K. Mills: ASM Handbook: Fractography, 9th ed., ASM International, Materials Park, OH, 1989, p. 21
Acknowledgment
The authors gratefully acknowledge the financial support from the Alexander von Humboldt Foundation (AvH), Federal Republic of Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted January 29, 2007.
Rights and permissions
About this article
Cite this article
Elrefaey, A., Tillmann, W. Interface Characteristics and Mechanical Properties of the Vacuum-Brazed Joint of Titanium-Steel Having a Silver-Based Brazing Alloy. Metall Mater Trans A 38, 2956–2962 (2007). https://doi.org/10.1007/s11661-007-9357-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-007-9357-5