Abstract
Summary
A meta-analysis to investigate the difference in fracture risk between individuals with and without HIV infection was performed. People living with HIV had lower bone mineral density (BMD) and greater risks of overall fractures and fragility fractures. Reducing fragility and maintaining skeletal strength for PLWH are urgently needed for this population.
Purpose
The introduction of effective antiretroviral therapy increased the life expectancy of people living with HIV (PLWH). This population now faces problems related to aging such as decreased bone mineral density (BMD) and increased fracture risk. Some antiretroviral therapies may also negatively impact bone health. We performed a meta-analysis to investigate the difference in the fracture risk between individuals with and without HIV infection.
Methods
We compared BMD, risk of fragility fracture, and risk of all fracture between the two groups. This study included 35 articles with 106,994 PLWH and 228,794,335 controls.
Results
PLWH had lower lumbar spine and hip BMD than controls. PLWH had a higher prevalence of all fracture events (4.08% versus 0.44%) and fragility fractures (2.66% versus 2.19%). The relative risks of all and fragility fractures of PLWH were 1.91 (95% confidence interval (CI), 1.46–2.49; p < 0.001) and 1.68 (95% CI: 1.40–2.01; p < 0.001). PLWH also had more vertebral fractures (1.26% versus 0.37%; RR, 1.97; 95% CI: 1.22–3.2; p < 0.05), hip fractures (1.38% versus 0.81%; RR, 1.88; 95% CI: 0.99–3.57; p = 0.05), and wrist fractures (1.38% versus 1.29%; RR, 1.67; 95% CI: 1.13–2.45; p < 0.05) than healthy controls. The pooled incidence of fractures was 1.72 per 100 person-years in PLWH and 1.29 in healthy controls.
Conclusion
PLWH had lower BMD and greater risks of all fractures and fragility fractures. Reducing fragility and maintaining skeletal strength for PLWH are urgently needed for this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of effective antiretroviral therapy (ART) has much improved the outcomes of human immunodeficiency virus (HIV) infection. HIV-infected individuals treated with ART can achieve a near-normal life expectancy and experience improved quality of life because of immune system restoration and viral suppression, which in turn reduces mortality and comorbidity [1, 2]. Therefore, potential related complications arising from long-term treatment have been a topic of interest.
Regarding bone health, osteoporosis is thought of as a silent disease, since bone loss usually arises without any symptoms or signs. It is also characterized by deterioration of bone microarchitecture, increased bone turnover, and low bone mineral density (BMD) [3]. Some studies found that HIV-infected patients had a decreased BMD [4,5,6].
A meta-analysis including 12 cross-sectional studies showed that the prevalence of osteoporosis in HIV-infected patients is 3.7 times higher than that in HIV-uninfected controls [7].
Although multiple analyses have demonstrated decreased BMD in HIV-infected persons and raised concerns for increased fracture risk, the effect of low BMD on fracture risk among patients diagnosed with HIV infection is not well-recognized because of the limited data.
We performed a systematic review and meta-analysis to investigate whether the risk of fracture increased in individuals with HIV infection. The primary goal of the study was to compare the risks of all fractures and fragility fractures between HIV-infected patients and controls. We also estimated the prevalence of fractures in the HIV population.
Materials and methods
We conducted this meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8].
Search method
We looked up PubMed, Medline, Embase, and the Cochrane Library using the keywords “human immunodeficiency virus,” “bone mineral density,” and “fracture” for the period 2000 to 2018.
Google search engine was also used with the following terms to search for studies: “human immunodeficiency virus,” “acquired immunodeficiency syndrome,” “fracture,” “fragility fracture,” “vertebral fracture,” “wrist fracture,” “hip fracture,” “bone mineral density,” “osteopenia,” “osteoporosis,” “prevalence,” and “epidemiology”.
We also reviewed the reference lists of relevant articles for any further relevant studies.
Study selection
The search for relevant studies and exclusion of papers was executed by two authors (CJ Chang, TW Tai) independently. Three authors (CJ Chang, YL Chan, TW Tai) performed data extraction and article appraisal. When discrepancies occurred, we reached consensus through discussion.
Articles designed without a control group, with unclear patient characteristics, or insufficient available data were excluded. We then appraised the studies and identified those eligible for inclusion in our analysis. The authors assessed the quality of each article by using the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement [9].
Data extraction
Two authors (CJ Chang, YL Chan) independently extracted the data from the articles.
We contacted the authors of the included articles for missing data. The following data were extracted from the included articles: (1) characteristics of patients, (2) body mass index, (3) fracture type, (4) fracture incidence and prevalence, (5) duration of follow-up, and (6) lumbar spine and hip bone mineral density.
Statistical analysis
We used the Mantel–Haenszel method and variance-weighted means to analyze the outcomes. The authors used I2 to figure out and quantify the effect of heterogeneity [10]. The range of I2 was from 0 to 100%. An I2 value > 50% was considered as obvious heterogeneity [11]. A random-effects analysis [12] was used to compare studies showing heterogeneity, and a fixed-effects analysis [13] was used to compare studies without obvious heterogeneity. We calculated the heterogeneity, mean difference, and relative risk (RR) for all outcomes in this meta-analysis. By using Egger funnel plots, we also assessed the possibility of publication bias [14]. We conducted this meta-analysis with the Review Manager version 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The pooled data on fracture incidence were analyzed using the MedCalc Statistical Software version 19.0.5 (MedCalc Software bvba, Ostend, Belgium).
Results
Identified trials
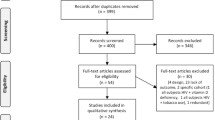
We found 1013 potentially relevant articles by using our search strategy initially. The full texts of 75 articles were reviewed. After applying the inclusion and exclusion criteria, 35 articles were identified and included in our meta-analysis (including 228,901,329 people; 106,994 people living with HIV and 228,794,335 non-infected controls) (Fig. 1). Of these included articles, 18 studies focused on the influence of HIV infection on BMD in HIV patients compared with that in healthy controls (Table 1), and 11 studies discussed differences in the fracture prevalence between these two groups (Table 2). Six articles mentioned both issues [5, 31, 39, 41, 45, 46].
Bone mineral density analysis
Twenty-two articles provided lumbar spine BMD data, and 19 articles provided hip BMD data for both HIV-infected patients and non-HIV-infected controls.
The means of lumbar spine BMD ranged from 0.85 to 1.25 g/cm2 in the HIV-infected group and 0.92 to 1.31 g/cm2 in the non-HIV-infected group. The pooled data showed that lumbar spine BMD was significantly lower in HIV-infected patients than that in non-HIV-infected controls (−0.04, 95% confidence interval (CI): −0.05~−0.03, p < 0.00001, random effect model) (Fig. 2a).
The means of hip BMD ranged from 0.8 to 1.05 g/cm2 in HIV-infected patients and 0.81 to 1.12 g/cm2 in non-HIV-infected controls. The pooled result revealed that HIV-infected patients also had significantly lower hip BMD compared with controls (−0.04, 95% CI: −0.05~−0.04, p < 0.00001, random effect model) (Fig. 2b).
Among the 22 articles that provided lumbar spine BMD data, there were 9 studies in which age and body mass index (BMI) were well-matched between the HIV-infected and HIV-uninfected subjects. The pooled data from these 9 studies showed that lumbar spine BMD was significantly lower in HIV-infected patients than in controls (p < 0.05) (Fig. 3a). In addition, seven age- and BMI-matched studies reported that hip BMD was also significantly lower in HIV-infected patients than that in controls (p < 0.05) (Fig. 3b).
Fracture risk analysis
The all fracture prevalence in HIV-infected people and controls was reported in 17 studies (Table 3). Among HIV-infected people, the prevalence was 4.08% for all fractures, 2.66% for fragility fractures, 1.26% for vertebral fractures, 1.38% for hip fracture, and 1.38% for wrist fractures.
The pooled data revealed that HIV-infected patients had a higher prevalence of all fractures than controls (4.08% versus 0.44%; relative risk (RR): 1.91, 95% CI: 1.46–2.49, p < 0.00001) (Fig. 4a). The prevalence of fragility fractures (including vertebral, hip, and wrist fractures) was provided in 13 studies. The pooled results found a significantly higher prevalence of fragility fractures in the HIV-infected group than in controls (2.66% versus 2.19%, RR: 1.68, 95% CI: 1.40–2.01, p < 0.00001) (Fig. 4b).
In addition to the analysis of fragility fractures, we also performed subgroup analyses of vertebral, hip, and wrist fractures. Six studies mentioned vertebral fracture rates, and the pooled results showed that the prevalence of vertebral fractures was significantly higher in HIV-infected patients than in controls (1.26% versus 0.37%; RR: 1.97, 95% CI: 1.22–3.2, p = 0.006) (Fig. 5a). Six articles provided data on the prevalence of hip fractures. The pooled data indicated a trend in that HIV-infected people were more likely to have hip fractures than healthy controls (1.38% versus 0.81%, RR: 1.88, 95% CI: 0.99–3.57, p = 0.05) (Fig. 5b). The pooled results for wrist fractures from four studies found that HIV-infected people were more likely to have wrist fractures than controls (1.38% versus 1.29%, RR: 1.67; 95% CI: 1.13–2.45; p = 0.009) (Fig. 5c).
Six articles provided information about fracture incidence. The pooled data showed that the incident fracture rate per 100 person-years was higher in HIV-infected patients than that in healthy controls (1.72 versus 1.29 per 100 person-year) (Fig. 6).
Discussion
The most important findings of this meta-analysis were that people living with HIV had lower lumbar spine and hip BMD and a higher prevalence of all fractures as well as fragility fractures, vertebral fractures, and wrist fractures than controls. People with HIV also had a higher incidence of fractures than controls.
Because of the increased life expectancy of people living with HIV, osteoporosis and fragility fractures may become emerging causes of morbidity. Low BMD and increased fracture risk in HIV-infected patients have been reported in a number of studies (studies included in Tables 1 and 2) [4,5,6, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Previous meta-analysis conducted by Schiau et al. revealed that people with HIV infection have increased risk for all fractures and fragility fractures compared with non-HIV-infected population [47]. However, the relationship between low BMD and increased fracture risks in patients with HIV is not well-established. Whether low bone density leads to more fractures in people living with HIV is inconclusive [5, 48].
HIV-infected people have three-fold increase in osteoporosis with abnormal cortical and trabecular microarchitecture, which diminishes the bone stiffness. The risk of fracture, especially vertebral fracture, is higher in HIV-infected person, which is consistent with our results [7, 41, 49]. A recent systematic review performed by Ilha et al. showed that the risk of vertebral fracture in HIV-infected individuals is approximately double when compared with HIV-uninfected patients [50], which resembled the finding in our study (RR, 1.97). The proposed mechanisms include immune system reaction, vitamin D metabolism, insulin resistance, and lipodystrophy. However, a complex of involved mechanisms transforms the mechanical properties and affects bone quality and turnover. [51,52,53]. The complex regulation involved may transform bone mechanical properties and thus affect quality and turnover rate. HIV was found to lower BMD and cause bone fragility [54]. Several studies have assessed serum markers of bone formation and resorption in patients with HIV infection [55,56,57]. The serum bone formation marker, osteocalcin, was decreased in advanced stages of the disease and was correlated with CD4 cell counts [55, 58]. Increased IL-6 plasma levels were associated with greater BMD loss among HIV-infected individuals but not uninfected controls [59]. Increased levels of soluble CD14, which is responsible for macrophage activation, was inversely correlated with bone mineral density and content in HIV-infected males [60].
Highly active antiretroviral therapy has been proved to be related to reduced BMD in most studies. Most antiretroviral drugs produce complications that interfere with bone metabolism [61]. Brown TT et al. reported a 1.6 times greater loss in BMD among those on a protease inhibitor (PI) regimen compared to those on highly active antiretroviral therapy without a protease inhibitor [7].
Nucleoside reverse transcriptase inhibitors, such as ritonavir, have been associated with low bone mass when compared with non-nucleoside reverse transcriptase inhibitors [62]. Tenofovir, also a nucleoside reverse transcriptase inhibitor, could possibly lead to proximal tubule toxicity and was associated with reduced BMD in people with HIV [63]. Tenofovir may also cause an acute decrease in BMD [64]. A recent longitudinal study including 3251 people living with HIV in Japan concluded that the risk of fractures must be considered regardless of patient age and gender if they had tenofovir disoproxil fumarate (TDF) administration more than 5 years [65].
Low BMI was reported as the strongest predictor of low BMD, regardless of HIV status, in some populations of men who have sex with men [29]. In this meta-analysis, HIV-infected patients had significantly lower lumbar spine BMD than controls in age-matched and BMI-matched studies. These findings suggested that both HIV infection and low BMI are independent risk factors for low bone mass. The Fracture Risk Assessment Tool (FRAX) has been used to estimate fracture risk. However, a cross-sectional study revealed that FRAX had poor sensitivity and specificity for low bone mass among HIV-infected men with a median age of 45 years [66]. The ideal screening protocols of bone health among HIV-infected individuals are not clearly defined and are still controversial. The European AIDS Clinical Society recommends screening fracture risk in HIV-infected adults for individuals older than 40 years, while other authors have suggested screening people older than 50 years [46, 61, 67]. In our opinion, we suggest that the age of fracture risk screening should be lower in HIV-infected groups than in non-HIV-infected people.
This study had some limitations, despite being the largest scale systemic review of the impact of HIV infection on bone health and fracture prevalence. Some included articles were not specifically designed to evaluate fracture prevalence or BMD as their primary purpose. The information we extracted from those studies may have been influenced by confounding factors. This meta-analysis included different types of studies, including case-control, cross-sectional, and cohort studies, which could also lead to clinical heterogeneity.
People with HIV infection had lower lumbar spine and hip BMD and a higher prevalence of all fractures as well as fragility fractures, vertebral fractures, and wrist fractures than controls. People with HIV also had a higher incidence of fractures than controls. Targeted screening with management to maintain skeletal strength and reduce fragility is urgent for people with HIV.
References
Harrison KM, Song R, Zhang X (2010) Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr 53(1):124–130. https://doi.org/10.1097/QAI.0b013e3181b563e7
van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F, study Anoc (2010) Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24(10):1527–1535. https://doi.org/10.1097/QAD.0b013e32833a3946
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4(6):368–381
Amiel C, Ostertag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, Neit-Ngeilh L, Rozenbaum W, De Vernejoul MC (2004) BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res 19(3):402–409. https://doi.org/10.1359/JBMR.0301246
Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS (2007) Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS 21(5):617–623. https://doi.org/10.1097/QAD.0b013e3280148c05
Yin M, Dobkin J, Brudney K, Becker C, Zadel JL, Manandhar M, Addesso V, Shane E (2005) Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos Int 16(11):1345–1352. https://doi.org/10.1007/s00198-005-1845-0
Brown TT, Qaqish RB (2006) Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 20(17):2165–2174. https://doi.org/10.1097/QAD.0b013e32801022eb
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 12(12):1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127(9):820–826
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE (2000) Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS 14(4):F63–F67. https://doi.org/10.1097/00002030-200003100-00005
Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A (2001) Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS 15(6):807–808. https://doi.org/10.1097/00002030-200104130-00022
Huang JS, Mulkern RV, Grinspoon S (2002) Reduced intravertebral bone marrow fat in HIV-infected men. AIDS 16(9):1265–1269. https://doi.org/10.1097/00002030-200206140-00009
Loiseau-Peres S, Delaunay C, Poupon S, Lespessailles E, Ballouche N, Arsac P, Benhamou CL (2002) Osteopenia in patients infected by the human immunodeficiency virus. A case control study. Joint Bone Spine 69(5):482–485. https://doi.org/10.1016/s1297-319x(02)00433-5
Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J (2003) Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS 17(13):1917–1923. https://doi.org/10.1097/00002030-200309050-00010
Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S (2004) Reduced bone density in HIV-infected women. AIDS 18(3):475–483. https://doi.org/10.1097/00002030-200402200-00014
Bolland MJ, Grey AB, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, Woodhouse AF, Gamble GD, Reid IR (2006) Bone mineral density is not reduced in HIV-infected Caucasian men treated with highly active antiretroviral therapy. Clin Endocrinol 65(2):191–197. https://doi.org/10.1111/j.1365-2265.2006.02572.x
Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE (2006) HIV infection and bone mineral density in middle-aged women. Clin Infect Dis 42(7):1014–1020. https://doi.org/10.1086/501015
Teichmann J, Lange U, Discher T, Lohmeyer J, Stracke H, Bretzel RG (2009) Bone mineral density in human immunodeficiency virus-1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART). Eur J Med Res 14:59–64. https://doi.org/10.1186/2047-783x-14-2-59
Yin MT, McMahon DJ, Ferris DC, Zhang CA, Shu A, Staron R, Colon I, Laurence J, Dobkin JF, Hammer SM, Shane E (2010) Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab 95(2):620–629. https://doi.org/10.1210/jc.2009-0708
Yin MT, Lu D, Cremers S, Tien PC, Cohen MH, Shi Q, Shane E, Golub ET, Anastos K (2010) Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr 53(2):202–208. https://doi.org/10.1097/QAI.0b013e3181bf6471
Sharma A, Flom PL, Weedon J, Klein RS (2010) Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS 24(15):2337–2345. https://doi.org/10.1097/QAD.0b013e32833d7da7
Badie BM, Soori T, Kheirandish P, Izadyar S, SeyedAlinagh S, Foroughi M, Rostamian A, Mohraz M (2011) Evaluation of bone mineral density in Iranian HIV/AIDS patients. Acta Med Iran 49(7):460–467
Mulligan K, Harris DR, Emmanuel P, Fielding RA, Worrell C, Kapogiannis BG, Monte D, Sleasman J, Wilson CM, Aldrovandi GM, team ATNP (2012) Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: Adolescent Trials Network Study 021B. Clin Infect Dis 55(3):461–468. https://doi.org/10.1093/cid/cis455
Grijsen ML, Vrouenraets SM, Wit FW, Stolte IG, Prins M, Lips P, Reiss P, Prins JM (2013) Low bone mineral density, regardless of HIV status, in men who have sex with men. J Infect Dis 207(3):386–391. https://doi.org/10.1093/infdis/jis687
Negredo E, Domingo P, Ferrer E, Estrada V, Curran A, Navarro A, Isernia V, Rosales J, Perez-Alvarez N, Puig J, Bonjoch A, Echeverria P, Podzamczer D, Clotet B (2014) Peak bone mass in young HIV-infected patients compared with healthy controls. J Acquir Immune Defic Syndr 65(2):207–212. https://doi.org/10.1097/01.qai.0000435598.20104.d6
Prior J, Burdge D, Maan E, Milner R, Hankins C, Klein M, Walmsley S (2007) Fragility fractures and bone mineral density in HIV positive women: a case-control population-based study. Osteoporos Int 18(10):1345–1353. https://doi.org/10.1007/s00198-007-0428-7
Triant VA, Brown TT, Lee H, Grinspoon SK (2008) Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 93(9):3499–3504. https://doi.org/10.1210/jc.2008-0828
Yin MT, Shi Q, Hoover DR, Anastos K, Sharma A, Young M, Levine A, Cohen MH, Shane E, Golub ET, Tien PC (2010) Fracture incidence in HIV-infected women: results from the Women’s Interagency HIV Study. AIDS 24(17):2679–2686. https://doi.org/10.1097/QAD.0b013e32833f6294
Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F (2011) Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 53(11):1120–1126. https://doi.org/10.1093/cid/cir627
Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS (2011) Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis 52(8):1061–1068. https://doi.org/10.1093/cid/ciq242
Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC, Veterans Aging Cohort Study Project T (2011) Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One 6(2):e17217. https://doi.org/10.1371/journal.pone.0017217
Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, Obel N (2012) Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS 26(3):285–293. https://doi.org/10.1097/QAD.0b013e32834ed8a7
Torti C, Mazziotti G, Soldini PA, Foca E, Maroldi R, Gotti D, Carosi G, Giustina A (2012) High prevalence of radiological vertebral fractures in HIV-infected males. Endocrine 41(3):512–517. https://doi.org/10.1007/s12020-011-9586-7
Pepe J, Isidori AM, Falciano M, Iaiani G, Salotti A, Diacinti D, Del Fiacco R, Sbardella E, Cipriani C, Piemonte S, Romagnoli E, Lenzi A, Minisola S (2012) The combination of FRAX and Ageing Male Symptoms scale better identifies treated HIV males at risk for major fracture. Clin Endocrinol 77(5):672–678. https://doi.org/10.1111/j.1365-2265.2012.04452.x
Lo Re V 3rd, Volk J, Newcomb CW, Yang YX, Freeman CP, Hennessy S, Kostman JR, Tebas P, Leonard MB, Localio AR (2012) Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology 56(5):1688–1698. https://doi.org/10.1002/hep.25866
Peters BS, Perry M, Wierzbicki AS, Wolber LE, Blake GM, Patel N, Hoile R, Duncan A, Kulasegaram R, Williams FM (2013) A cross-sectional randomised study of fracture risk in people with HIV infection in the probono 1 study. PLoS One 8(10):e78048. https://doi.org/10.1371/journal.pone.0078048
Guerri-Fernandez R, Vestergaard P, Carbonell C, Knobel H, Aviles FF, Castro AS, Nogues X, Prieto-Alhambra D, Diez-Perez A (2013) HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res 28(6):1259–1263. https://doi.org/10.1002/jbmr.1874
Prieto-Alhambra D, Guerri-Fernandez R, De Vries F, Lalmohamed A, Bazelier M, Starup-Linde J, Diez-Perez A, Cooper C, Vestergaard P (2014) HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr 66(1):90–95. https://doi.org/10.1097/QAI.0000000000000112
Sharma A, Shi Q, Hoover DR, Anastos K, Tien PC, Young MA, Cohen MH, Golub ET, Gustafson D, Yin MT (2015) Increased fracture incidence in middle-aged HIV-infected and HIV-uninfected women: updated results from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 70(1):54–61. https://doi.org/10.1097/QAI.0000000000000674
Guerri-Fernandez R, Molina D, Villar-Garcia J, Prieto-Alhambra D, Mellibovsky L, Nogues X, Gonzalez-Mena A, Guelar A, Trenchs-Rodriguez M, Herrera-Fernandez S, Horcajada JP, Diez-Perez A, Knobel H (2016) Brief report: HIV infection is associated with worse bone material properties, independently of bone mineral density. J Acquir Immune Defic Syndr 72(3):314–318. https://doi.org/10.1097/QAI.0000000000000965
Gonciulea A, Wang R, Althoff KN, Palella FJ, Lake J, Kingsley LA, Brown TT (2017) An increased rate of fracture occurs a decade earlier in HIV+ compared with HIV- men. AIDS 31(10):1435–1443. https://doi.org/10.1097/QAD.0000000000001493
Shiau S, Broun EC, Arpadi SM, Yin MT (2013) Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. Aids 27(12):1949–1957. https://doi.org/10.1097/QAD.0b013e328361d241
Collin F, Duval X, Le Moing V, Piroth L, Al Kaied F, Massip P, Villes V, Chene G, Raffi F, group ACA-Cs (2009) Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS 23(8):1021–1024. https://doi.org/10.1097/QAD.0b013e3283292195
Yin MT, Lund E, Shah J, Zhang CA, Foca M, Neu N, Nishiyama KK, Zhou B, Guo XE, Nelson J, Bell DL, Shane E, Arpadi SM (2014) Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 28(3):345–353. https://doi.org/10.1097/QAD.0000000000000070
Ilha T, Comim FV, Copes RM, Compston JE, Premaor MO (2018) HIV and vertebral fractures: a systematic review and metanalysis. Sci Rep 8(1):7838. https://doi.org/10.1038/s41598-018-26312-9
Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, Ofotokun I, Weitzmann MN (2010) Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A 107(31):13848–13853. https://doi.org/10.1073/pnas.1003020107
Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS (2008) Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205(8):1797–1805. https://doi.org/10.1084/jem.20072683
Brown TT, Ruppe MD, Kassner R, Kumar P, Kehoe T, Dobs AS, Timpone J (2004) Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab 89(3):1200–1206. https://doi.org/10.1210/jc.2003-031506
Walker Harris V, Brown TT (2012) Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis 205(Suppl 3):S391–S398. https://doi.org/10.1093/infdis/jis199
Teichmann J, Stephan E, Discher T, Lange U, Federlin K, Stracke H, Friese G, Lohmeyer J, Bretzel RG (2000) Changes in calciotropic hormones and biochemical markers of bone metabolism in patients with human immunodeficiency virus infection. Metabolism 49(9):1134–1139. https://doi.org/10.1053/meta.2000.8609
McNurlan MA, Garlick PJ, Frost RA, Decristofaro KA, Lang CH, Steigbigel RT, Fuhrer J, Gelato M (1998) Albumin synthesis and bone collagen formation in human immunodeficiency virus-positive subjects: differential effects of growth hormone administration. J Clin Endocrinol Metab 83(9):3050–3055. https://doi.org/10.1210/jcem.83.9.5076
Aukrust P, Haug CJ, Ueland T, Lien E, Muller F, Espevik T, Bollerslev J, Froland SS (1999) Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab 84(1):145–150. https://doi.org/10.1210/jcem.84.1.5417
Jacobson MA, Gambertoglio JG, Aweeka FT, Causey DM, Portale AA (1991) Foscarnet-induced hypocalcemia and effects of foscarnet on calcium metabolism. J Clin Endocrinol Metab 72(5):1130–1135. https://doi.org/10.1210/jcem-72-5-1130
Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA (2014) Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS 28(12):1759–1767. https://doi.org/10.1097/QAD.0000000000000320
Ruan A, Tobin NH, Mulligan K, Rollie A, Li F, Sleasman J, Aldrovandi GM (2016) Brief report: Macrophage activation in HIV-infected adolescent males contributes to differential bone loss by sex: Adolescent Trials Network Study 021. J Acquir Immune Defic Syndr 72(4):372–375. https://doi.org/10.1097/QAI.0000000000000953
McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, Aldrovandi GM, Cardoso SW, Santana JL, Brown TT (2010) Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 51(8):937–946. https://doi.org/10.1086/656412
Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D, group AHs (2009) Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 23(7):817–824. https://doi.org/10.1097/QAD.0b013e328328f789
Haskelberg H, Hoy JF, Amin J, Ebeling PR, Emery S, Carr A, Group SS (2012) Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS One 7(6):e38377. https://doi.org/10.1371/journal.pone.0038377
Calmy A, Fux CA, Norris R, Vallier N, Delhumeau C, Samaras K, Hesse K, Hirschel B, Cooper DA, Carr A (2009) Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis 200(11):1746–1754. https://doi.org/10.1086/644785
Komatsu A, Ikeda A, Kikuchi A, Minami C, Tan M, Matsushita S (2018) Osteoporosis-related fractures in HIV-infected patients receiving long-term tenofovir disoproxil fumarate: an observational cohort study. Drug Saf 41(9):843–848. https://doi.org/10.1007/s40264-018-0665-z
Short CE, Shaw SG, Fisher MJ, Gilleece YC, Walker-Bone K (2014) Comparison of peripheral forearm DXA and clinical risk factor screening using FRAX(R) to assess the risk of HIV-associated low bone mass: a cross-sectional study. Arch Osteoporos 9:181. https://doi.org/10.1007/s11657-014-0181-4
(2003) The EACS Euroguidelines Group European guidelines for the clinical management and treatment of HIV-infected adults in Europe. AIDS 17:S3–S26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reprint requests to: Ta-Wei Tai, MD, PhD; Department of Orthopaedics, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Rights and permissions
About this article
Cite this article
Chang, CJ., Chan, YL., Pramukti, I. et al. People with HIV infection had lower bone mineral density and increased fracture risk: a meta-analysis. Arch Osteoporos 16, 47 (2021). https://doi.org/10.1007/s11657-021-00903-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-00903-y