Abstract
Summary
We determined the prospective 10-year association among incident fragility fractures and four glucocorticoid (GC) treatment groups (Never GC, Prior GC, Baseline GC, and Ever GC). Results showed that GC treatment is associated with increased 10-year incident fracture risk in ambulatory men and women across Canada.
Purpose
Using the Canadian Multicentre Osteoporosis Study dataset, we determined the prospective 10-year association between incident fragility fractures and GC treatment.
Methods
We conducted a 10-year prospective observational cohort study at nine sites across Canada. A total of 9,263 ambulatory men and women 25 years of age and older were included in the analysis. Multivariable Cox proportional hazards analyses were conducted to determine the relationship among GC treatment groups in four levels that included Never GC, Prior GC, Baseline GC, and Ever GC (combined baseline and prior groups) and time to fracture.
Results
In each of the Never GC, Prior GC, Baseline GC, and Ever GC treatment groups, the number of participants were 8,832 (95.4 %), 303 (3.3 %), 128 (1.4 %), and 431 (4.7 %), respectively. Of the 9,263 individuals enrolled, incident fragility non-spine, hip, spine, and any fractures were experienced by a total of 896 (9.67 %), 157 (1.69 %), 130 (1.40 %), and 1,102 (11.90 %) over 10-years, respectively. For men and women combined, prior GC treatment was associated with a higher hazard ratio (HR) for time to incident non-vertebral (HR = 1.5, 95 % confidence interval [CI] = 1.1, 2.0), hip (HR = 2.1, 95 % CI = 1.1, 4.0), and any fracture (HR = 1.4, 95 % CI = 1.0, 1.8) compared with never GC treatment.
Conclusions
GC treatment is associated with increased 10-year incident fracture risk; this highlights the importance of considering therapy to prevent GC-induced fractures for patients who are using GC for various medical conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GC) are prescribed for a variety of inflammatory disorders including chronic obstructive pulmonary disease, rheumatologic and skin conditions [1], as well as for transplantation and some infectious diseases. GC therapy suppresses osteoblast function, increases bone resorption, decreases calcium gut absorption, and suppresses endogenous gonadal steroids, all of which lead to increased bone loss [2, 3]. Major adverse effects of prolonged GC use are decreased bone mineral density and increased fracture risk [4]. There is evidence that after GC initiation, bone loss occurs quickly within the first 3 to 12 months of beginning therapy and that the risk of GC-induced osteoporosis increases as the dose increases [4, 5]. However, low doses of GC (<2.5 mg/day) remain associated with increased fracture risk [5].
GC is considered as a major risk factor for new fractures in current osteoporosis clinical guidelines [6–8]. GC use is also utilized in fracture risk assessment tools in determining 10-year absolute fracture risk [9–12]. The fracture risk assessment tool (FRAX) developed by the World Health Organization, considers ever use (current or past) of GC as a part of the algorithm to calculate the 10-year fracture risk [9, 10]. In the model developed by the joint initiative of the Canadian Association of Radiologists and Osteoporosis Canada (CAROC), recent prolonged systemic GC use is considered to increase an individual’s 10-year fracture risk category by one level (i.e., from moderate to high) [11, 12].
To date, there have been limited prospective, population-based studies examining the relationship between the use of oral GC and the long-term risk of fracture. Utilizing the Canadian Osteoporosis Study (CaMos), a large randomly sampled, Canada-wide longitudinal study that includes men and women, our purpose was to ascertain any associations between incident clinical fractures and GC use over a 10-year period.
Methods
Study cohort
CaMos is a large, prospective population-based cohort of community dwelling adults that involves nine sites across Canada (St. John’s, Newfoundland and Labrador; Halifax, Nova Scotia; Québec City, Quebec; Kingston, Toronto, and Hamilton, Ontario; Saskatoon, Saskatchewan; Calgary, Alberta; and Vancouver, British Columbia). Details regarding the purpose and methodology have been published previously [13]. Briefly, adult CaMos participants ages 25 and older were recruited, beginning in 1995, from a randomly selected list of residential telephone numbers from all postal codes within 50 km of each study center. The sampling framework results in a population representing 40 % of the whole Canadian population. Informed consent was obtained from each individual and the study received approval by the institutional review boards at each participating center.
For the current study, only those participants with at least 1 year of prospective data were included in the analyses. The study includes 9,263 participants (2,819 men and 6,444 women).
Data sources
At study entry, an extensive interviewer-administered questionnaire was conducted in the following subject areas: sociodemographic and anthropometric, prescription and nonprescription medication use, dietary intake, medical and fracture history, family history of osteoporosis, and lifestyle data. The anthropometric and demographic characteristics were age, sex, height, weight, and educational status. Medications identified included ovarian hormone therapy, glucocorticoids, and bisphosphonates. Health-related habits included caffeine intake, alcohol intake, regular physical activity, and smoking status.
Glucocorticoid therapy
At baseline, related to their treatment with glucocorticoids, CaMos participants were divided into four groups. Group 1 consisted of individuals who were currently taking oral GC for any medical condition at the start of the study (baseline GC). The Baseline GC group, however, may have discontinued GC during the 10-year follow-up period. Group 2 included participants who reported previous GC therapy (Prior GC). Previous users are individuals who had to have been on GC daily for more than 1 month. Group 3 consisted of individuals who had never been on GC (never GC). Never users were defined as those individuals who were not included in the Baseline GC or Prior GC users groups. Group 4 included individuals in both the Baseline GC and Prior groups (Ever GC). The dose, type of medication, and precise duration of oral GC use were not available.
Fracture assessment: baseline and follow-up
Baseline, standardized radiographs of the thoracic and lumbar spine were obtained for participants ages 50 and older. Morphometric vertebral fractures at baseline were included in prevalent fractures. To identify morphometric fractures, radiographs were quantitatively examined for vertebral fractures using a digital graphics tablet. Vertebral bodies were examined by measuring the anterior, middle, and posterior heights of lateral thoracic and lumbar bodies on the radiographs. References norms for vertebral shape were determined from a subset of the CaMos population. Vertebral fractures were defined as a vertebral ratio at either the anterior, middle, or posterior heights, which is greater than three standard deviations below the normal group [14]. Baseline non-vertebral fractures were based on self-reports; prevalent non-vertebral fractures included fragility fractures (i.e., resulting from force of a fall from a standing height or less) and included hip, pelvis, ribs, forearm, and other sites (but excluded finger, toe, and face fractures). All participants with baseline vertebral morphometric and non-vertebral fractures were classified into the prevalent fracture category (yes/no).
The occurrence of clinically recognized incident, fragility fractures at non-spine, spine, hip, or other sites was determined over a period of 10 years based on a yearly postal questionnaire. Participants who reported experiencing a fracture during the intervening year were asked for consent to contact their treating physician or hospital for verification and for acquisition of further details. Non-vertebral fractures are as defined above. Incident vertebral fractures were clinically recognized vertebral fractures (based on self-reports and thus incident morphometric vertebral deformities were not included).
Statistical analysis
Continuous values are expressed as means and standard deviations (SD) and categorical values are expressed as counts and percentages. Two distinct GC variables were developed for this analyses: (1) included three categories: Baseline GC, Prior GC, and Never GC with the reference level being Never GC; (2) the other category included two levels: Ever GC (Baseline and Prior GC), and Never GC with Never GC as the reference. Chi-squared statistics were performed to determine differences in absolute 10-year incident clinical fragility fracture rates between Never GC versus Ever GC, and Baseline GC versus Prior GC groups. Multivariable Cox proportional hazards analyses were conducted to determine the relationship between GC use and time to fracture (at non-vertebral, spine, hip, or other sites). GC analyses were conducted separately for each GC variable for all participants combined and for men and women separately. We used the exact method to handle ties in the times-to-event analyses. For each model, we reported adjusted hazard ratios (HRs) and 95 % confidence intervals (CI). The analyses were adjusted for antiresorptive therapy (including any bisphosphonates, SERM, ovarian hormone therapy, or calcitonin) and categorized as yes/no, sex (men/women), prior fractures (yes/no), current smoking status (yes/no), alcohol intake (yes/no; >3 drinks/day), age (years), femoral neck bone mineral density T score, number of co-morbid conditions (including coronary artery disease or acute myocardial infarction, stroke, chronic obstructive lung disease, rheumatoid arthritis, liver disease, diabetes, kidney disease, breast cancer, uterine cancer, prostate cancer, and inflammatory bowel disease), and body mass index (kilogram per square meter). The covariates were selected a priori for their potential association with fractures and GC use. To assess the impact of individuals who were lost to follow-up prior to the 10-year follow-up period on the GC variables (Baseline GC, Prior GC, and Never GC), a sensitivity analysis was conducted for individuals who were followed for the entire 10-year period.
The proportionality assumption was assessed using martingale residuals. All statistical analyses were performed using the SAS/STAT (version 9.2; SAS Institute, Cary, NC, USA) software package running on Windows XP Professional. The criterion for statistical significance was set at α = 0.05.
Results
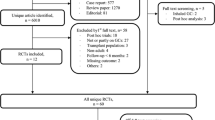
A total of 9,263 participants, 2,819 men and 6,444 women ages 25 and older, participated in this 10-year prospective study (Fig. 1). Most of the participants in the study (>95 %) had never used GC at baseline. Only 0.2 % of the men and 25 % of the women were on some form of antiresorptive therapy at the start of the study. Baseline participant characteristics are shown in detail in Table 1.
During the 10-year study period, more fractures occurred in those who reported treatment with GC as compared with the Never GC group. Fractures occurred for a total of 896 (9.67 %) of the cohort including non-vertebral fracture in 157 (1.69 %), hip fractures in 130 (1.40 %), and the total for any fractures was 1,102 (11.90 %) during the 10-year study period. The absolute fracture rate, for any fracture, was approximately 21 and 23 % of participants (men and women combined) for the Prior GC and Baseline GC groups compared with a rate of approximately 11 % in the Never GC group (Table 2).
Table 3 summarizes the multivariable Cox proportional hazards analyses expressed as adjusted hazard ratios and 95 % confidence intervals among those reporting Baseline GC, Prior GC, and never treatment with GC. Results showed that prior use of GC was associated with a higher hazard ratio for time to new fragility fracture at the non-spine, hip, and any fracture region compared with those who had never taken GC. Inconclusive results were found for Baseline GC users (all three groups and all fracture types) versus never GC users. The sensitivity analysis including only participants that completed all 10 years of follow-up showed similar results for the combined and men alone analyses. However, for women, prior use of GC was associated with a higher hazard ratio for time to new fragility fracture at the hip compared with those who had never taken GC (HR 2.95; 95 % CI: 1.31, 6.67). In our primary analysis (Table 3), the results were inconclusive for this comparison (HR = 1.91; 95 % CI: 0.91, 3.99).
Figure 2a, b, and c display the adjusted hazard ratio and 95 % confidence interval among GC status (two levels: Ever GC and Never GC) and time to each fracture type for the whole adult cohort, men and women, respectively. The ever GC group showed a higher rate of non-spine fracture and any fracture in the whole group (Fig. 1a). Inconclusive results were obtained for the hip and spine fractures in the combined group. In the analysis of men and women separately, significantly more non-spine fractures occurred in the Ever GC group as compared with the Never GC group (Fig. 2b and c).
Discussion
Our study is the first prospective population-based cohort study to examine the persistent effects of baseline or prior to baseline GC therapy on fracture risk. These data show that over 10 years, prior use of GC for a month or more significantly increased the risk of incident fragility fractures including those at the hip and at any site. This clinically meaningful increase in fracture risk in those who had previously been treated with GC (Prior GC) may indicate that an elevated fracture risk persists, although at a lower level, even after the discontinuation of GC. Although the exact determinants of elevated fracture risk in the prior GC group could not be determined from this study, the increased fracture risk may be due to deterioration of bone structure that cannot be repaired even with the discontinuation of GC therapy.
For Baseline GC users, although our results were inconclusive, the 10-year fracture hazard ratios were clinically relevant and similar to the results in the Prior GC group. In addition, in GC users, compared with those who had never been GC treated (Never GC), the absolute fracture rates for non-vertebral and all-fragility fractures were approximately doubled and for vertebral fractures increased about fourfold. Although current guidelines indicate that those taking glucocorticoids should be concurrently treated with bisphosphonates, they do not indicate that osteoporosis treatment is needed for previous GC use. The higher observed absolute fracture rate in those who had previously been GC treated is important given that osteoporotic fractures cause deterioration in patients’ quality of life and increase health care costs [15, 16]. Furthermore, vertebral fractures and hip fractures are associated with an increased risk of death in those over the age of 50 years [17]. Given the consequences of osteoporotic fractures, our study showed the importance of assessing and treating all of those who have previously been treated with GC. These results are similar to other research in different populations [5, 18–20].
The current analyses suggest that GC use is an important risk factor for future fracture risk regardless of whether a precise duration and dose of the therapy is known. This finding supports the FRAX and CAROC fracture risk assessment tools, which do not explicitly specify GC use by a threshold duration or dose [9–12]. Furthermore, our results showing a higher risk of incident fracture in the Ever GC group, as compared with the Never GC group, also further supports the FRAX model which includes the ever use of GC (defined as: patients who are currently exposed to oral GC or has been exposed to oral GC) as a risk factor for osteoporotic fracture. The confirmation of the association between past GC treatment and incident fractures is important given that the FRAX tool is used in 47 countries and the website receives approximately 35 million hits per year [19].
Although there have been clinically relevant associations with Baseline GC therapy and incident fragility fractures over a 10-year period, these results were inconclusive. This is contrary to several researchers who have showed that fracture risk increases rapidly after the start of oral GC therapy (within 3 to 6 months) [21–24]. It is possible that, if the Baseline GC group in our study discontinued the steroids after a few months of therapy, the individual’s fracture risk would also decrease and that this could have contributed to a non-significant increase in fracture risk. Another possible explanation for our finding is that only 128 participants were being treated with GC at baseline (n = 34 men, n = 94 women), and that these numbers may not have been large enough to detect statistically significant results.
Our study had several strengths that increase the generalizability of our findings. The study population was drawn from a randomly selected sample of men and women across Canada. The duration of the study was 10 years; the same time frame that is used in the CAROC and FRAX fracture risk assessment tools that are clinically used throughout Canada and the world. Finally, our study also adjusted for other medical conditions that can lead to changes in fracture risk allowing for a more precise estimate of the relationship between former GC use and incident fractures.
Nonetheless, this study has a few limitations. Very few men who were taking GC developed hip fractures, and there may have been an under reporting of vertebral fractures given that only incident clinically recognized vertebral fractures, and not morphometric fractures, were examined in this analysis over the 10-year study period. The small sample size in these groups may have contributed to the inconclusive results.
The doses and durations of GC therapy in our participants are not known and both amount and time on GC therapy relate to fracture risk [15–18]. Our study also included participants who only were treated with oral GC and did not use parenteral and higher dose forms of GC. Thus, the results from our study cannot be extrapolated to persons taking inhaled, intra-articular, pulse, or other forms of non-oral GC, nor can these data assess fractures and different oral GC formulations [25, 26]. Furthermore, individuals in the Baseline GC and Never GC group may have stopped or started GC after the baseline assessment. This may have reduced the association between GC and new fractures between the GC groups (causing inconclusive results). In addition, GC treatment and incident fracture assessment were based on self-reports and thus the reliability of the data may depend on a number of factors [27, 28]. While a majority of underlying diseases for which GC were prescribed were included in these analyses, we may not have included all potential diseases that may by independently associated with fracture. Finally, only ambulatory, community dwelling participants were enrolled in this study and thus, results cannot be generalized to those residing in long-term health facilities.
In conclusion, future prospective studies should aim to delineate the relationship of dose, duration, and delivery of GC therapy to incident fragility fracture risk. This study shows that past or baseline use of GC is associated with an increased 10-year incident fragility fracture risk and supports fracture assessment tools (FRAX, CAROC). In addition, the findings highlight the importance for physicians to consider therapy to prevent GC-related fractures for people who report previous GC treatment as well as those who now require GC therapy for a variety of medical conditions.
References
Van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fracture. J Bone Miner Res 15:993–1000
Chavassieux P, Pastoureau P, Chapuy MC, Delmas PD, Meunier PJ (1993) Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos int 2:97–102
Barbarino A, DeMarinis L, Folli G, Tofani A, Della Casa S, D’Amico C et al (1989) Corticotrophin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism 38:504–506
Van Staa TP, Leufkens HGM, Cooper C (2000) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 39(12):1383–1389
Papaioannou A, Morin S, Cheung A, Atkinson S, Brown JP, Feldman S et al (2010) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1872
Kanis, Burlet N, On behalf of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
National Osteoporosis Foundation (2008) Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation. www.nof.org. Accessed September 2013
Kanis JA on behalf of the World Health Organization Scientific Group (2008) assessment of osteoporosis at the primary health-care level. Technical report. Who collaborating Centre, University of Sheffield, UK. Accessed at www.shef.ac.uk/FRAX/. Accessed September 2013
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Siminoski K, Leslie W, Frame H, Hodsman A, Josse RG, Khan A et al (2005) Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J 56:178–188
Leslie WD, Berger C, Langsetmo L, Lix LM, Adachi JD, Hanley G et al (2011) Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: from the CaMos and Manitoba cohorts. Osteoporos Int 6:1873–1883
Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP et al (1999) The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging 18:376–387
Jackson SA, Tenenhouse A, Robertson L, CaMos Study Group (2000) Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos). Osteoporos Int 8:680–687
Tarride JE, Hopkins RB, Leslie WE, Morin S, Adachi JD, Papaioannou A et al (2012) The burden of illness of osteoporosis in Canada. Osteoporos Int 23:2591–25600
Adachi JD, Ioannidis G, Berge C, Joseph L, Papaioannou A, Pickard L et al (2001) The influence of osteoporotic fractures on Health related quality of life in community dwelling men and women across Canada. Osteoporos Int 12:903–908
Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pichard L et al (2009) Relation between fractures and mortality: results from the Canadian multicentre osteoporosis study. CMAJ 181:265–271
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ III et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Kanis JA, Johansson H, Oden A, McCloskey M (2011) Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 22:809–816
Steinbuch M, Youket TE, Cohen S (2004) Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int 15:323–328
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Salto JK, Davis JW, Wasnich RD, Ross PD (1995) Users of low-dose glucocorticoids have increases bone loss rates: a longitudinal study. Calcif Tissue Int 57:115–119
Adachi JD, Bensen WG, Bell MJ et al (1990) Corticosteroid induced osteoporosis: follow-up over 3 years. In: Christiansen C, Overgaard K (eds) Osteoporosis 1990 (3). Third International Symposium on Osteoporosis, Copenhagen, pp 1745–1747
Natsui K, Tanaka K, Suda M, Yasoda A, Sakuma Y, Ozasa A et al (2006) High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int 17:105–108
Gonnelli S, Caffarelli C, Maggi S, Guglielmi G, Siviero P, Rossi S et al (2010) EOLO study group. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int 87:137–147
Vestergaard P, Rejnmark L, Mosekilde L (2008) Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int 82:249–257
Cadarette SM, Jaglal SB, Raman-Wilms L, Beaton DE, Paterson JM (2011) Osteoporosis quality indicators using healthcare utilization data. Osteoporos Int 22:1335–1342
Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ et al (2004) Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause 11(3):264–274
Conflicts of interest
G. Ioannidis, S. Pallan, M. Mulgund, L. Rios, J. Ma, L. Thabane, K. S. Davison, C. S. Kovacs, N. Kreiger, J. C. Prior, and T. Towheed declare that they have no conflict of interest.
A. Papaioannou: Speaker's bureau: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi Aventis, Warner Chilcott. Research grants: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi Aventis, Warner Chilcott. Consulting fees or other remuneration (payment): Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth.
R. G. Josse: Advisory board member: Amgen, Lilly, Merck, Novartis, BMS/AZ, Janssen. Speaker honoraria: Amgen, Lilly, Merck, Novartis, BMS/AZ, Janssen. Research grants: Amgen, Janssen, BMS/AZ.
W. P. Olszynski: Consultant or on a speaker's bureau for Amgen, Eli Lilly, Merck Frosst Canada, Novartis, Pfizer, Procter & Gamble Pharmaceuticals and sanofi-aventis.
J. D. Adachi: Speaker's bureau: Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott. Research grants: Amgen, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth. Consulting fees or other remuneration (payment): Amgen, Eli Lilly, GSK, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi Aventis, Warner Chilcott, Wyeth.
Grant support
The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR), Amgen, Merck Frosst Canada Ltd., Eli Lilly Canada Inc., Novartis Pharmaceuticals Inc., The Alliance: sanofi-aventis and Procter and Gamble Pharmaceuticals Canada Inc., The Dairy Farmers of Canada, and The Arthritis Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ioannidis, G., Pallan, S., Papaioannou, A. et al. Glucocorticoids predict 10-year fragility fracture risk in a population-based ambulatory cohort of men and women: Canadian Multicentre Osteoporosis Study (CaMos). Arch Osteoporos 9, 169 (2014). https://doi.org/10.1007/s11657-013-0169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-013-0169-5