Abstract
Summary
There is a paucity of normative bone mineral density (BMD) data in healthy African women. Baseline total hip and lumbar spine BMD was measured in premenopausal women. BMD distribution was comparable to that of a reference population and was impacted by several factors including contraception and duration of lactation.
Introduction
Normative data on bone mineral density (BMD) and the cumulative impact of lactation, contraceptive use, and other factors on BMD in healthy African women have not been well studied.
Objectives
The objective of this study was to determine the factors associated with BMD in healthy premenopausal women in Uganda and Zimbabwe.
Methods
Baseline total hip (TH) and lumbar spine (LS) BMD was measured by dual x-ray absorptiometry in 518 healthy, premenopausal black women enrolling in VOICE, an HIV-1 chemoprevention trial, at sites in Uganda and Zimbabwe. Contraceptive and lactation histories, physical activity assessment, calcium intake, and serum vitamin D levels were assessed. Independent factors associated with BMD were identified using an analysis of covariance model.
Results
The study enrolled 331 women from Zimbabwe and 187 women from Uganda. Median age was 29 years (IQR 25, 32) and median body mass index (BMI) was 24.8 kg/m2 (IQR 22.2, 28.6). In univariate analyses, lower TH BMD values were associated with residence in Uganda (p < 0.001), lower BMI (p < 0.001), and any use of and duration of depot-medroxyprogresterone acetate. Use of oral contraceptives, progestin-only implants, and higher physical activity levels were protective against reduced BMD. Similarly, lower LS BMD values were associated with these same factors but also higher parity and history of breastfeeding. In a multivariable analysis, lower TH and LS BMD values were associated with enrollment in Uganda, lower BMI, and lower physical activity level; contraceptive use was associated with lower spine BMD, and breastfeeding contributed to lower total hip BMD.
Conclusions
Among healthy premenopausal women, TH and LS BMD was higher in Zimbabwe than Uganda. Additional factors independently associated with BMD included BMI, physical activity level, contraceptive use, and lactation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthy women generally achieve peak bone mass at approximately 30 years of age, though the process of bone accumulation may continue slowly until 50 years of age [1–3]. Factors traditionally associated with lower bone mineral density (BMD) include pregnancy, lactation, low body mass index, use of depot-medroxyprogesterone acetate (DMPA) for contraception, low physical activity (PA), low socioeconomic status, and a sedentary lifestyle [4–11]. Environmental, nutritional, and genetic factors have also been shown to contribute to variations in bone metabolism [2, 10].
Nearly all prior studies establishing normative BMD levels for various racial and ethnic subpopulations have been conducted in middle- to high-resource settings. Petitti et al. explored the association of hormonal contraception and BMD among women, aged 30 to 34 years, in an international cross-sectional study conducted at family planning clinics [6]. They found that out of a total of 2474 participants, 78 women from Zimbabwe had higher BMD than those in other parts of the world, but normative reference levels for this subpopulation have not been established. In their study evaluating the impact of daily oral tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) for HIV prevention on BMD among healthy, young heterosexual men and women in Botswana, Kasonde et al. [9], demonstrated a higher than previously expected prevalence of low BMD (defined as a Z-score of more than 2.0 standard deviations below the mean at any anatomic site; hip, spine, or forearm) of 6.8 % (CI 3.4–11.0). Prevalence of low baseline BMD was higher in men compared with women (11.3 versus 2.6 %, p = 0.02.) It was not clear whether the high prevalence of low BMD was due to biological factors or whether it arose from use of reference ranges that were not appropriate for the population under study.
Chantler et al. found black South African women to have higher femoral neck and total hip but lower lumbar spine BMD compared with white South African women [5]. In that study, racial differences in BMD were attributed to body fat composition, diet, socioeconomic status, contraceptive use, physical activity, and lifestyle (smoking, alcohol consumption). Black South African women were found to be less involved in vigorous physical activity, consume high dietary fat, have lower calcium (Ca) intake, and were four times more likely to use injectable contraceptives compared with white South Africa women [5]. Additionally, factors affecting BMD differed by ethnic group within South Africa and may also differ across different ethnic black populations elsewhere in sub-Saharan Africa (SSA) [5, 12, 13].
Numerous factors may contribute to differences in BMD among black African women, including genetics, nutrition, socioeconomic status, lifestyle, contraceptive use, parity, and lactation. Gene polymorphisms affecting peak bone mass and the rate of adult bone loss may contribute to the differences in BMD between ethnic groups after adjusting for predefined variables [12, 14]. In most studies, variability is only partially explained by measureable factors, and some studies have even failed to explain the ethnic/racial differences in BMD [10].
The majority of SSA women have access to modern methods of contraception, and injectable contraceptives (most commonly DMPA) account for approximately 23 and 57 % of contraceptive methods ever used by women in Zimbabwe and Uganda, respectively [15]. The SSA region still has a significant unmet need for contraception among 25 % of women overall, 13 % in Zimbabwe, and 38 % in Uganda [16]. Women in SSA continue to have relatively high fertility rates averaging 4.94 births per woman, ranging from 3.22 in Zimbabwe, 4.68 in Kenya, and 6.05 in Uganda to 6.28 in Zambia [16]. Most policies in the region set optimal infant feeding duration at extended lactation periods of 12 to 24 months, and the SSA region has made great strides at attaining optimal infant feeding practices.
In view of the possible negative effect on BMD of the high rate of fertility, extended lactation, and frequent use of DMPA among SSA women, and the paucity of normative BMD data for this population, we evaluated baseline BMD among a subset of women in Zimbabwe and Uganda enrolled in the VOICE study, a large randomized trial of oral tenofovir disoproxil fumarate (TDF), oral tenofovir disoproxil fumarate-emtricitabine (TDF-FTC), and tenofovir 1 % vaginal gel for HIV-1 prevention [17].
Methods
Study population
From September 2009 through June 2012, 5029 women aged 18–45 years were enrolled in the VOICE study at 15 sites in South Africa (Durban, Johannesburg, Klerksdorp,) Uganda (Kampala), and Zimbabwe (Harare, Chitungwiza) [17]. A total of 952 heterosexual women at risk for HIV were identified from target populations of women of child-bearing age 15–49 years in Chitungwiza, Harare, and Kampala through community sensitization, snow-balling, self-referral, health facility-based referral, door-to-door recruitment, and night recruitment strategies. Eligible participants were HIV-negative, sexually active, had normal urinalysis, and no laboratory evidence of hepatic, renal, or hematological disease. Use of an effective method of contraception provided at the study site at enrollment was an additional inclusion criterion. Exclusion criteria were current or recent pregnancy or current breastfeeding, any history of non-traumatic bone fracture, current injection drug use, chronic hepatitis B, any ongoing medical condition known to affect bone (e.g., hyperparathyroidism, bone cancer), or taking any medication known to affect bone (e.g., glucocorticoids, heparin, warfarin, cyclosporine, cytotoxic drugs, and thyroid hormone). All women randomly assigned to receive oral TDF, TDF-FTC, or placebo in the VOICE trial at the four research clinics in Uganda and Zimbabwe were offered participation in the bone mineral density substudy.
Ethical approval and study oversight
The study was reviewed and approved by the ethics committees and institutional review boards at each site (ClinicalTrials.gov Identifier NCT00729573). Each participant provided a written informed consent prior to taking part in study procedures. Study oversight was provided by the US National Institutes of Health (NIH).
Study procedures
Self-reported contraceptive and lactation history was collected for all study participants using an interviewer-administered questionnaire. Anthropometric measurements (height and weight) were recorded using standardized procedures. Dietary calcium was estimated using an abbreviated food frequency questionnaire tailored to calcium-rich foods commonly available in the research communities. Site staff used plastic models of common food items to determine intake of various food groups.
The physical activity (PA) assessment recorded time spent engaging in daily vigorous activity including walking for travel and load bearing on back and head, and was estimated using the International Physical Activity Questionnaires (IPAQ) short form (August 2002; available at www.ipaq.ki.se) [18].
Baseline bone mineral density (BMD) of the lumbar spine (LS) and total hip (TH) were measured at study entry, within 14 days of first exposure to oral TDF, TDF-FTC, or placebo, by dual-energy x-ray absorptiometry (DXA) using identical densitometers at the two sites (Hologic Explorer, Bedford, MA; software version 2.3.2). Site staff were trained to conduct scans by Vertec (SA, Third Party Support Group) personnel, and analysis was monitored by a technician and physician trained and certified by the International Society of Clinical Densitometry (ISCD) from the Osteoporosis Research Center at the University of Pittsburgh. In order to standardize DXA scans across the two densitometers and different operators, sites used a common phantom to calibrate the DXA scanners. In addition, each site performed daily quality control checks. Scans were read locally by trained staff then reviewed centrally at the Osteoporosis Research Center of the University of Pittsburgh; any scans requiring correction were sent back electronically to replace the original scan. DXA scans were performed in duplicate to reduce measurement errors. For the LS, L1–L4 were analyzed to improve precision. BMD for each participant was the arithmetic mean of the two DXA scans. The mean difference (standard deviation, SD) in BMD between the two scans was 4.0 × 10−4 (0.012) g/cm2 for LS and 2.1 × 10−4 (0.016) g/cm2 for TH. T- and Z-scores were derived from the National Health and Nutrition Examination Survey (NHANES) III reference database for total hip and lumbar spine measurements in Caucasian women aged 20–29 years [19].
25′hydroxy-vitamin D was measured from stored sera collected at the baseline visit at the University of Pittsburgh Medical Center Presbyterian Hospital laboratory by mass spectrometry; results were categorized as: deficiency (0–19 mg/dl), insufficiency (20–29 mg/dl), or sufficiency (>30 mg/dl).
Statistical analysis
Demographic characteristics and other factors of interest were summarized descriptively. Distributions of these factors for each site were compared and p values were provided from chi-squared test for categorical variables and from Wilcoxon two-sample test for continuous variables. Independent factors associated with baseline BMD were identified using an analysis of covariance model. Additionally, logistic regression models were used to identify independent factors associated with low T-scores (<−1.0) for BMD. Factors with a p value of 0.20 or less in univariate modeling were entered into a multivariable model. Thus, parameter estimates (ANCOVA) and odds ratios (logistic) reflect adjustment for all co-factors deemed relevant by univariate analysis, even if the co-factors were ultimately non-significant in the multivariable model. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics
Among the 576 HIV-negative, premenopausal women randomized to the oral arms at these four sites, 518 (90 %) were enrolled into the bone density substudy: 187 in Uganda and 331 in Zimbabwe. The 58 women who did not enroll included 20 women ineligible due to having >14 days elapsed since randomization in VOICE, one pregnant, and 37 declined enrollment. The majority of the 37 women who declined to enroll were in Zimbabwe, and the major reason was the long distance to the study DXA scanner. The characteristics of the study population are summarized in Table 1. There were no significant differences in the baseline characteristics for the women who did and did not enroll (data not shown). The median age of the study population was 29 years, the median parity was 2.0 and the median body mass index (BMI) was 24.8 kg/m2. Nearly all participants (98 %) had a history of breastfeeding with a cumulative duration of >5 years (30 %), 2–5 years (49 %), and <2 years (21 %). The elapsed time from cessation of breastfeeding to BMD measurement was >12 months for 80 % of the participants.
Current or prior use of hormonal contraceptives was common: 71 % of women reported any history of DMPA use, 81 % reported any history of combined oral contraceptive pill (COCP) use, and 27 % reported any history of progestin-only implant use. The median serum vitamin D concentration was 30 ng/mL, and 96 % of participants had a level ≥20 ng/mL.
Significant differences in demographic characteristics were observed between the participants in the two countries (Table 1). Zimbabwean women had a lower parity, had higher educational attainment, were more likely to be married, and less likely to have consumed alcohol in the 3 months prior to enrollment compared with their Ugandan counterparts. A majority of Ugandan women (73.8 %) earned their own income compared with less than half of the Zimbabwean women.
The participants from Zimbabwe were more likely to have used oral contraceptive and contraceptive implants whereas Ugandan women were more likely to have used DMPA. Since the use of an effective contraception was required for participation in the VOICE trial, some women had short durations of use for their current method at enrollment, particularly for implants. The median duration of implant use among the 139 women who reported use was 0.5 months (interquartile range [IQR] 0, 11). The total duration of DMPA use ranged from 0 to 180 months with a median of 3 months (median 12 months among those indicating current DMPA use).
Bone mineral density
The median lumbar spine (LS) BMD was 0.97 g/cm2 (IQR 0.90, 1.04). The median total hip (TH) BMD was 0.96 g/cm2 (IQR 0.88, 1.03), and BMD at both the LS and TH was significantly higher in the women from Zimbabwe (1.00 and 0.98 g/cm2, respectively) compared to Uganda (0.93 and 0.93 g/cm2, respectively) both with p < 0.0001 as shown in Table 1.
Correlates of BMD
In univariate analyses, lower TH BMD was associated with enrollment in Uganda (p < 0.001), lower BMI (p < 0.001), lower categorical physical activity (p = 0.01), and less education (p < 0.001). History of and duration of DMPA use were associated with lower TH BMD whereas use of oral contraceptives (COCP) or contraceptive implants was associated with higher BMD. Age, parity, history and duration of breastfeeding, and calcium intake were not associated with TH BMD, but serum vitamin D concentration was significantly associated with higher TH BMD (p = 0.04) (Table 2).
Similarly, lower LS BMD was associated with enrollment in Uganda (p < 0.001), lower BMI (p < 0.001), and lower categorical PA (p = 0.016), but also with higher parity (p = 0.04), less education (p < 0.001), and longer duration of breastfeeding (p = 0.02). For the LS, history and duration of use of DMPA were associated with lower BMD; and history and duration of use of COCP and contraceptive implants were associated with higher BMD (Table 2). Neither calcium intake nor vitamin D level was associated with LS BMD.
In a multivariable analysis, enrollment in Uganda, lower BMI, low or moderate level (versus high) of physical activity, and a history of ever breastfeeding were significantly associated with lower TH BMD (Table 3). A trend was detected for the association of longer duration of DMPA with lower TH BMD (p = 0.08). Parity, age, use of contraceptive implants, duration of lactation, and vitamin D level were not significant (p > 0.05). For the LS, enrollment in Uganda, lower BMI, longer duration of DMPA, shorter duration of implant use, lower levels of PA, and cumulative duration of breastfeeding (>5 years) were independently associated with lower BMD.
Country-specific analyses
In country-specific analyses, participants from Zimbabwe were similar to the overall group with the factors associated with lower TH BMD including lower BMI, longer duration of DMPA, low or moderate physical activity (versus high), and history of breastfeeding. The same factors were associated with lower LS BMD; additionally, longer duration of implant use was protective. For Ugandan women, however, the multivariable model demonstrated that only lower BMI and shorter duration of implant use were associated with lower TH BMD. These same factors plus less education were independently associated with lower LS BMD in Uganda.
Factors associated with low T-score (<−1.0)
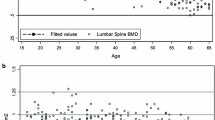
Overall, the proportions of women with T-score between −1 and −2.5 were 35.3 % for LS and 9.7 % for TH (Fig. 1). The proportion of women with a T-score less than −2.5 was low: 1.5 % for LS and 0.2 % for TH. Compared with their Zimbabwean counterparts, participants from Uganda had a greater than twofold increased risk of having a baseline T-score of ≤1.0 for LS (p < 0.01). Distributions of Z-scores were similar to those of T-scores.
In univariate analysis for the LS, country of residence (Uganda), any history of DMPA use, and duration of DMPA use were significantly associated with a greater risk of having a baseline T-score of <−1.0 while duration of implant use and high (vs. moderate) PA levels significantly reduced the risk of having a low T-score. In multivariable analyses, enrollment in Uganda and longer duration of DMPA use remained significantly associated with a low baseline T-score while higher BMI, longer duration of implant use, and high (vs. moderate or low) PA levels were protective.
For the TH, univariate analysis identified that residence in Uganda was associated with a greater risk of T-score <−1.0 (OR 2.33; 95 % CI 1.30, 4.19) and higher BMI and physical activity level were protective; however, in the multivariable analysis, only BMI and the physical activity level (total MET-hours/week) were significantly associated with TH T-score <−1.0.
Discussion
In this large cross-sectional study of premenopausal black African women in Uganda and Zimbabwe, the factors associated with lower bone mineral density include Uganda as country of domicile, lower BMI, lower levels of physical activity, a lifetime cumulative duration of breastfeeding >5 years, and longer duration of use of injectable DMPA. To date, this study represents the largest report of bone mineral density in non-South African black African women. These data provide an important resource to define the normal ranges of BMD in healthy premenopausal African women.
The overall BMD measures for our study population were normally distributed. Compared to the reference population, the distribution of T-scores for the LS BMD was shifted to the lower range whereas for the TH, the distribution was nearly identical to the reference NHANES data [19]. Our results were similar to those of Kasonde et al. [9] who demonstrated comparable levels of baseline LS and TH BMD among 114 Batswana women.
Our findings are supported by prior studies that have shown that fracture incidence is lower in black South African women compared to their white counterparts and that blacks have increased cortical thickness [13]. It has also been previously shown that African-American women achieve 5–15 % greater peak bone mass, have higher BMD, and have lower fracture incidence than their white counterparts [10, 14].
In this study, higher BMD at both the LS and TH was significantly associated with higher levels of physical activity. Load carrying on the head, which is common in African women, was included in the physical activity assessment in our study. According to Lloyd et al., the effects of load-bearing depended on the weight carried as well as duration of load bearing. Load bearing was shown to have osteogenic effects on the spine although not on the hip [13]. Our finding of a left (lower) shift in the distribution of T-scores at the spine is consistent with the findings of Chantler et al. [5] comparing black and white South African women and suggests a racial or genetic difference in the relative density of the spine and hip.
The observed difference between the bone density of the women from Uganda and Zimbabwe was unanticipated. In fact, we speculated that Zimbabwean women would have a lower BMI and lower BMD but actually both BMI and BMD were higher in Zimbabwe, even after controlling for other factors like calcium intake, contraceptive use and duration, and physical activity. The differences in BMD between Zimbabwean women and women from Uganda were small but consistent. These small differences might be attributable to cohort effects such as ethnic differences; however, Zimbabweans and the population in the region of Uganda from which this study enrolled are both of Bantu origin, so most, if not all, participants were of Bantu origin. It is possible that there could be pockets of more recently derived gene polymorphisms which could impact body morphology and bone composition. Zimbabwean women were more likely to have used oral contraceptive and contraceptive implants compared with Ugandan women who were more likely to have used DMPA.
Extended lactation as well as both use and duration of use of DMPA were associated with lower BMD in this study, similar to the findings of Petitti et al. in their international cross-sectional study of the impact of contraception on BMD. In the study of Petitti et al. (which included 78 Zimbabwean women), multivariate analyses revealed that both recent and total duration of lactation and use of DMPA were associated with lower BMD [6]. Similarly, several other studies have confirmed the association of use of DMPA and lower BMD [7, 8, 11, 20]. We showed that longer duration of breastfeeding had a negative impact on LS BMD. Parity was found to be significantly associated with lower LS BMD in the univariate model but was no longer significant after controlling for other factors. We might have been unable to discern significant differences because most women had two or three children. This lower parity in the study population, as compared to the Zimbabwean and Ugandan fertility rate averages of 3.22 and 6.05, respectively, may have potentially introduced bias in the determination BMD by shifting the distribution to the right. Interestingly, Rahman et al. showed that never having been pregnant had a negative effect on BMD [21].
We found no correlation between age and BMD. The lack of correlation between age and BMD and parity and BMD is likely due to the narrow age range in our study with all participants being premenopausal and expected to have a stable bone mass.
There is a paucity of data on the effect of progestin implants on BMD, and the available data are inconsistent. Similar to results from two prior studies [22, 23], we found that any use of progestin implants was associated with higher bone density at the LS but had no association with TH BMD. In contrast, two other previous studies found no significant difference in BMD between users and non-users of progestin-only implants respectively [24, 25]. The duration of implant use could have a potential impact on the effect of progestin implants on BMD. Our current data are limited by the relatively short median duration of implant use; longitudinal data acquired during progestin implant use by this same cohort will be important to clarify any potential associations with BMD gain or loss.
Data on effects of oral contraceptives on BMD are also variable; with the number of previous studies that have shown an increase in BMD with oral contraceptive use being almost equal to that of prior studies that have shown no effect [26, 27]. This inconsistency in findings is possibly explained by the different study populations studied (age, ethnicity, sample size) skeletal site assessed and the type of oral contraception [6]. We found that COCP use was associated with higher BMD, but that this association was no longer significant in the multivariate model after controlling for other factors.
In our study population, there was no correlation between vitamin D levels and BMD after controlling for other factors. There is little data on the correlation between vitamin D levels and BMD in adults with most studies examining the effect of supplementary vitamin D on BMD. Assessment of vitamin D in previous studies has reported inconsistent findings, likely because of different reference ranges used by different authorities, varying assays, and seasonal effects. Similarly, we did not find any correlation between Ca intake and BMD. These inconsistent findings are not surprising given the interdependence of Ca, vitamin D, and other nutrients on BMD. As previously described, diets rich in Ca were shown to be high in vitamin D, protein, saturated fatty acids, magnesium, and phosphorus all of which can act as confounding variables [28].
Our study had several limitations which deserve to be mentioned. First and foremost, the dietary calcium information collected was approximate due to lack of a validated tool for local foods available at the sites. Second, the accuracy of the calcium intake may have been affected by translation of foods and difficulty in calculating quantities by participant self-report and recall bias. Recall bias may also have affected the validity of other variables such as physical activity, although a validated international survey tool was utilized. Third, some variables associated with BMD in prior studies such as caffeine, smoking, and detailed menstrual history (age at menarche) were not measured in our study. Fourth, despite our calibration procedures, it is possible that differences in the machine setup and calibration may have contributed to differences we observed. However, we followed similar procedures used by the standard clinical trials for across-site calibration. Finally as with all cross-sectional studies, this study is prone to ecological fallacy and causality cannot be inferred. Longitudinal follow-up of this group of women will provide additional data on the impact of these variables on changes in BMD.
This study also had several strengths. Bone mineral density was assessed by state-of-the-art measurements by dual x-ray absorptiometry that was carefully monitored and centrally reviewed. The large sample size of healthy premenopausal women residing in two separate countries in sub-Saharan Africa provides robust estimates of the normal range of bone mineral density and the major factors associated with BMD. This well-controlled set of measurements contributes to establishing normative values for bone density in these two countries for which little or no other data exists.
In conclusion, this large cross-sectional study of premenopausal black African women showed that the bone mineral density of healthy African women in Uganda and Zimbabwe was comparable to that of a US reference population. The factors associated with lower bone mineral density include Uganda as country of domicile, lower BMI, lower levels of physical activity, a lifetime cumulative duration of breastfeeding >5 years, and longer duration of use of injectable DMPA. As far as we know, to date, our study represents the largest report of bone mineral density in black African women outside of South Africa. These data provide an important resource to define the normal ranges of BMD in healthy premenopausal African women.
References
Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK et al (1994) Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest 93(2):799–808
Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N et al (2010) Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 25(9):1948–1957
IQ Solutions I. Osteoporosis: peak bone mass in women [Internet]. Available from: http://www.niams.nih.gov/Health_Info/Bone/Osteoporosis/bone_mass.asp. Accessed 28 Aug 2014
Karlsson C, Obrant KJ, Karlsson M (2001) Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 12(10):828–834
Chantler S, Dickie K, Goedecke JH, Levitt NS, Lambert EV, Evans J et al (2012) Site-specific differences in bone mineral density in black and white premenopausal South African women. Osteoporos Int 23(2):533–542
Petitti DB, Piaggio G, Mehta S, Cravioto MC, Meirik O (2000) Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO study of hormonal contraception and bone health. Obstet Gynecol 95(5):736–744
Cundy T, Cornish J, Evans MC, Roberts H, Reid IR (1994) Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ 308(6923):247–248
Banks E, Berrington A, Casabonne D (2001) Overview of the relationship between use of progestogen-only contraceptives and bone mineral density. BJOG Int J Obstet Gynaecol 108(12):1214–1221
Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, et al. (2014) Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS ONE 13;9(3):e90111
Ettinger B, Sidney S, Cummings SR, Libanati C (first), Bikle DD, Tekawa IS, et al. Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metabol 82(2): 429–434
Scholes D et al (2005) Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med 159:139–144
Nelson DA, Pettifor JM, Barondess DA, Cody DD, Uusi-Rasi K, Beck TJ (2004) Comparison of cross-sectional geometry of the proximal femur in white and black women from Detroit and Johannesburg. J Bone Miner Res 19(4):560–565
Lloyd R, Hind K, Micklesfield A, Lisa K, Sean C, Truscott JG, Parr B et al (2010) A pilot investigation of load-carrying on the head and bone mineral density in premenopausal, black African women. J Bone Miner Metab 28:185–190
Finkelstein JS, Lee M-LT, Sowers M, Ettinger B, Neer RM, Kelsey JL et al (2002) Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87(7):3057–3067
Cleland JG, Ndugwa RP, Zulu EM (2011) Family planning in sub-Saharan Africa: progress or stagnation? Bull World Health Organ 89(2):137–143
The World Bank (2011). Unmet need for contraception [Internet]. The World Bank. Available from: http://go.worldbank.org/PCDPQW7Y70. Accessed 28 Aug 2014
Marrazzo J et al (2015). Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med 372:509–518. doi:10.1056/NEJMoa1402269
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395
Kanis JA, Adachi JD, Cooper C, Clark P, Cummings SR, Diaz-Curiel M et al (2013) Standardising the descriptive epidemiology of osteoporosis: recommendations from the epidemiology and quality of life working group of IOF. Osteoporos Int 24(11):2763–2764
Kaunitz A, Miller P, Rice V, Ross D, McClung M (2006) Bone mineral density in women aged 25–35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 74(2):90–99
Rahman M, Berenson AB (2010) Predictors of higher bone mineral density loss and use of depot medroxyprogesterone acetate. Obstet Gynecol 115(1):35–40
Naessen T, Olsson SE, Gudmundson J (1995) Differential effects on bone density of progestogen-only methods for contraception in premenopausal women. Contraception 52(1):35–39
Di X, Li Y, Zhang C, Jiang J, Gu S (1999) Effects of levonorgestrel-releasing subdermal contraceptive implants on bone density and bone metabolism. Contraception 60(3):161–166
Beerthuizen R, van Beek A, Massai R, Mäkäräinen L, Hout J, Bennink HC (2000) Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod Oxf Engl 15(1):118–122
Díaz S, Reyes MV, Zepeda A, González GB, López JM, Campino C et al (1999) Norplant((R)) implants and progesterone vaginal rings do not affect maternal bone turnover and density during lactation and after weaning. Hum Reprod Oxf Engl 14(10):2499–2505
Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE (1995) Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception 51(4):221–224
Mehta S (1993) Bone loss, contraception and lactation. Acta Obstet Gynecol Scand 72(3):148–156
Holbrook TL, Barrett-Connor E (1991) Calcium intake: covariates and confounders. Am J Clin Nutr 53(3):741–744
Acknowledgments
We are grateful to the research participants for their participation in our study. We would also like to thank the Microbicide Trials Network (MTN)-003B Protocol Team, the MTN-003 (VOICE) protocol team, and the UZ-UCSF and MU-JHU research site teams for sample and data collection as well as SCHARP, our data management center. We acknowledge Karen B. Patterson for her dedication in quality control of the scans and other study data. This work would not have been possible without the support of staff of the Osteoporosis Research Center at the University of Pittsburgh.
Funding
The Microbicide Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Visit www.mtnstopshiv.org.
UZ-UCSF Clinical Trials Unit Grant Number - UM1 AI 0694361 I
MU-JHU Clinical Trials Unit Grant Number - UM1 AI069530
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Sources of Funding: NIAID (5UM1AI068633), NICHD, and NIMH, all of the US National Institutes of Health.
Rights and permissions
About this article
Cite this article
Mgodi, N.M., Kelly, C., Gati, B. et al. Factors associated with bone mineral density in healthy African women. Arch Osteoporos 10, 3 (2015). https://doi.org/10.1007/s11657-015-0206-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-015-0206-7