Abstract
Osteoporosis is characterized by an increasing osseous fragility and fracture resulting from the low mass and deteriorated microarchitecture in the bone tissue. The hormone replacement therapy and alendronate were frequently used to treat osteoporosis as the primary therapeutic strategy, but their adverse effects have severely limited their extensive clinical application, therefore, it is urgent to develop alternative or complementary therapeutic agents for anti-osteoporosis. Interestingly, with more people focusing on the complementary and alternative medicine, traditional Chinese herbs and formulas are being gradually recognized as safe and effective agents in the treatment of osteoporosis. In particular, a notable trend is that increasing studies are making efforts to clarify the anti-osteoporotic effects and mechanism of the tonifying kidney-yin herbs and formulas, a category of agents identified as effective therapy. Therefore, the purpose of this study is to comprehensively review the tonifying kidney-yin herbs and formulas that have been reported in the treatment of osteoporosis as well as how the agents play their roles in detail. This current study not only will advance our understanding of the actions of tonifying kidney-yin herbs and formulas, but also provide new evidence for the clinic use of the tonifying kidney-yin herbs and formulas in the treatment of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a complex and degenerative bone metabolism disease, which is characterized by the reduced bone mass and deteriorated microarchitecture of bone tissue [1]. Osteoporosis-induced fracture has been a major health hazard, especially the fracture in hip and vertebrae [2]. Actually, Si et al. [3] predicted that the number of osteoporosis-related fracture in China would double annually by 2035 and could increase to 5.99 million in 2050. Meanwhile, in the United States, the national expenses on bone fracture care will increase from $17 billion in 2005 to $474 billion by 2025 for the enhancing rate of osteoporosis [4].
It is conventionally considered that menopause and old age are the major determinant factors for bone loss due to the reduction in gonadal function [5, 6]. Additionally, epidemiological data showed that the common factors including excessive glucocorticoids use [7], diabetes [8], kidney diseases [9], weightlessness [10] and smoking [11] were proved to lead to osteoporosis. As fully demonstrated by animal and cell experiments as well as clinical evidence, the central research parameters for osteoporosis largely involved the reduction in bone quality, changes in serum and urine biochemical markers, a decrease in bone formation, and an increase in bone resorption. Moreover, from the cell and molecular level, the mechanism of bone loss is generally attributed to the altered osteoclast differentiation and bone resorption over osteoblasts bone formation [12]. The research indexes and relevant parameters of osteoporosis were described in detail in Table 1.
Recently, hormone replacement therapy (HRT) drugs and bisphosphates are the major agents to treat osteoporosis in clinical practice due to their inhibition to bone resorption. However, several lines of researches have demonstrated a series of potential adverse effects in the process of treatment with HRT. For example, the long-term treatment with estrogen will pose increasing risk for the morbidity of the secondary diseases including breast cancer, endometrial carcinoma, heart attack, blood clots and stroke [13, 14]. Similarly, it has been reported that bisphosphates partly would result in the complication of osteonecrosis in jaw [15, 16]. Thus, to reduce these adverse effects, a new insight into complementary and alternative medicine is becoming an indispensable exploration in the treatment of osteoporosis.
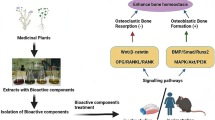
Traditional Chinese medicine (TCM) are being gradually recognized and approved in dealing with osteoporosis as one of the remarkable complementary and alternative therapy, because of their own less adverse reactions as well as significant curative effects [17]. TCM theory pointed out that the growth and development of bones are closely related to a normal function of the kidney-yin, an essential substance for nourishing the bones [18]. Alternatively, in case the kidney-yin is insufficient, there will be increasing risk of some TCM syndromes like “Guwei”, “Guku” and “Gukong”, which are similar to the disease of osteoporosis in western medicine [19, 20]. Thus, the traditional Chinese herbs categorized into tonifying the kidney-yin played crucial roles in the clinical treatment and preclinical researches of osteoporosis. A large number of researches have been carried out on this topic. However, only scattered rather than systematic reports are available in previous literature with regard to anti-osteoporotic effects of tonifying kidney-yin herbs and formulas. Therefore, the purpose of this study is to comprehensively review the previous researches on the effective herbs and formulas including Fructus Ligustri Lucidi, Eclipta prostrata, Fructus Schisandrae Chinensis, Fructus Corni, Rhizoma Polygonati, Radix Polygoni Multiflori, Radix Angelicae Sinensis, Erzhi Wan, Siwu Tang, Liuwei Dihuang Wan and Zuogui Wan, as well as their potential targets.
Fructus Ligustri Lucidi
Fructus Ligustri Lucidi (FLL) is mostly used to nourish kidney-yin for maintaining healthy energy and treating age-related disease, which was initially recorded in Shennong Bencaojing, the oldest Chinese materia medica monograph in China (Anonymous, CA. 200 BC). Many studies have confirmed that FLL had various pharmacological effects including anticancer [21], immunoloregulation [22], antioxidant [23], and anti-osteoporosis [24].
Bone density is determined by the peak value reached during young adulthood. Furthermore, reaching the higher peak bone mass (PBM) may prevent and reduce the risk of osteoporosis in the later years [24]. In vivo studies, with the administration of FLL extract for 4 months till PBM time point, the FLL-treated rats exhibited higher bone mineral density (BMD) on femur than the control ones. The μCT testing indicated that FLL improved their bone microarchitecture with higher trabecular bone volume fraction and thickness. Additionally, FLL-treated group had higher dry weight value, calcium retention and calcium absorption. The serum parameters including 25(OH)D3, 1,25(OH)2D3, and osteocalcin (OCN) were remarkably elevated, while the level of C-terminal telopeptide of type I collagen (CTX-I) was decreased by FLL when compared to the control group [25, 26]. And, the bone biomechanical parameters in ultimate load and ultimate deformation were also increased by FLL treatment [25]. These effects could be elucidated that, on the one hand, the ratio of receptor activator of NF-κB ligand (RANKL) to osteoprotegerin (OPG) in tibia was decreased and the genes expression of 1α-hydroxylase (1-OHase), transient receptor potential vanilloid 6 (TRPV6), calcium transporter calbindin-D9k (CaBP-9 k) and vitamin D receptor (VDR) in kidney and duodenum were enhanced, consequently the treatment with FLL regulating the absorption of calcium [25, 26]. On the other hand, FLL inhibited the high bone turnover rate of biochemical markers including the increased urinary deoxypyridinoline (DPD) and serum OCN in ovariectomized (OVX) rats, which are the special bone resorption and formation markers respectively [27]. Moreover, a combination of FLL with high calcium managed to synergistically increase calcium level in serum and bone, despite the report from Zhang et al. that the decreased bone calcium level in OVX mice was difficult to be rectified by single high calcium administration. This significant effect on calcium balance was mainly attributed to the up-regulation of CaBP-9 k and down-regulation of calcium-sensing receptor (CaSR) in kidney [28].
In vitro studies, FLL administration realized the anti-osteoporotic effects by significantly stimulating the differentiation of osteoblasts. FLL enhanced Alkaline phosphatase (ALP) activity in mesenchymal stem cells (MSCs) and significantly attenuated the mineralization time for MSCs. These actions may be related to the increased genes expression of β-Catenin, BMP2, Cyclin D1, MT1-MMP, OPG, and TBX3 by FLL administration, which were the differentiation regulators of osteoblasts [29]. It has already showed that when the osteoblast MC3T3-E1 cells were treated with FLL for 7 days, the mRNA levels of ALP, bone sialoprotein (BSP) and OCN were elevated, and the ratio of RANKL to OPG was decreased, resulting in the positive effect of FLL on the cells differentiation [25].
In addition, the anti-osteoporotic effects of FLL were determined by means of studying the interaction between FLL and other positive anti-osteoporosis herbs, such as Herba Epimedii (HE) and Radix Puerariae (RP) [30, 31]. For example, Liu et al. has demonstrated that the osteoporosis induced by retinoic acid was alleviated by a combination therapy of FLL with HE, because they enhanced the levels of bone mineral content, biomechanical parameters in the model rats, and the bone formation markers, as well [32]. But, when FLL and RP were applied into treating the OVX rats together, their respective positive effects including inhibiting the bone loss and regulating mineral metabolism wore off [33]. Although more researches about the herb-herb interaction should be further done, after all, the above effects have proved that FLL will be a potential candidate for the prevention and treatment of osteoporosis.
Eclipta Prostrata
Eclipta prostrata (EP) is originated from dried aerial parts of Eclipta prostrata L. in compositae family. It possesses many pharmacological effects, including anti-breast tumor [34], hypolipidemic capacity [35] and promotion of hair growth [36]. Recently, researchers attached too much importance to its anti-osteoporotic effect.
The EP extract exhibited positive effects on inhibiting osteoporosis in similar ways of FLL. When the OVX female rats were administrated with EHE for 3 months, they had remarkably higher BMD than the control rats, which was equivalent to the positive drugs administration group. The impaired histomorphology of bone was also ameliorated by EP extract [37]. These vivo findings that EP extract reversed the increased interleukin-6 (IL-6) and decreased calcitonin (CT) as well as RANKL in tibiae were partly responsible for its protective effects on the bones of OVX rats [37]. Furthermore, Deng et al. found that Echinocystic acid (EA), one of bioactive components in EP, significantly improved the damages in the bones induced by estrogen deficiency, such as lower BMD, impaired trabecular architecture and biomechanical properties, and the excessive expression of pro-inflammatory cytokines such as IL-1β and TNF-α. Consequently, they concluded that EA may be an important substance for EP to play roles in inhibiting bone loss [38].
Meanwhile, in the vitro study, it has been found that wedelolactone (extracted from EP) may be another important bioactive compound to treat osteoporosis. Liu et al. [39] found that wedelolactone significantly inhibited the proliferation and differentiation of pre-osteoclastic RAW264.7 cells, while it was capable of stimulating the proliferation and differentiation of BMSCs. They inferred that wedelolactone still showed an excellent anti-osteoporotic effect at low concentration in spite of the fact that the high dose of wedelolactone leaded to some cytotoxic effects on BMSCs [39]. These findings indicated that wedelolactone may be another potential bioactive compound to work in EP for anti-osteoporosis.
Fructus Schisandrae Chinensis
Fructus Schisandrae Chinensis (FSC) is supposedly categorized as the tonic due to its powerful and effective action on nourishing kidney-yin [40]. Currently, the FSC extract showed anti-inflammatory effects to treat asthma and sepsis [40, 41]. FSC also exerted oxidant inhibition to treat alcoholic liver injury by preventing hepatocyte apoptosis and fatty degeneration [42]. But more importantly, the FSC was attracting a lot of interests from the researches on the osteoporosis.
In vivo studies, the treatment of FSC not only attenuated the pathomorphological changes in femur head and condyles of OVX mice, such as growth plate hypertrophy and bone marrow pores, but also reversed some key parameters due to the lack of estrogen including the involvement in bone loss as well as the deficiency of uterus. The data proved that FSC indeed enhanced the levels of BMD and serum OCN, and, moreover, the level of serum estradiol and the expression of ER-α and ER-β in uterus were significantly increased by FSC. Additionally, FSC obviously downregulated the relevant oncogene proteins in uterus including c-fos and c-Jun [43]. Schisantherin A (SA) is one kind of lignans extracted from FSC. He et al. found that SA inhibited osteoclast formation and Ti particle-induced osteolysis by decreasing osteolysis area, osteoclasts activity and osteoclasts number [44].
In vitro studies, He et al. further explored whether the SA worked at the cellular level. It turned out that SA suppressed the differentiation and osteoclastogenesis of BMMs and RAW 264.7 cells. This group also clarified the result that SA destroyed osteoclast-induced bone resorption may be fully understood by its roles in RANKL signaling pathways involving the down-regulation of the phosphorylation of IκB, JNK and ERK1/2, as well as the genes expression of NFATc1 and c-Fos [44]. Parallel study has demonstrated that FSC stimulated the proliferation of osteoblasts UMR 106 cells and increased the activity of ALP, indicating the positive effects of FSC on osteoblasts [45]. These data proved that FSC is a promising herb for the treatment of bone loss induced by osteoclast.
Fructus Corni
Fructus Corni (FC) is usually used in the Orient as a source of food resulting from redundant nutrition greatly enriched in sarcocarp. In China, however, it is often known in medical literature that the core medicinal uses of FC involve treating the deficiency of kidney-yin. In recent decades, the uses of FC have been expanded into treating diabetes [46], neurotoxicity [47] and platelet aggregation [48]. But, from the nourishing kidney-yin viewpoint, FC has recently been researched as an ideal medicinal component for anti-osteoporosis.
In vivo studies, it is believed that the spinal cord-injured (SCI) surgery was considered as another promising osteoporotic model. As long as 6 weeks after SCI surgery, the rats encountered the deterioration of biomechanical parameters, such as maximal load, energy absorption and structural stiffness, which is similar to those of OVX model, the main osteoporotic model. But, these abnormal biomechanics were effectively improved by the administration of FC with a dose-dependent manner. Meanwhile, SCI surgery brought about the lower BMD and BMC and a certain amount of morphological damage including inner diameter, internal and external areas in tibia diaphysis, all of which were also partly reserved by FC [49]. Several lines of studies further found that FC served as a rich source of cornel total glycoside, which is well-known for its ability to significantly elevate the levels of serum E2 and BMD and improve the damaged bone trabecula, especially that of rat model of OVX [50, 51].
In vitro studies, Sweroside extracted form FC significantly increased the proliferation of human MG-63 cells and osteoblasts, and enhanced the activity of ALP and osteocalcin, as well. In addition to its proliferation effects, the involvement of anti-apoptosis effect on the osteoblast was found in the treatment of sweroside [52]. The data manifested that developing FC as well as its extracts could potentially provide certain benefits for osteoporosis that have been implied by laboratory animal and cell studies.
Rhizoma Polygonati
Rhizoma Polygonati (RP) belongs to the genus polygonatum family of plants. Huge amount of RP are consumed as food-medicine dual plants in the East Asia countries, despite the fact that it is widely distributed from Asia to Europe. In China, RP is the ideal tonic for the ones who need to strengthen body’s kidney-yin. Current studies showed that RP was usually used to prevent and treat metabolic disorders such as obesity [53], diabetes [54] and cardiovascular disease [55]. Additionally, some progress has been made in its anti-osteoporotic effect and its pharmacological mechanism.
In vivo studies, the administration of RP at a dose of 0.4 g/kg/day for 35 days significantly enhanced the level of BMD, and decreased the occurrence of malalignment in the tibia trabeculae of the OVX rats, which is similar to that of 17-ethinylestradiol, the excellent conventional agent for deficiency of estrogen. These effects have been proven to be related to the up-regulation of basic fibroblast growth factor (bFGF) and BMP and the suppression on excessive expression of bone Gla protein (BGP) and TRAP in tibiae [56]. This animal experiment indeed indicated the anti-osteoporotic activity of RP.
In vitro studies, Zong et al. [57] reported that RP increased the viability of BMSCs with the greatest stimulation at a dose of 0.5 g/ml. They then clarified this relevant mechanism by detecting the effects of RP on the levels of ALP, OCN, PINP and BMP-2 in BMSCs as well as the protein expression of BSP (bone sialoprotein) and SPARC (secreted protein, acidic and rich in cysteine). The results demonstrated that RP showed powerful and effective effects on these parameters, indicating a potential effect from RP on promoting the proliferation and differentiation of osteoblasts [57]. The vivo and vitro studies have confirmed the RP might be a potential alternative medicine for treatment of osteoporosis.
Radix Polygoni Multiflori
Radix Polygoni Multiflori (RPM) is originated from the dried root tuber of Polygonum multiflorum Thunb. (Fam. Polygonaceae). In China, RPM is used in the prevention and treatment of deficiency of the kidney-yin, which has been documented in detail by Bencao Gangmu (Shizhen Li, AD 1578), one of most authoritative Chinese pharmacopeias. Previously, the modern medicinal uses of RPM have been found that include the promotion of hair growth [58], increasing longevity [59], the prevention and treatment of cerebrovascular diseases [60], and anti-osteoporotic ability.
In vivo studies, it was reported that OVX rats experienced 15 days of RPM administration showed an increase in the bone ALP. Subsequently, the level of ALP in serum was elevated by RPM when treating the OVX rats for 30 days, and the TRAP level in RPM group exhibited a decreased trend. Meanwhile, the impaired bone morphological features were significantly improved by the intervention of RPM [61], suggesting the anti-osteoporotic effect of RPM on animal experiments.
In vitro studies, exposure to H2O2 remarkably suppressed the differentiation of MC3T3-E1 osteoblast cells by reducing the genes expression of ALP, collagen I (COL-I) and osteocalcin (OCN), resulting in the increase of dead cells. However, 2,3,5,40-tetrahydroxystilbene-2-O-b-D-glucoside (TSG), one of useful ingredients in RPM, prolonged the survival of cells to maintain their function, which was proven to work by significantly inhibiting the increased levels of RANKL, IL-6, reactive oxygen species (ROS) and lipid peroxide (MDA) that were induced by H2O2 [62]. And the suppressed expressions of osteogenic differentiation genes (ALP, COL-I and OCN) were significantly enhanced by TSG. These evidences suggested that the role of RPM in treating osteoporosis is mainly associated with its antioxidant activities.
Radix Angelicae Sinensis
Radix Angelicae Sinensis (RAS) is the dried root of Angelica sinensis (Oliv.) Diels. RAS is a well-known herb with the efficacy of nourishing the liver-blood. However, according to the principle of traditional Chinese medicine, the homogeny of the liver-blood and kidney-essence or kidney-yin expanded the clinical uses of RAS into treating the deficiency of the kidney-yin, even if RAS has been alleged to be useful for the treatment of deficiency of live-blood [63]. In vitro and in vivo studies have shown that RAS and its extracts exhibited antioxidant activity [64], neuroprotective role in ischemic injury [65], and the improvement of memory impairment in Alzheimer [66]. Additionally, there are a number of reports concerning the uses of RAS in the treatment of osteoporosis in recent years.
In vivo studies, 0.3 g/kg of the RAS extract daily for 4 weeks increased the BMD of the femur in OVX rats, which is similar to that of 17β-estradiol, another excellent conventional agent for deficiency of estrogen. Moreover, RAS extract also significantly reserved OVX-induced excessive expressions of ALP, CTx, and OCN in serum, but it has been verified that the RAS extract did not bring about uterus impairment even if adding the dose of RAS extract to 1 g/kg or 2 g/kg. Results of this study suggested that RAS extract inhibited OVX-induced bone loss without adverse toxicity [67]. In other studies, more successful treatment of osteoporosis induced by estrogen deficiency was further found after administration of the ultrafine RAS when compared with the coarse RAS. Administration of RAS for 8 weeks was effective in up-regulating the level of serum estradiol and reducing the ratio of bone-alkaline phosphatase (BALP) to total-alkaline phosphatase in bone (TALP), especially the ultrafine RAS, indicating the improvement of bone formation. More importantly, the ultrafine RAS reversed the low BMD and the trabecular and cortical bone loss that were induced by estrogen deficiency, and consequently ultrafine RAS attenuated the biomechanics impairment. These positive activities of ultrafine RAS were deemed to be associated with its more active ingredients such as decursin and decursinol than the coarse RAS [68]. In another study, Kim et al. [69] proved the above inference by exploring the effects of decursin on lipopolysaccharide (LPS)-induced bone loss. Results demonstrated that the treatment of decursin effectively inhibited the bone loss induced by LPS by enhancing the BMD, trabecular thickness (Tb.Th) and trabecular number (Tb.N).
In vitro studies, decursin isolated from the RAS inhibited NFATc1 that directly and positively related to RANKL signaling pathway [69]. Since the RANKL signaling pathway are known to have pronounced effects on the differentiation of osteoclasts, the inhibitory activities to RANKL may explain some of the reported effects of RAS extract on osteoclastogenesis. Furthermore, the decursin showed significantly suppressed effects on pre-osteoclasts. This mechanism of action appeared to be via down-regulation of the genes of DC-STAMP and β3 integrin, leading to less fusion and migration of pre-osteoclasts. In another study, Kong et al. and Ahn et al. [70, 71] pointed out that the administration of RAS extract and Angelica tenuissima water extract (ATWE) inhibited the formation of filamentous actin (F-actin) ring and the differentiation of osteoclasts, as determined by reducing the phosphorylation of p38, ERK, JNK, p65, and I-κB, as well as the protein expression of c-Fos, c-Jun and NFATc1. These results indicated that RAS and its extracts will be potential candidate for anti-resorptive agent in osteoporosis via RANKL pathway.
Erzhi Wan
The traditional Chinese formula Er Zhi Wan (EZW) was famous for its tonifying kidney-yin effect that was firstly recorded in Fushou Jingfang (AD 1530). EZW was consisted of FLL and EP at a ratio of 1:1. Besides the pharmacological applications in liver injury [72], menopausal syndrome [73] and breast cancer [74], EZW also showed some potent inhibition effects on bone loss.
In vivo studies, the treatment with EZW for consecutive 26 weeks significantly increased the BMD level of OVX rats by inhibiting the bone loss on femur, tibia and the 4th lumbar vertebra, and ameliorated the impaired trabecular parameters including BV/TV, connectivity density (Conn.D), trabecula number (Tb.N), trabecular thickness (Tb.Th), trabecula separation (Tb.Sp) and structure model index (SMI) in femur, with the results that the OVX-induced osteoporosis was effectively dealt with by EZW. In addition, the bone biomechanical parameters including maximum load, energy, maximum stress and elastic modulus were enhanced by EZW administration, but there was rarely side effects such as the endometrial hyperplasia [75]. Similarly, Sun et al. found that EZW was capable of inhibiting the degradation of trabecular microarchitecture and bone loss in mandibles induced by estrogen deficiency, because of its regulation effects on the important bone metabolism markers for osteoporosis, such as serum E2, bone-specific alkaline phosphatase (BALP) and tartrate-resistant acid phosphatase 5b (TRAP5b). Furthermore, these actions of EZW in osteoporosis induced by estrogen deficiency may be related to the up-regulation of wingless-related MMTV integration site 3a (Wnt3a), lipoprotein receptor-related protein 5 (LRP5), β-catenin expression, and the down-regulation of dickkopf homolog 1 (DKK1) expression [76].
In vitro studies, although EZW-containing serum showed few effect on the proliferation of primary cultural osteoblasts and osteoblast-like UMR106 cells, the proliferation and differentiation of osteoclast precursors RAW264.7 induced by M-CSF and RANKL were positively inhibited by EZW-containing serum administration [77]. These positive effects indicated that EZW was beneficial to inhibit bone resorption and treat osteoporosis.
Siwu Tang
Siwu Tang (SWT) is a classical formula that is widely used for the treatment of gynecological diseases in Chinese medicine. SWT consists of four herbs, Radix Angelicae Sinensis, Radix Rehmanniae Preparata, Radix Paeoniae Alba and Rhizoma Ligustici Chuanxiong (usually in mixture ratio 1:1:1:1). Many evidences have shown that SWT has special clinical significance in the relief of women’s diseases such as emmeniopathy, climacteric syndrome and dysmenorrhea [78, 79]. The latest studies reported that SWT exhibited promising efforts in the treatment of cancers including breast and primary colorectal cancers [80, 81]. Furthermore, due to the valuable efficacy for nourishing the yin and blood, SWT has been verified as a potential treatment method for osteoporosis.
In vivo studies, administration of SWT extract to OVX rats increased the levels of ALP, BMP-2 and OPN in serum that were related to the bone formation, and conversely inhibited the level of C-terminal telopeptides of type I collagen, a biomarker of bone resorption. These results of treatment of SWT extract ultimately brought about a higher BMD in OVX rats, leading to significant inhibition for the bone loss [82].
In vitro studies, Wu et al. [82] found that SWT promoted the differentiation of osteoblast by increasing the differentiation-related genes including BMP-2, ALP, and osteopontin (OPN). Meanwhile, administration of SWT inhibited the protein expression of phosphatidylinositol 3-kinase (PI3K), Akt and NF-κB in osteoblasts, resulting in the stimulation of bone mineralization nodules. There was no evidence to support that SWT affected the osteoblasts viability with examined MTT examination, but the positive action of SWT for osteoblast differentiation suggested a potential therapeutic effect on osteoporosis.
Liuwei Dihuang Wan
Liuwei Dihuang Wan (LWDHW) was initially formulated in Song Dynasty, and has been widely used till now as basic tonic prescription for the deficiency of kidney-yin [83]. It is made up of six crude herbs, including Radix Rehmanniae Preparata, Fructus Corni, Rhizoma Dioscoreae, Cortex Moutan Radicis, Rhizoma Alismatis and Poria. Modern pharmacological studies showed that LWDHW was effective to improve spatial memory and neurogenesis [84]. In other studies, the novel medicinal purposes of LWDHW were found that included the prevention and treatment of diabetes [85], breast cancer [83] and osteoporosis.
In vivo studies, administration of LWDHW (0.4 g/kg body weight) daily for 12 weeks elevated the decreased BMD in right femurs induced by OVX, leading to the attenuation of bone loss. Meanwhile, the estrogen deficiency caused the morphologic impairments of femur including trabecular malalignment, empty bone lacunae and fractures, and they were significantly improved after the treatment of LWDHW. Moreover, the result of biomechanical test in L2 vertebra showed that the administration of LWDHW to OVX rats reversed the suppression of maximum loading as well as elastic modulus. Results of this study indicated that LWDHW is effective to the prevention and treatment of osteoporosis [86].
In vitro studies, LWDHW-containing serum studies demonstrated that administration of LWDHW to osteoblasts significantly increased the cells viability, proliferation and ALP activity. LWDHW also stimulated the formation of mineralization nodules when administered to the osteoblasts. LWDHW enhanced the OVX-mediated downregulation of Lrp-5, β-catenin, Runt-related transcription factor-2 (Runx2) and Osx genes, as well. These results suggested that the up-regulation of the canonical Wnt/β-catenin signaling pathway induced by LWDHW administration produced a stimulation on bone formation that facilitating the treatment of osteoporosis [86]. Further study found that administration of morroniside and loganin isolated from LWDHW stimulated the differentiation of MC3T3-E1 cells in the whole process, as determined by the increased production of ALP and osteocalcin [87]. Meanwhile, the morroniside and loganin inhibited the apoptosis when administered to the osteoblasts. The mechanism of action appeared to be via the inhibition of caspase-3, capase-9 and RANKL genes as well as the upregulation of bcl-2 gene [87]. These studies may explain the mechanism of anti-osteoporotic effect in LWDHW.
Zuogui Wan
Zuogui Wan (ZGW) was firstly conceived and developed by Jingyue Zhang during Ming Dynasty in Jingyue Quanshu that has been listed as an authorized Chinese medicine book. There are 8 ingredients in ZGW: Radix Rehmanniae Preparata, Rhizoma Dioscoreae, Fructus Corni, Fructus Lycii, Semen Cuscutae, Colla Corni Cervi, Carapax et Plastrum Testudinis, and Radix Achyranthis Bidentatae, with mixed weight ratio of 8 : 4 : 4 : 4 : 4 : 4 : 4 : 3 [88]. Recent studies has proven that ZGW exhibited neuroprotective effects on autoimmune encephalomyelitis [89] and hypoglycemic effects on gestational diabetes mellitus [88]. However, the clinical applications of ZGW were extensive due to the excellent effects on nourishing the kidney-essence, which was consistent with the traditional Chinese medicine theory in Huangdi Neijing that the kidney is responsible for the birth, growth and reproduction in body, including bone growth of course. So, ZGW is indispensable to researchers interested in the prevention and treatment of osteoporosis with traditional Chinese formula. Actually, a number of studies are trying to clarify how ZGW managed to inhibit bone loss and benefit the osteoporosis.
In vivo studies, it has been reported that ZGW was capable of improving OVX-induced rat model of osteoporosis by up-regulating the levels of BMD and serum calcitonim (CT). It was believed that the inhibition on high bone turnover rate of BGP and TRAP in serum may partly explain the mechanism of action for ZGW [90, 91]. Another supplementary study found that ZGW with their ability to inhibit the bone loss was largely responsible for the downregulation of TGF-β1/Smad 4 gene [92]. Since the glucocorticoid can exert a rapid and powerful damage in rat bone histomorphology, glucocorticoid-induced rodents are developed as a stable and repeatable osteoporotic model. It has been reported that as long as 8 weeks for intraperitoneal injection of glucocorticoid, the rat tibia tissue showed the reduction of trabecula volume percent (TBV%) and trabecular forming surface percent (TFS%), as well as the enhancement of trabecula absorption surface percent (TRS%), indicating a worse bone histomorphology. But, these impairments caused by glucocorticoid were ameliorated by administration of ZGW [18]. However, the mechanism of action may be further understood by other studies that ZGW upregulated glucocorticoid-induced decrease in serum bone r-carboxyaluatamic-acid-containing proteins (BGP), insulin-like growth factors (IGF-I) and E2, and inhibited the increased serum parathyroidhormon (PTH), as well [93, 94].
In vitro studies, administration of ZGW mainly regulated the relevant protein of osteoblasts and MSCs in canonical Wnt/β-catenin signaling pathway including Wnt1, LRP-5 and β-catenin, resulting in promoting the differentiation, development and proliferation of osteoblasts that facilitated to maintain the normal bone metabolism [18]. These studies may help to understand the mechanism of anti-osteoporotic effect in ZGW.
Conclusion
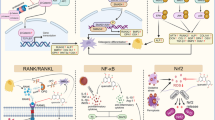
In summary, with the increasing number and accelerated aging in the world population, osteoporosis has become an urgent healthy and social problem. However, the side effects of current agents to treat osteoporosis promote the development of new drugs. The tonifying kidney-yin herbs and formulas have been identified as the effective anti-osteoporosis agents with few adverse effects. Thus, the present study comprehensively reviewed the effects of tonifying kidney-yin herbs and formulas on improving bone density, bone microstructure, biomechanics and other parameters as well as their potential mechanisms. Their relevant applications and mechanism were described in detail in Tables 2 and 3. But the mechanisms of the imbalance between bone formation and resorption in osteoporosis are really complicated. In addition to the canonical Wnt pathway and the RANKL/RANK pathway (Fig.1), some papers have reported that there were several other signaling pathways liking BMP/Smad signaling pathways [95], TGF-β signaling pathways [96], PTH pathways [97] and Notch signaling pathways [98] being involved in the metabolism of bone. And some tonifying kidney-yin herbs and formulas exhibit duplicate effects via different pathways on the treatment of osteoporosis. Therefore more studies examining the intricate process of anti-osteoporotic effects with particular pathway in kidney-yin herbs and formulas are required. However, this current study will contribute to understand the functions of tonifying kidney-yin herbs and formulas in inhibiting osteoporosis, and also provide direct evidence for promoting the use of tonifying kidney-yin herbs and formulas in the treatment of osteoporosis.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BGP:
-
bone Gla protein
- BMP2:
-
bone morphogenetic protein 2
- CT:
-
calcitonin
- CTX-I:
-
C-terminal telopeptide of type I collagen
- DPD:
-
deoxypyridinoline
- ERK:
-
extracellular signal-regulated kinase
- IL-6:
-
interleukin-6
- JNK:
-
c-JunN-terminal kinase
- LRP5:
-
lipoprotein receptor-related protein 5
- NFATc1:
-
nuclear factor of activated T cells c1
- OCN:
-
osteocalcin
- OPG:
-
osteoprotegerin
- OPN:
-
osteopontin
- PI3K:
-
phosphatidylinositol 3-kinase
- RANKL:
-
receptor activator of NF-kB ligand
- RUNX2:
-
Runt-related transcription factor 2
- TNF-α:
-
Tumor Necrosis Factor-α
- TRACP:
-
tartrate-resistant acid phosphatase
- Wnt:
-
wingless-related MMTV integration site
References
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet (London, England) 377(9773):1276–1287. doi:10.1016/s0140-6736(10)62349-5
Genant HK, Engelke K, Bolognese MA, Mautalen C, Brown JP, Recknor C, Goemaere S, Fuerst T, Yang YC, Grauer A, Libanati C (2016) Effects of Romosozumab compared with Teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. doi:10.1002/jbmr.2932
Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ (2015) Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporosis international : a Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 26(7):1929–1937. doi:10.1007/s00198-015-3093-2
AAOS (2008) Burden Of Musculoskeletal Diseases In The United States: Prevalence, Societal And Economic Cost. 1st edn. Amer Academy Of Orthopaedic, Rosemont
Zhou Z, Gao M, Liu Q, Tao MD (2015) Comprehensive transcriptome analysis of mesenchymal stem cells in elderly patients with osteoporosis. Aging Clin Exp Res 27(5):595–601. doi:10.1007/s40520-015-0346-z
Shanbhogue VV, Brixen K, Hansen S (2016) Age- and sex-related changes in bone microarchitecture and estimated strength: a three-year prospective study using HRpQCT. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 31(8):1541–1549. doi:10.1002/jbmr.2817
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29(4):403–440. doi:10.1210/er.2007-0038
Reni C, Mangialardi G, Meloni M, Madeddu P (2016) Diabetes stimulates osteoclastogenesis by acidosis-induced activation of transient receptor potential cation channels. Scientific Reports 6. doi:10.1038/srep30639
Fidan N, Inci A, Coban M, Ulman C, Kursat S (2016) Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research 64(4):861–866. doi:10.1136/jim-2015-000043
Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR (2012) Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 27(9):1896–1906. doi:10.1002/jbmr.1647
Ko CH, Chan RL, Siu WS, Shum WT, Leung PC, Zhang L, Cho CH (2015) Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif Tissue Int 96(5):389–400. doi:10.1007/s00223-015-9966-8
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science (New York, NY) 289(5484):1504–1508
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s health initiative randomized controlled trial. JAMA 288(3):321–333
Meyer MR, Barton M (2016) Estrogens and coronary artery disease: new clinical perspectives. Advances in Pharmacology (San Diego, Calif) 77:307–360. doi:10.1016/bs.apha.2016.05.003
O’Ryan FS, Lo JC (2012) Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: clinical course and outcomes. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 70(8):1844–1853. doi:10.1016/j.joms.2011.08.033
Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC (2013) Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 88(4):418–419. doi:10.1016/j.mayocp.2013.01.002
Rufus P, Mohamed N, Shuid AN (2013) Beneficial effects of traditional Chinese medicine on the treatment of osteoporosis on ovariectomised rat models. Curr Drug Targets 14(14):1689–1693
Liu MJ, Li Y, Pan JH, Liu H, Wang SJ, Teng JR, Zhao HY, DH J (2011) Effects of zuogui pill (see text) on Wnt singal transduction in rats with glucocorticoid-induced osteoporosis. Journal of traditional Chinese medicine Chung i tsa chih ying wen pan / sponsored by All-China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine 31(2):98–102
Shu B, Shi Q, Wang YJ (2015) Shen (kidney)-tonifying principle for primary osteoporosis: to treat both the disease and the Chinese medicine syndrome. Chinese Journal of Integrative Medicine 21(9):656–661. doi:10.1007/s11655-015-2306-z
Chen YY, Hsue YT, Chang HH, Gee MJ (1999) The association between postmenopausal osteoporosis and kidney-vacuity syndrome in traditional Chinese medicine. The American Journal of Chinese Medicine 27(1):25–35. doi:10.1142/s0192415x99000057
Hu B, Du Q, Deng S, An HM, Pan CF, Shen KP, Xu L, Wei MM, Wang SS (2014) Ligustrum lucidum Ait. Fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21. Oncol Rep 32(3):1037–1042. doi:10.3892/or.2014.3312
Wang J, Shan A, Liu T, Zhang C, Zhang Z (2012) In vitro immunomodulatory effects of an oleanolic acid-enriched extract of Ligustrum lucidum fruit (Ligustrum lucidum supercritical CO2 extract) on piglet immunocytes. Int Immunopharmacol 14(4):758–763. doi:10.1016/j.intimp.2012.10.006
Jeong SC, Tulasi R, Koyyalamudi SR (2016) Antioxidant capacities of hot water extracts and Endopolysaccharides of selected Chinese medicinal fruits. Cancers 8(3). doi:10.3390/cancers8030033
Tsuboyama T, Takahashi K, Matsushita M, Okumura H, Yamamuro T, Umezawa M, Takeda T (1989) Decreased endosteal formation during cortical bone modelling in SAM-P/6 mice with a low peak bone mass. Bone and Mineral 7(1):1–12
Lyu Y, Feng X, Zhao P, Wu Z, Xu H, Fang Y, Hou Y, Denney L, Xu Y, Feng H (2014) Fructus ligustri lucidi (FLL) ethanol extract increases bone mineral density and improves bone properties in growing female rats. J Bone Miner Metab 32(6):616–626. doi:10.1007/s00774-013-0536-8
Feng X, Lyu Y, Wu Z, Fang Y, Xu H, Zhao P, Xu Y, Feng H (2014) Fructus ligustri lucidi ethanol extract improves bone mineral density and properties through modulating calcium absorption-related gene expression in kidney and duodenum of growing rats. Calcif Tissue Int 94(4):433–441. doi:10.1007/s00223-013-9825-4
Zhang Y, Lai WP, Leung PC, Wu CF, Yao XS, Wong MS (2006) Effects of fructus ligustri lucidi extract on bone turnover and calcium balance in ovariectomized rats. Biol Pharm Bull 29(2):291–296
Zhang Y, Mukwaya E, Pan H, Li XM, Yang JL, Ge J, Wang HY (2015) Combination therapy of Chinese herbal medicine fructus ligustri lucidi with high calcium diet on calcium imbalance induced by ovariectomy in mice. Pharm Biol 53(7):1082–1085. doi:10.3109/13880209.2014.950388
Li G, Zhang XA, Zhang JF, Chan CY, Yew DT, He ML, Lin MC, Leung PC, Kung HF (2010) Ethanol extract of fructus ligustri lucidi promotes osteogenesis of mesenchymal stem cells. Phytotherapy Research : PTR 24(4):571–576. doi:10.1002/ptr.2987
Chen G, Wang C, Wang J, Yin S, Gao H, Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, Yang LI, Zhang R (2016) Antiosteoporotic effect of icariin in ovariectomized rats is mediated via the Wnt/beta-catenin pathway. Experimental and Therapeutic Medicine 12(1):279–287. doi:10.3892/etm.2016.3333
Udomsuk L, Chatuphonprasert W, Monthakantirat O, Churikhit Y, Jarukamjorn K (2012) Impact of Pueraria Candollei Var. Mirifica and its potent phytoestrogen miroestrol on expression of bone-specific genes in ovariectomized mice. Fitoterapia 83(8):1687–1692. doi:10.1016/j.fitote.2012.09.024
Liu RH, Kang X, LP X, Nian HL, Yang XW, Shi HT, Wang XJ (2015) Effects of the combined extracts of herba Epimedii and fructus ligustri lucidi on bone mineral content and bone turnover in osteoporotic rats. BMC Complement Altern Med 15(112). doi:10.1186/s12906-015-0641-4
Dong XL, Cao SS, Gao QG, Feng HT, Wong MS, Denney L (2014) Combination treatment with fructus ligustri lucidi and Puerariae radix offsets their independent actions on bone and mineral metabolism in ovariectomized rats. Menopause (New York, NY) 21(3):286–294. doi:10.1097/GME.0b013e3182966fd3
Arya RK, Singh A, Yadav NK, Cheruvu SH, Hossain Z, Meena S, Maheshwari S, Singh AK, Shahab U, Sharma C, Singh K, Narender T, Mitra K, Arya KR, Singh RK, Gayen JR, Datta D (2015) Anti-breast tumor activity of Eclipta extract in-vitro and in-vivo: novel evidence of endoplasmic reticulum specific localization of Hsp60 during apoptosis. Scientific Reports 5:18457. doi:10.1038/srep18457
Zhao Y, Peng L, Lu W, Wang Y, Huang X, Gong C, He L, Hong J, Wu S, Jin X (2015) Effect of Eclipta prostrata on lipid metabolism in hyperlipidemic animals. Exp Gerontol 62:37–44. doi:10.1016/j.exger.2014.12.017
Begum S, Lee MR, Gu LJ, Hossain J, Sung CK (2015) Exogenous stimulation with Eclipta alba promotes hair matrix keratinocyte proliferation and downregulates TGF-beta1 expression in nude mice. Int J Mol Med 35(2):496–502. doi:10.3892/ijmm.2014.2022
Zhang ZG, Bai D, Liu MJ, Li Y, Pan JH, Liu H, Wang WL, Xiang LH, Xiao GG, Ju DH (2013) Therapeutic effect of aqueous extract from Ecliptae herba on bone metabolism of ovariectomized rats. Menopause (New York, NY) 20(2):232–240. doi:10.1097/gme.0b013e318265e7dd
Deng YT, Kang WB, Zhao JN, Liu G, Zhao MG (2015) Osteoprotective effect of Echinocystic acid, a Triterpone component from Eclipta prostrata, in ovariectomy-induced osteoporotic rats. PLoS One 10(8):e0136572. doi:10.1371/journal.pone.0136572
Liu YQ, Zhan LB, Liu T, Cheng MC, Liu XY, Xiao HB (2014) Inhibitory effect of Ecliptae herba extract and its component wedelolactone on pre-osteoclastic proliferation and differentiation. J Ethnopharmacol 157:206–211. doi:10.1016/j.jep.2014.09.033
Kook M, Lee SK, Kim SD, Lee HY, Hwang JS, Choi YW, Bae YS (2015) Anti-septic activity of alpha-cubebenoate isolated from Schisandra Chinensis. BMB Rep 48(6):336–341
Lee KP, Kang S, Park SJ, Kim JM, Lee JM, Lee AY, Chung HY, Choi YW, Lee YG, Im DS (2015) Anti-allergic effect of alpha-cubebenoate isolated from Schisandra Chinensis using in vivo and in vitro experiments. J Ethnopharmacol 173:361–369. doi:10.1016/j.jep.2015.07.049
Li W, XN Q, Han Y, Zheng SW, Wang J, Wang YP (2015) Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra Chinensis on alcoholic liver oxidative injury in mice. Int J Mol Sci 16(2):2446–2457. doi:10.3390/ijms16022446
Kim MH, Choi YY, Han JM, Lee HS, Hong SB, Lee SG, Yang WM (2014) Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)-alpha/−beta. Food & Function 5(7):1594–1601. doi:10.1039/c4fo00133h
He Y, Zhang Q, Shen Y, Chen X, Zhou F, Peng D (2014) Schisantherin a suppresses osteoclast formation and wear particle-induced osteolysis via modulating RANKL signaling pathways. Biochem Biophys Res Commun 449(3):344–350. doi:10.1016/j.bbrc.2014.05.034
Caichompoo W, Zhang QY, Hou TT, Gao HJ, Qin LP, Zhou XJ (2009) Optimization of extraction and purification of active fractions from Schisandra Chinensis (Turcz.) and its osteoblastic proliferation stimulating activity. Phytotherapy Research : PTR 23(2):289–292. doi:10.1002/ptr.2585
Park CH, Noh JS, Tanaka T, Roh SS, Lee JC, Yokozawa T (2015) Polyphenol isolated from Corni fructus, 7-O-galloyl-D-sedoheptulose, modulates advanced glycation endproduct-related pathway in type 2 diabetic db/db mice. Arch Pharm Res 38(6):1270–1280. doi:10.1007/s12272-014-0457-7
Hong SY, Jeong WS, Jun M (2012) Protective effects of the key compounds isolated from Corni fructus against beta-amyloid-induced neurotoxicity in PC12 cells. Molecules (Basel, Switzerland) 17(9):10831–10845. doi:10.3390/molecules170910831
Zhang QC, Zhao Y, Bian HM (2013) Antiplatelet activity of a novel formula composed of malic acid, succinic acid and citric acid from Cornus officinalis fruit. Phytotherapy Research : PTR 27(12):1894–1896. doi:10.1002/ptr.4934
Meng QX, Wang BL, Yu P, Shan QQ, Mao ZH, Zhang F, Li J, Zhao TB (2015) Extract of Cornus officinalis SIEB ameliorates osteoporosis in spinal cord-injured rats. Chin. J Osteoporos 21(5):627–633
Li P, Li J, Wang HM (2007) Effect of cornel total glycoside on bone metabolism and bone density in caponized rats. Tianjin. J Tradit Chin Med 24(4):315–317
Li J, JY W, Wang HM, Bai RX, SL Y, Li P (2010) Effects of cornel Total Glycocide on bone morphological metrology in ovariectomized rats. Shanghai. J Tradit Chin Med 1:69–72
Sun H, Li L, Zhang A, Zhang N, Lv H, Sun W, Wang X (2013) Protective effects of sweroside on human MG-63 cells and rat osteoblasts. Fitoterapia 84:174–179. doi:10.1016/j.fitote.2012.11.010
Ko JH, Kwon HS, Yoon JM, Yoo JS, Jang HS, Kim JY, Yeon SW, Kang JH (2015) Effects of Polygonatum Sibiricum rhizome ethanol extract in high-fat diet-fed mice. Pharm Biol 53(4):563–570. doi:10.3109/13880209.2014.932393
Wang J, CS L, Liu DY, YT X, Zhu Y, HH W (2016) Constituents from Polygonatum Sibiricum and their inhibitions on the formation of advanced glycosylation end products. J Asian Nat Prod Res 18(7):697–704. doi:10.1080/10286020.2015.1135905
Yang JX, Wu S, Huang XL, Hu XQ, Zhang Y (2015) Hypolipidemic activity and Antiatherosclerotic effect of polysaccharide of Polygonatum Sibiricum in rabbit model and related cellular mechanisms. Evidence-Based Complementary and Alternative Medicine : eCAM 2015:391065. doi:10.1155/2015/391065
Zeng GF, Zhang ZY, Lu L, Xiao DQ, Xiong CX, Zhao YX, Zong SH (2011) Protective effects of Polygonatum Sibiricum polysaccharide on ovariectomy-induced bone loss in rats. J Ethnopharmacol 136(1):224–229. doi:10.1016/j.jep.2011.04.049
Zong S, Zeng G, Zou B, Li K, Fang Y, Lu L, Xiao D, Zhang Z (2015) Effects of Polygonatum Sibiricum polysaccharide on the osteogenic differentiation of bone mesenchymal stem cells in mice. International Journal of Clinical and Experimental Pathology 8(6):6169–6180
Begum S, LJ G, Lee MR, Li Z, Li JJ, Hossain MJ, Wang YB, Sung CK (2015) In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice. Pharm Biol 53(8):1098–1103. doi:10.3109/13880209.2014.959614
Shen B, Truong J, Helliwell R, Govindaraghavan S, Sucher NJ (2013) An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Complement Altern Med 13(373). doi:10.1186/1472-6882-13-373
Lee SV, Choi KH, Choi YW, Hong JW, Baek JU, Choi BT, Shin HK (2014) Hexane extracts of Polygonum multiflorum improve tissue and functional outcome following focal cerebral ischemia in mice. Mol Med Rep 9(4):1415–1421. doi:10.3892/mmr.2014.1943
Zhang HX, Yin ZW, Li FF, Shi ZX, Li E (2006) The effect of polygonam multififloruma extract on ovariotomized rat’ s bone dynamic change. Joumal of China-Japan Friendship Hospital 20(4):217–221
Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J (2012) Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol 689(1–3):31–37. doi:10.1016/j.ejphar.2012.05.045
Liu C, Li J, Meng FY, Liang SX, Deng R, Li CK, Pong NH, Lau CP, Cheng SW, Ye JY, Chen JL, Yang ST, Yan H, Chen S, Chong BH, Yang M (2010) Polysaccharides from the root of Angelica Sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement Altern Med 10(79). doi:10.1186/1472-6882-10-79
Wang Q, Huang Y, Qin C, Liang M, Mao X, Li S, Zou Y, Jia W, Li H, Ma CW, Huang Z (2016) Bioactive peptides from Angelica Sinensis protein Hydrolyzate delay senescence in Caenorhabditis elegans through antioxidant activities. Oxidative Med Cell Longev 2016:8956981. doi:10.1155/2016/8956981
Kuang X, Yao Y, JR D, Liu YX, Wang CY, Qian ZM (2006) Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res 1102(1):145–153. doi:10.1016/j.brainres.2006.04.110
Duan MH, Wang LN, Jiang YH, Pei YY, Guan DD, Qiu ZD (2016) Angelica Sinensis reduced Abeta-induced memory impairment in rats. J Drug Target 24(4):340–347. doi:10.3109/1061186x.2015.1077848
Lim DW, Kim YT (2014) Anti-osteoporotic effects of Angelica Sinensis (Oliv.) Diels extract on ovariectomized rats and its oral toxicity in rats. Nutrients 6(10):4362–4372. doi:10.3390/nu6104362
Choi KO, Lee I, Paik SY, Kim DE, Lim JD, Kang WS, Ko S (2012) Ultrafine Angelica Gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: particle size effect. J Med Food 15(10):863–872. doi:10.1089/jmf.2011.2047
Kim KJ, Yeon JT, Choi SW, Moon SH, Ryu BJ, Yu R, Park SJ, Kim SH, Son YJ (2015) Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre-osteoclasts. Bone 81:208–216. doi:10.1016/j.bone.2015.07.023
Kong L, Zhao Q, Wang X, Zhu J, Hao D, Yang C (2014) Angelica Sinensis extract inhibits RANKL-mediated osteoclastogenesis by down-regulated the expression of NFATc1 in mouse bone marrow cells. BMC Complement Altern Med 14(481). doi:10.1186/1472-6882-14-481
Ahn SJ, Baek JM, Cheon YH, Park SH, Lee MS, Oh J, Kim JY (2015) The inhibitory effect of Angelica Tenuissima water extract on receptor activator of nuclear factor-kappa-B ligand-induced osteoclast differentiation and bone resorbing activity of mature osteoclasts. The American Journal of Chinese Medicine 43(4):715–729. doi:10.1142/s0192415x15500445
Yao W, Gu H, Zhu J, Barding G, Cheng H, Bao B, Zhang L, Ding A, Li W (2014) Integrated plasma and urine metabolomics coupled with HPLC/QTOF-MS and chemometric analysis on potential biomarkers in liver injury and hepatoprotective effects of Er-Zhi-wan. Anal Bioanal Chem 406(28):7367–7378. doi:10.1007/s00216-014-8169-x
Xu H, ZR S, Huang W, Choi RC, Zheng YZ, Lau DT, Dong TT, Wang ZT, Tsim KW (2012) Er Zhi wan, an ancient herbal decoction for woman menopausal syndrome, activates the estrogenic response in cultured MCF-7 cells: an evaluation of compatibility in defining the optimized preparation method. J Ethnopharmacol 143(1):109–115. doi:10.1016/j.jep.2012.06.009
Shang GB, Zeng LP, Zhao Y, Zhang QY, Dong W, Tang XL, GL X, Zhu WF, Liu HN (2013) Effect of Erzhi pills on expressions of VEGF and MMP- 9 in induced rat. Breast Cancer 19(13):270–273
Cheng M, Wang Q, Fan Y, Liu X, Wang L, Xie R, Ho CC, Sun W (2011) A traditional Chinese herbal preparation, Er-Zhi-wan, prevent ovariectomy-induced osteoporosis in rats. J Ethnopharmacol 138(2):279–285. doi:10.1016/j.jep.2011.09.030
Sun W, Wang YQ, Yan Q, Lu R, Shi B (2014) Effects of Er-Zhi-wan on microarchitecture and regulation of Wnt/beta-catenin signaling pathway in alveolar bone of ovariectomized rats. Journal of Huazhong University of Science and Technology Medical sciences Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban Huazhong keji daxue xuebao Yixue Yingdewen ban 34(1):114–119. doi:10.1007/s11596-014-1241-0
Zhang H, Xing WW, Li YS, Zhu Z, JZ W, Zhang QY, Zhang W, Qin LP (2008) Effects of a traditional Chinese herbal preparation on osteoblasts and osteoclasts. Maturitas 61(4):334–339. doi:10.1016/j.maturitas.2008.09.023
Chang PJ, Lin CC, Chen YC, Chuang CH, Tseng YC, Hsieh WS, Lin SJ, Chen PC (2013) Use of herbal dietary supplement si-wu-tang and health-related quality of life in postpartum women: a population-based correlational study. Evidence-based Complementary and Alternative Medicine : eCAM 2013:790474. doi:10.1155/2013/790474
Yeh LL, Liu JY, Lin KS, Liu YS, Chiou JM, Liang KY, Tsai TF, Wang LH, Chen CT, Huang CY (2007) A randomised placebo-controlled trial of a traditional Chinese herbal formula in the treatment of primary dysmenorrhoea. PLoS One 2(8):e719. doi:10.1371/journal.pone.0000719
Liu L, Ma H, Tang Y, Chen W, Lu Y, Guo J, Duan JA (2012) Discovery of estrogen receptor alpha modulators from natural compounds in Si-Wu-tang series decoctions using estrogen-responsive MCF-7 breast cancer cells. Bioorg Med Chem Lett 22(1):154–163. doi:10.1016/j.bmcl.2011.11.041
Zhang Y, Li A, Peng W, Sun J, Xu F, Xu J (2015) Efficient inhibition of growth of metastatic cancer cells after resection of primary colorectal cancer by soluble Flt-1. Tumour biology : The journal of the International Society for Oncodevelopmental Biology and Medicine 36(10):7399–7407. doi:10.1007/s13277-015-3434-y
CM W, Chen PC, Li TM, Fong YC, Tang CH (2013) Si-Wu-tang extract stimulates bone formation through PI3K/Akt/NF-kappaB signaling pathways in osteoblasts. BMC Complement Altern Med 13(277). doi:10.1186/1472-6882-13-277
Zheng L, Liu H, Gong Y, Meng X, Jiang R, Wang X, Wang Q, Wang Y (2015) Effect of Liuweidihuang pill and Jinkuishenqi pill on inhibition of spontaneous breast carcinoma growth in mice. Journal of traditional Chinese medicine Chung i tsa chih ying wen pan / sponsored by All-China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine 35(4):453–459
Lee KS, Lim BV, Chang HK, Yang HY, Bahn GH, Paik EK, Park JH, Kim CJ (2005) Liuweidihuang-tang improves spatial memory function and increases neurogenesis in the dentate gyrus in rats. Fitoterapia 76(6):514–519. doi:10.1016/j.fitote.2005.04.022
Qian Y, Xue YM, Li J, Zhu B, Pan YH, Zhang Y (2010) Effect of Liuweidihuang pills in preventing diabetes mellitus in OLETF rats. Nan fang yi ke da xue xue bao Journal of Southern Medical University 30(1):21–24
Xia B, Xu B, Sun Y, Xiao L, Pan J, Jin H, Tong P (2014) The effects of Liuwei Dihuang on canonical Wnt/beta-catenin signaling pathway in osteoporosis. J Ethnopharmacol 153(1):133–141. doi:10.1016/j.jep.2014.01.040
Li M, Wang W, Wang P, Yang K, Sun H, Wang X (2010) The pharmacological effects of morroniside and loganin isolated from Liuweidihuang wan, on MC3T3-E1 cells. Molecules (Basel, Switzerland) 15(10):7403–7414. doi:10.3390/molecules15107403
Wang Y, Feng Q, Niu X, Liu X, Xu K, Yang X, Wang H, Li Q (2014) The therapeutic effect of zuogui wan in gestational diabetes mellitus rats. Journal of Analytical Methods in Chemistry 2014:737961. doi:10.1155/2014/737961
Kou S, Zheng Q, Wang Y, Zhao H, Zhang Q, Li M, Qi F, Fang L, Liu L, Ouyang J, Zhao H, Wang L (2014) Zuo-Gui and you-Gui pills, two traditional Chinese herbal formulas, downregulated the expression of NogoA, NgR, and RhoA in rats with experimental autoimmune encephalomyelitis. J Ethnopharmacol 158(Pt A):102–112. doi:10.1016/j.jep.2014.10.007
Ju DH, Wu P, Jia HW, Yu MZ (2003) Effect of zuogui pill on the content of bone Gla -containing protein and calcitonim in ovariectomy-induced osteoporosis rats. Chinese journal of information on traditional. Chin Med 10(1):16–17
Lv HB, Ren YL, Wang Y, Zhao JR, Liu LP, Ma XD (2010) Experimental research of the preventive and therapeutic effect of zuogui pill on ovariectomized rats. Chin. J Osteoporos 16(11):847–850
Ren YL, Li YL, Lv HB, Liu LP, Zhao JR, Wang LC (2012) Expressions of TGF-β1 /Smad4 mRNA of kidney by zuogui wan in ovariectomy-induced osteoporosis rats. Chinese Journal of Experimental Traditional Medical Formulae 18(10):190–194
Liu MJ, Pan JH, Li Y, Liu H, Teng JR, Wang SJ, Zhang Y, Du ZP, Yu Z, Ju DH (2011) Effects of Zuoguiwan on BGP and IGF-I in serum of glucocorticoid-induced osteoporosis rats. Chinese Journal of Experimental Traditional Medical Formulae 17(16):133–136
Li HH, DH J, Teng JR, Li Y, Wang SJ, Pan JH, Yu Z, Liu MJ (2011) Effects of zuogui pill on E2 and PTH in serum of glucocorticoid-induced osteoporosis rats. Chinese journal of basic medicine in traditional. Chin Med 17(7):744–745
Liu Y, Liu Y, Zhang R, Wang X, Huang F, Yan Z, Nie M, Huang J, Wang Y, Wang Y, Chen L, Yin L, He B, Deng Z (2014) All-trans retinoic acid modulates bone morphogenic protein 9-induced osteogenesis and adipogenesis of preadipocytes through BMP/Smad and Wnt/beta-catenin signaling pathways. Int J Biochem Cell Biol 47:47–56. doi:10.1016/j.biocel.2013.11.018
van Zoelen EJ, Duarte I, Hendriks JM, van der Woning SP (2016) TGFbeta-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: identification of drug targets for prevention of fat cell differentiation. Stem Cell Research & Therapy 7(1):123. doi:10.1186/s13287-016-0375-3
Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ (2015) PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol 11(12):712–724. doi:10.1038/nrendo.2015.139
Fan JZ, Yang L, Meng GL, Lin YS, Wei BY, Fan J, HM H, Liu YW, Chen S, Zhang JK, He QZ, Luo ZJ, Liu J (2014) Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway. Mol Cell Biochem 392(1–2):85–93. doi:10.1007/s11010-014-2021-7
Acknowledgements
Jian-Bo He conceived the idea and wrote the manuscript paper. Ding-Kun Lin supervised the research and contributed to the final draft of the paper. Mei-Hui Chen collected the relevant literature. We thank Xiao-Juan Li and Shu-Dong Chen for the help of this manuscript. All authors reviewed and approved the final manuscript. This work was supported by National Natural Science Foundation of China (no. 81673992), and National Natural Science Foundation of China (no. 81273782).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Jian-Bo He, Ding-Kun Lin and Mei-Hui Chen declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
He, JB., Chen, MH. & Lin, DK. New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch Osteoporos 12, 14 (2017). https://doi.org/10.1007/s11657-016-0301-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-016-0301-4