Abstract
Summary
Trabecular bonescore (TBS) helps to predict fracture risk in older adults. In this registry-based cohort study of patients aged 40 years and older, reduction in bone mineral density (BMD) and TBS are complementary for fracture risk prediction enhancement with lower BMD imparting greater risk than reduction in TBS.
Purpose
Trabecular bone score (TBS) enhances fracture risk prediction independent of bone mineral density (BMD) in older adults. The purpose of this study was to further evaluate the gradient of fracture risk based on TBS tertile categories and WHO BMD categories, adjusted for other risk factors.
Methods
Using the Manitoba DXA registry, patients aged 40 years and older with spine/hip DXA and L1-L4 TBS were identified. Any incident fractures, major osteoporotic fractures (MOF), and hip fractures were identified. Cox regression models were used to estimate unadjusted and covariate-adjusted hazard ratios (HR, 95%CI) for incident fracture by BMD and TBS category and for each SD decrease in BMD and TBS.
Results
The study population included 73,108 individuals, 90% female with mean age 64 years. Mean (SD) minimum T-score was − 1.8 (1.1), and mean L1-L4 TBS was 1.257 (0.123). Lower BMD and TBS, both per SD, by WHO BMD category and by TBS tertile category, were significantly associated with MOF, hip, and any fracture (all HRs p < 0.001). However, the quantum of risk was consistently greater for BMD than TBS, with HRs showing non-overlapping CIs.
Conclusion
TBS is complementary to BMD in prediction of incident major, hip, and any osteoporosis-related fracture, but reductions in BMD impart greater risk than reductions in TBS on both continuous and categorical scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis has been defined as a systemic skeletal disease characterized by loss of bone mineral density (BMD) with microarchitectural deterioration. Operationally, this was subsequently defined as a BMD value of ≥ 2.5 SD below the young adult mean [1]. However, only a minority of “osteoporosis-related” fractures occur in those with a T-score below − 2.5 [2] which has led to approaches to enhance fracture risk prediction by inclusion of clinical fracture risk factors, most widely with the FRAX tool, and also by enhancements to skeletal status assessment.
One DXA-based approach to enhance skeletal assessment is trabecular bone score (TBS), a measure of bone texture derived from lumbar spine DXA images [3]. TBS has been extensively studied as a complementary approach to DXA BMD to improve fracture risk prediction. Indeed, TBS predicts fracture risk independent of FRAX clinical risk factors and femoral neck BMD [4,5,6]. As such, TBS has been integrated into the FRAX calculator and is increasingly utilized to facilitate patient management and recommendation of pharmacologic therapy [7,8,9].
Both the WHO classification of BMD (normal, osteopenia, and osteoporosis) and meta-analysis-based tertiles of TBS [10] (often reported as normal, partially degraded, and degraded bone microarchitecture) have been widely studied. Recently, a combination of WHO BMD category and TBS tertile has been integrated into DXA software output which is based upon complementary and additive importance of TBS and BMD categories. However, as TBS and BMD show low correlations [11, 12], it is not rare for these categorizations to be discordant. This raises clinical uncertainty as to which has the greater impact on fracture risk. As such, the purpose of this work is to evaluate the gradient of fracture risk based on these TBS and BMD categories, and for each SD decrease in TBS and BMD, adjusted for multiple clinical risk factors.
Methods
Study population
DXA is provided as an insured service in the Canadian province of Manitoba for all qualifying residents. Via this program, all DXA results are maintained in a database with completeness and accuracy exceeding 99% [13]. These data can be anonymously linked to other provincial health databases through an anonymous personal identifier. From this database, initial fan-beam DXA scans obtained in adults aged 40 years and up were identified from February 28, 1999, to March 29, 2018. Incident fractures were captured to March 31, 2018. This study was approved by the Research Ethics Board of the University of Manitoba and the Health Information Privacy Committee of Manitoba Health.
DXA performance
Lumbar spine and hip DXA scans were performed with a narrow fan-beam DXA configuration using GE Healthcare densitometers (Madison, WI, USA), Prodigy before November 2012, iDXA from November 2012 forward, and analyzed following manufacturer recommendations. T-scores were defined at the hip using NHANES III White female reference values; at the lumbar spine, these were derived using the manufacturer’s White female database. All densitometers used in this study were BMD, but not TBS, cross-calibrated.
TBS measurements at L1-L4 were obtained at the Bone Disease Unit of the University of Lausanne, Switzerland, using TBS iNsight Software, Version 3.x, (Medimaps group, Geneva, Switzerland). No TBS phantom was available for scanner cross-calibration given the retrospective study design; therefore, scanner type was included as a statistical adjustment.
All lumbar spine DXA files were anonymized for this analysis, thereby blinding these investigators to any clinical or fracture data. All TBS analyses included L1-L4; no vertebral body exclusions were performed. Lumbar BMD analyses included L1-L4 in 49,927 (68.3%), with one or more vertebral exclusions performed in the remainder. Eligible patients included those with DXA data who had a body mass index (BMI) between 15 and 37 kg/m2. BMI was determined based upon height and weight measured at the time of their DXA scan. Those outside the 15–37 BMI range were excluded as recommended by the TBS manufacturer. No other inclusion/exclusion criteria were applied.
Fracture identification
Manitoba health records from the index DXA scan date forward to March 31, 2018, were evaluated for fracture diagnosis codes, thus identifying incident fractures of the hip, vertebra (clinical), humerus, forearm, pelvis, and other sites (excluding those of the head, neck, ankle, hand, and foot) not associated with severe trauma through a combination of hospital discharge abstracts (diagnoses and procedures coded using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] prior to 2004 and International Classification of Diseases, Tenth Revision, Canadian Enhancements [ICD-10-CA] thereafter) and physician billing claims (coded using ICD-9-CM). For this analysis, it was required that hip and forearm fracture codes be associated with site-specific fracture reduction, fixation, or casting codes to augment specificity for an acute fracture event. To minimize potential misclassification with prior incident fractures, we conservatively required that there be no hospitalization or physician visit(s) with the same fracture type in the 6 months preceding an incident fracture diagnosis. Analysis considered any fractures as listed above and the subgroups of major osteoporotic fractures (MOF; hip, clinical vertebral, forearm, and humerus) and hip fracture separately.
Statistical analysis
Statistical analyses were performed with IBM SPSS for Windows (Version 27). Descriptive statistics for demographic characteristics are presented as mean ± SD for continuous variables or number (%) for categorical variables. Skeletal status was divided into 9 mutually exclusive categories based upon WHO BMD lowest T-score classification at the lumbar spine, femoral neck, or total hip (i.e., normal ≥ − 1.0, osteopenia − 1.1 to − 2.4, and osteoporosis ≤ − 2.5) and TBS tertile (lowest ≤ 1.230, middle 1.230–1.310, and highest ≥ 1.310). Crude fracture rates per 1000 person years were estimated by category. Unadjusted and covariate-adjusted Cox regression models were used to estimate hazard ratios (HR, 95% CI) for incident fracture by BMD-TBS category (9 levels; referent normal BMD, highest TBS tertile), by BMD category (3 levels; referent normal BMD), and TBS tertile (3 levels; referent highest TBS tertile) with assessment for linear trend. Unadjusted and covariate-adjusted Cox regression models were also used to estimate HRs for each SD decrease in BMD and SD decrease in TBS. Covariate adjustments considered technical and clinical factors that could impact TBS and fracture risk: type of scanner (Prodigy vs iDXA), age, sex, BMI, any previous fracture, parental hip fracture, glucocorticoid exposure (greater than 3-month use in the prior year), smoking status, high alcohol intake, rheumatoid arthritis, and other causes of secondary osteoporosis (aromatase inhibitor use, androgen deprivation therapy, hyperthyroidism, ankylosing spondylitis, celiac disease, chronic pancreatitis, chronic liver disease, inflammatory bowel disease, cerebrovascular disease, multiple sclerosis, muscular dystrophy, Parkinson disease, solid organ or bone marrow transplantation). Medication use was obtained from the provincial pharmacy system [14]. Other variables were from a combination of self-report, hospital discharge abstracts, and physician billing claims as previously described [15]. Preliminary analysis did not show significant interaction between TBS and sex; therefore, results are not sex stratified.
Results
Study population
The study cohort included 73,108 individuals (90% female) with mean (SD) age of 64 years (10.8) and BMI 26.4 (4.4) kg/m2. Table 1 summarizes the baseline characteristics of these subjects. Mean (SD) minimum T-score was − 1.8 (1.1), and mean L1-L4 TBS was 1.257 (0.123). Over a mean follow-up period of 8.7 (5.2) years, 7048 (9.6%) had an incident MOF, 2157 (3%) an incident hip fracture, and 9446 (12.9%) any incident fracture.
BMD/TBS category and fracture incidence
Based on WHO classification, normal BMD, osteopenia, and osteoporosis were present in 24.5%, 50.5%, and 25.1%, respectively (Table 1). Applying TBS tertile categories, 35.0%, 26.1%, and 38.9% were in the highest, middle, and lowest tertile. Unadjusted incident fracture rates for MOF, hip, and any fracture across all three BMD strata increased with lower TBS tertile (Table 2). The crude rates were used to estimate average increase in fracture risk for a single category worsening in BMD or TBS. The rate ratios for BMD are 1.85, 2.69, and 1.75 for MOF, hip fracture, and any fracture, respectively. Corresponding rate ratios for TBS are 1.34, 1.54, and 1.28, respectively.
BMD/TBS category and fracture hazard ratios
Based on WHO classification with normal BMD as the reference, unadjusted HRs (95% CI) in those with osteopenia and osteoporosis for MOF were 2.19 (2.02–2.38) and 4.11 (3.78–4.48), respectively, with a significant overall linear trend (p < 0.001) across BMD categories (Table 3). In the same model, there was also significant linear trend (p < 0.001) across TBS categories; compared with the highest tertile, HRs were 1.30 (1.21–1.40) for the middle and 1.80 (1.69–1.92) for the lowest TBS tertiles. For hip fracture, HRs for osteopenia and osteoporosis were 3.70 (3.02–4.53) and 9.08 (7.41–11.13), respectively, and for any incident fracture were 1.96 (1.83–2.09) and 3.57 (3.33–3.83), respectively. As noted for MOF, in the same models, linear trends (p < 0.001) were present across TBS categories with HRs of 1.35 (1.18–1.56) and 2.17 (1.92–2.45) for hip fracture and 1.26 (1.18–1.33) and 1.72 (1.63–1.81) for any fracture in the middle and lowest tertiles, respectively (Table 3). Corresponding HRs for BMD were greater than for TBS, with non-overlapping CIs.
After covariate adjustment (Table 3), the HRs were slightly attenuated, but the patterns were otherwise similar. For those with osteopenia and osteoporosis, MOF HRs were 1.82 (1.67–1.98) and 2.73 (2.49–2.99), for hip fracture were 2.42 (1.97–2.97) and 3.56 (2.88–4.41), and for any incident fracture were 1.65 (1.54–1.77) and 2.46 (2.28–2.65), respectively. In the same models, significant linear trends (p < 0.001) across TBS categories were observed. For MOF, compared with the highest tertile, HRs were 1.14 (1.06–1.22) for the middle and 1.38 (1.29–1.47) for the lowest TBS tertiles. Similar results were observed for hip fracture and any fracture (Table 3).
In both unadjusted and adjusted analyses, a significant gradient in fracture risk for MOF, hip fracture, and any fracture was seen for each SD decrease in BMD (Table 4). Following covariate adjustment, HRs (95% CI) for each SD decrease in minimum T-score were 1.38 (1.35–1.42), 1.35 (1.29–1.42), and 1.37 (1.34–1.40) for incident MOF, hip, or any fracture, respectively. HRs were slightly greater when femoral neck T-score was used rather than minimum T-score, especially for hip fracture (HR 1.86, 1.75–1.97) (Table 4). Lower TBS was also related to MOF, hip, and any fracture, with HRs (CI) per SD decrease of 1.14 (1.11–1.17), 1.16 (1.10–1.22), and 1.13 (1.10–1.16), respectively. Once again, corresponding HRs for BMD were greater than for TBS, with non-overlapping CIs. Similar patterns were seen when results were stratified as age < 65 years or > 65 years (Supplemental Table 1).
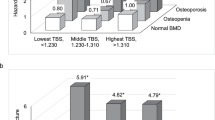
Finally, we estimated covariate-adjusted HRs for fracture in models that considered all 9 combinations BMD of TBS category (referent normal BMD, highest TBS tertile). This showed an increase in MOF, hip, and any fracture risk with each stepwise decrease in BMD from normal to osteoporosis and each stepwise decrease in TBS from highest to lowest tertile (Fig. 1a–c). Across each individual WHO BMD stratum and each individual TBS tertile stratum, there was a significant linear trend in the complementary parameter (p < 0.05).
a–c Adjusted hazard ratios for fracture by BMD and TBS category. Decreasing BMD (normal, osteopenia, osteoporosis) and TBS categories (lowest ≤ 1.230, middle 1.230–1.310, highest ≥ 1.310) were independently associated with stepwise increased risk for incident major osteoporotic fracture (a), hip fracture (b), and any fracture (c). Note: Numeric values with 95% CI and significance, age-stratified results, and sex-stratified results are presented in Supplemental Table 2. Adjusted for type of scanner, age, sex, BMI, previous fracture, parental hip fracture, glucocorticoid exposure in the prior year, smoking status, high alcohol intake, rheumatoid arthritis, and secondary osteoporosis
Discussion
In this registry-based cohort study of older adults, BMD and TBS were predictive of future major osteoporosis-related fracture (MOF), hip fracture, and any incident fracture. Specifically, lower BMD, whether categorized by WHO classification or per SD decline, and lower TBS, whether categorized by tertile or per SD decline, were significantly associated with incident fracture risk. These relationships were attenuated but still significant when adjusted for multiple covariates including FRAX risk factors. This confirms previous analyses showing independent information from TBS and BMD, justifying their use in combination with clinical risk factors to improve fracture risk prediction [4, 10]. Notably, this study demonstrated that the gradient of risk for MOF, hip, and any fracture was greater for a decrease in WHO BMD category compared to a decrease in TBS tertile and also for per SD decrease in BMD versus TBS. Thus, TBS and BMD are complementary but not of equal weight in fracture risk evaluation.
The role of TBS in facilitating fracture risk assessment in both women and men is widely recognized. As examples, Martineau et al. reported that incorporating TBS into FRAX calculations led to a small but significant improvement in hip and MOF risk assessment. Furthermore, they found that risk reclassification improvement was greatest in women who were close to an intervention threshold and women younger than age 65 years [16]. Another study using the Manitoba DXA database evaluated 33,352 women, mean age 63 years, and validated that TBS is able to predict incident MOF independent of FRAX clinical risk factors and femoral neck BMD (5). Schousboe et. al. reported similar results in older men where they found that TBS was associated with incident MOF after adjustment for FRAX 10-year risk with BMD [17]. Su et al. studied the association of TBS with fracture risk in 1923 men and 1950 women from Mr. OS and Ms. OS Hong Kong studies and reported lower TBS predicted MOF risk independent of FRAX score [18]. A meta-analysis of 17,809 men and women in 14 prospective cohorts found the MOF hazard ratio per SD change in TBS to be similar in women and men [10].
Iki et al. evaluated the use of TBS in Japanese men age ≥ 65 years from the FORMEN study cohort and determined that the combination of TBS and FRAX may improve MOF risk prediction [19]. Other studies have shown the association of TBS with fracture risk prediction even in patients receiving osteoporosis medications. As an example, a recent study by Leslie et al. evaluated the effect of fracture prediction from TBS in patients who are on anti-resorptive osteoporosis treatment; it was reported that there was no association and TBS can be used to assess fracture risk in patients receiving anti-resorptive therapy [20].
The utility of TBS in patients with normal BMD T-score and incident fragility fracture has been assessed by a few studies including Binkley et al., where they evaluated postmenopausal women (n = 4649) with any osteoporotic fracture 5 years prior and following a baseline DXA. They reported that addition of TBS to BMD significantly reduced the percentage of women with any prior fracture who were previously identified as having normal bone from 11 to 6% (p < 0.001) [21].
The TBS software algorithm has and continues to evolve over time. Schacter et al. [22] compared the original (v1) which had been optimized for women and a newer (v2) TBS algorithm. In 47,736 women and 4,348 men aged > 40 years with v1 was greater in women than men who have greater abdominal soft tissue thickness which reduces the TBS value. Software version v2 was modified for use in men and resulted in mean TBS being higher in men than in women. To further address the effect of soft tissue, a forthcoming TBS software (v4, utilized thus far only in research studies) [23] will utilize soft-tissue thickness measured by the densitometer.
This study has strengths and limitations. A major strength is study size; this is the single largest cohort to evaluate the relationship of TBS in fracture risk prediction and is ~ fourfold larger than the McCloskey meta-analysis [10]. Moreover, this is a clinical population cohort, and thus these results are likely broadly applicable to clinical care. However, study limitations exist including use of densitometers from a single manufacturer and study of a primarily White female cohort. Moreover, TBS categorization was based upon simple tertiles and has not been optimized for lifetime fracture prediction as were BMD T-scores. An additional important limitation is that these data were generated using currently available TBS software that adjusts for BMI. Similar analyses are warranted when future iterations of TBS software adjusted for tissue thickness become commercially available.
In conclusion, BMD and TBS are predictive of incident MOF, hip, and any fracture and in combination enhance fracture risk prediction independent of other clinical risk factors. In this regard, TBS is complementary to BMD, but does not have equal weight as reductions in BMD impart greater risk than reductions in TBS on both continuous and categorical scales.
References
Kanis JA et al (1994) Perspective. The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Schuit SCE et al (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone 34:195–202
Harvey NC et al (2015) Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78:216–224
McCloskey EV et al (2015) Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96(6):500–509
Leslie WD et al (2014) Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25(9):2271–2277
Shevroja E et al (2017) Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J Clin Densitom 20(3):334–345
LeBoff MS et al (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 33(10):2049–2102
Shepherd JA et al (2015) Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom 18(3):274–286
Compston J et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12(1):43
McCloskey EV et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31(5):940–948
Hans D et al (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14(3):302–312
Padlina I et al (2017) The lumbar spine age-related degenerative disease influences the BMD not the TBS: the Osteolaus cohort. Osteoporos Int 28(3):909–915
Leslie WD et al (2005) Construction and validation of a population-based bone densitometry database. J Clin Densitom 8:25–30
Kozyrskyj AL, Mustard CA (1998) Validation of an electronic, population-based prescription database. Ann Pharmacother 32(11):1152–1157
Leslie WD et al (2019) Performance of FRAX in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. J Bone Miner Res 34(8):1428–1435
Martineau P et al (2017) Clinical utility of using lumbar spine trabecular bone score to adjust fracture probability: the Manitoba BMD Cohort. J Bone Miner Res 32(7):1568–1574
Schousboe JT, et al. Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. LID - J Bone Miner Res. 2015;(1523–4681 (Electronic))https://doi.org/10.1002/jbmr.2713
Su Y et al (2017) Added clinical use of trabecular bone score to BMD for major osteoporotic fracture prediction in older Chinese people: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int 28(1):151–160
Iki M et al (2015) Trabecular bone score may improve FRAX(R) prediction accuracy for major osteoporotic fractures in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Osteoporos Int 26(1433–2965 (Electronic)):1841–8
Leslie WD, Hans D, Silva BC (2023) Fracture prediction from trabecular bone score is unaffected by anti-resorptive treatment: a registry-based cohort study. J Clin Densitom 26(1):10–15
Binkley N et al (2020) Frequency of normal bone measurement in postmenopausal women with fracture: a registry-based cohort study. Osteoporos Int 31(12):2337–2344
Schacter GI et al (2017) Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int 28(11):3199–3203
Cosman F et al (2023) Effect of abaloparatide on bone microarchitecture assessed by trabecular bone score in women with osteoporosis: post hoc analysis of ACTIVE and ACTIVExtend. J Bone Miner Res
Acknowledgements
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017- 29). The results and conclusions are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Healthy Living, and Seniors or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Heenam Goel and William Leslie declare no conflict of interest. Neil Binkley declares no conflict of interest for the content of this paper; research funding from Radius; consultant Amgen. Didier Hans: Co-ownership in the TBS patent. Stock options or royalties: Medimaps. Research grants: Amgen, Agnovos.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goel, H., Binkley, N., Hans, D. et al. Fracture risk gradient assessed by categories of bone mineral density and trabecular bone score: the Manitoba BMD Registry. Arch Osteoporos 18, 73 (2023). https://doi.org/10.1007/s11657-023-01284-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-023-01284-0