Abstract
Heavy use of chemical fertilizer causes increasing soil and environmental crisis, and the use of organic fertilizer increases obvious in recent years. In this study, mineral organic fertilizer (MOF) and compound fertilizer (CF) were applied in amaranth culture to explore the effects of these two kinds of fertilizers on soil quality and the potential function for CO2 fixation. Some soil parameters were tested, e.g. pH value, organic carbon content, microbial biomass, urease activity, and available potassium content. In addition, some parameters of soil infiltration water were also determined, such as pH and HCO3 − concentration. Experimental results showed that MOF improved soil quality and amaranth biomass and increased possible soil carbon sink. On the contrary, the utilization of CF worsened soil quality and made the soil acidize. These results suggested that MOF can partially replace CF to improve plant growth, soil quality and possible CO2 sink.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is widely accepted that the excessive use of chemical fertilizer (CF) have led to harden and acidify soil. Application of organic fertilizer has been proved to improve soil quality and it is beneficial for the crop growth (Kang et al. 2016). Considering the close correlation between atmospheric CO2 concentration and the large-scale agricultural land use, the investigation that what kinds of fertilizer practices are feasible and effective in blocking the trend of increasing atmosphere greenhouse gas concentrations attracts more and more attention (Liping and Erda 2001; Dhadli and Brar 2016). Large quantities of carbon are exuded through plant root in the form of organic matter and degraded to gaseous form, which will return to the atmosphere ultimately (Ryan et al. 2001). It is the dominant carbon flux, which transports approximately 120 Gt carbon a−1, in the global carbon cycle and is responsible for transporting 20 times the quantity of anthropogenic emissions each year (Renforth et al. 2009). Therefore, soil carbon sink is very important for atmospheric CO2 cycle.
Increased sequestration of carbon in agricultural soils can potentially mitigate the greenhouse effect (Young 2003). Previous studies showed that mineral weathering can stimulate CO2 sink (Schuiling 2014; Xiao et al. 2014, 2015). However, soil carbon sequestration through the practice of agriculture is something that we should value (Lal 2004a), and reasonable agricultural activities may be valuable for the sustainable use of soil and slowing down CO2 release. Agricultural operations affect the carbon cycle mainly through uptake, fixation, emission and transfer of carbon among different pools (Lal 2004b). Manipulation of agricultural land developed great potential to increase soil carbon stocks (Coutinho et al. 2015). It was achievable by adopting appropriate agricultural management practices, such as application of fertilizers or organic amendments, conservation tillage and crop rotation (Lal 2004a). At present, chemical fertilizers are to use in large doses,but long-term application of inorganic fertilizers are inadequate to maintain soil organic carbon (SOC) levels and nutrients if no aboveground crop residues return to the soil (Su et al. 2006). Recommended management practices are integrated nutrient management with compost, biosolids and nutrient cycling. In general, the SOC pool will be enhanced greater by the use of organic manures and compost than the application of inorganic fertilizers with the same amount of nutrients. Triberti et al. (2008) confirmed the valuable efficacy of manure in increasing both SOC content and soil fertility in the long-term. Recently, manure is applied to increase carbon sequestration in many kinds of soils (Fan et al. 2014; Mahmoodabadi and Heydarpour 2014). However, Schlesinger (1999) pointed out that manure is not likely to yield a net sink for carbon. Unpleasantly, those operations even accelerated CO2 release (Rochette and Gregorich 1998). Schuiling and Andrade (1999) proposed that mixing the fines from olivine mining with a slow-release fertilizer like struvite in the right proportion was a promising solution to the recovery of phosphate with the formation of carbon sink. Other possible mitigation strategies included the use of organic manures and slow-release mineral fertilizers (Metz 2001). However, the related studies are relatively lacking. Seeking such useful and cost-effective ways is very meaningful, although it is long and arduous process.
Potassium is one of the three major plant nutrient elements and plays a pivotal role in agricultural production. Most soils in southern China are deficient in K (Xie 1998). Fortunately, Potassium-bearing mineral deposit is very rich in China, but direct absorption of potassium in its feldspathic or other silicate forms is impossible for plants (Sun et al. 2013). Amaranth is a common vegetable that is planted widely in China and has a strong ability to enrich K ions (Li et al. 2006). Therefore, this kind of plant was used in this study. The aim of the research reported here is to investigate if the application of mineral organic fertilizer (MOF) can improve the quality of the soil, promote amaranth growth, and meanwhile accelerate the possible fixation of CO2 from atmosphere or soil respiration.
2 Materials and methods
2.1 Mineral organic fertilizer and compound fertilizer
Mineral organic fertilizer (MOF) was made in a bio-organic fertilizer Ltd. in Puding, Guizhou Province, China. Corn and sorghum straw and chicken manure were the organic matters, which were mixed with K-bearing silicate mineral power (80 mesh) at a ratio of 3:1 as the matrix. One kg microbial fermentation agents, which contained photosynthetic bacteria, Lactobacillus, yeast, Bacillus, Acetobacter, Bifidobacterium and Actinomycetes, was added into 10 ton matrix, followed by fermentation and turning. This process lasted about 30 days. The percentage of organic content was about 34%. The content of N, P2O5, and K2O in MOF is 1.9%:1.9%:2.5%. pH was obvious alkaline about 8.48. Two kinds of compound fertilizer (CF) were selected. The proportion of N, P2O5, and K2O in one kind of CF is 15%:15%:15%. The proportion of N, P2O5, and K2O in another CF is 11%:11%:18%. In order to make the proportion of N, P2O5, and K2O in CF be consistent with that of MOF, two kinds of CF were mixed in the ratio of 1:1.343 in the study. The content of N, P, and K in 50 g MOF is equal to that in 7.476 g CF. The experiment with no fertilizer treatment was named as WF in this study.
2.2 Summary of experimental operation
XRD results showed that soil was composed of quartz (SiO2), muscovite (KAl2(Si3Al)O10(OH,F)2), albite (Na(Si3Al)O8), orthoclase (KAlSi3O8), and kaolinite-1A (Al2Si2O5(OH)4). Each device (supplemental materials) contained 2000 g soil and different amounts of fertilizer listed in Table 1. To increase the permeability of soil, 450 g glass bead was added to those devices. The amaranth was watered timely according to the soil moisture. Water (500 ml) was regularly added to the device every 6 days, and the soil infiltration water (SIW) collected the next day.
2.2.1 Determination of organic carbon content of amaranth
Whole amaranth plants were collected and dried overnight at 105 °C. The dry weight was then measured. The percentage of carbon present was measured using an elemental analyzer (ElementarVario MACRO, Germany).
2.2.2 Determination of soil parameters
At the end of the experiment, soil moisture, pH, and urease activity were tested. Portions of the naturally air-dried soil samples were used to determine SOC and microbial biomass.
To measure soil moisture content, an aliquot of moist soil (about 5 g) was dried at 105 °C. After 5 h, the sample was placed in a desiccator to cool for 30 min. Samples were dried and weighed repeatedly until the weight no longer decreased. pH was determined according to Fierer and Jackson (2006). Dry soil sample was mixed with water at a ratio of 1:1, then samples were shaken for 15 min, left to settle for 30 min, and tested with a pH-meter.
Urease activity was test using colorimetric determination of ammonium (Kandeler and Gerber 1998). For this test, toluene (1 ml) was mixed with 5 g natural air-dried soil sample for 15 min. Then, urea solution (10 ml, 5%) and citrate buffer (20 ml, 0.96 M, pH 6.7) were added. Meanwhile, as a control, a repeat experiment was performed using an equal volume of distilled water instead of the urea solution. After incubation for 24 h at 37 °C, the solution was centrifuged (4000g, 10 min). Supernatant (1 ml) was mixed with sodium phenoxide solution (4 ml, 2.7 M) and sodium hypochlorite solution (3 ml, 0.9%) in a 50 ml volumetric flask. After 20 min, the reaction solution was diluted to 50 ml and the absorbance was measured at 460 nm. Urease activity was expressed according to the milligrams of NH3–N in 1 g soil.
SOC determination was followed by a previous report (Page 1982), dry soil (2 g) was added to HCl solution (40 ml, 5%), and the mixture blended for 10 min. The tubes were then spun at 8000g for 5 min. After this, the samples were rinsed 3 times with ultrapure water. After drying at 105 °C, the residual solids were weighed and the carbon content tested using the elemental analyzer (ElementarVario MACRO, Germany).
The microbial biomass in the soil was measured using the method outlined by Vance et al. (1987) involving chloroform–fumigation and extraction. Moist soil was fumigated in a sealed desiccator using ethanol-free chloroform for 24 h at 25 °C. Water (20 ml) and the same amount of NaOH (1 M) were placed in the bottom to trap any evolved CO2. Non-fumigated soil was used as a control. Fumigated and non-fumigated soils were subjected to extraction using 0.5 M K2SO4 solution for 30 min using an ‘end-over-end’ shaker at 350 rpm. After centrifugation, supernatant was filtered through a 0.45 μm membrane. The filtrate was tested using a total organic carbon analyzer (Shimadzu TOC-VCSN, Japan). The soil microbial biomass carbon (BC) was estimated from Bc = Ec/kEC, where Ec = [organic carbon extracted by K2SO4 from fumigated soil-carbon extracted by K2SO4 from non-fumigated soil] (Wu et al. 1990). The quantity kEC (the proportion of the extracted microbial biomass carbon evolved as organic carbon)was taken to be 0.45 following the work of Wu et al. (1990).
To test available potassium content, a portion (0.5 g) of crushed dry sample (50–80 mesh) and ammonium acetate solution (50 ml, 1 M) were mixed in an extraction bottle (flask) following the method of Zhu et al. (2013). Then, the bottle was stoppered and oscillated for 30 min. The mixture was filtered using a filter paper, and the filtrate collected for testing using a full-spectrum, direct-reading plasma emission spectrometer (Thermo Fisher Scientific, UK).
2.2.3 Determination of SIW parameters
pH and temperature of the SIW was measured using a pH meter (S20 SevenEasy, Mettler-Toledo). The concentrations of certain cations (K+, Na+, Ca2+, and Mg2+) were determined using an atomic absorption analyzer (AA900F, PerkinElmer, US). Anion concentrations (Cl− and SO4 2−) were determined using ion chromatography (DIONEX ICS-90, US). An acid–base titration method was used to measure the content of the bicarbonate in the aqueous solution according to the published literature with a little modification (Verma 2004). The SIW was filtered using a 0.45 μm microporous membrane and 20 ml titrated with a standardized HCl solution. The above parameters (pH, water temperature, ion concentrations) were imported into appropriate software (MINTEQ) to calculate the saturation index of the calcite.
2.3 Statistical analysis
STATISTICA 6.0 software was used to analyze the data. The significance of the differences between the treatments was tested separately using one-way ANOVA tests followed by Fisher LSD tests for mean comparisons. The data were shown in the form of average value of at least three independent experiments. The standard deviation was presented as well.
3 Results
3.1 Carbon content of Amaranth
For the average carbon content, CF and MOF were both conducive to amaranth growth (see Fig. 1). Those fertilizers thus improved organic carbon fixation. With the increase of MOF applied, carbon content of amaranth raised. From a statistical point of view, only adding 50 g MOF (50F) was beneficial to the promotion of organic carbon content. Anyhow, it was enough to prove that application of MOF was more conducive to the growth of amaranth than CF under the premise of consistent usage amount of N, P, and K.
3.2 Soil parameters
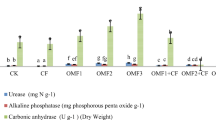
Soil moisture content was not significantly different (statistically) among the five different kinds of treatment (ranging from 11.6% to 16.8%). MOF significantly increased soil pH in all four treatments (Fig. 2a). Different addition amounts of MOF did not result in the difference of soil pH, except for the 50F treatment. The maximum amount of MOF added gave rise to the highest pH value. On the contrary, the addition of CF made the pH value decrease obviously (Fig. 2a).
As can be seen from Fig. 2b, the addition of CF and a relatively minor amount of MOF (12.5F) did not contribute to the accumulation of soil organic carbon. Nonetheless, it was significantly boosted after adding a large amount of MOF (25F, 37.5F, and 50F). Compared 37.5F with 50F, it suggested that adding too much MOF did not increase SOC content, i.e. 37.5 g MOF had made the content of SOC reach a high level.
The application of CF did not cause the change of microbial biomass (Fig. 2c). Obviously, MOF was benefited to microbial reproduction and growth. Compared to WF treatment, it increased fourfolds after adding 37.5 g MOF. Regardless of 50F, it showed that some positive correlation between microbial biomass and the amount of MOF. To our surprise, adding 50 g MOF caused the soil microbial biomass to decline a certain amount, but it was still notably higher than WF and CF treatments. Excess MOF was against to microbial proliferation and had a dilution effect on the ratio of microbial biomass in soil. It indicated that appropriate implement of MOF had greatest benefits to microbial biomass and plant production.
Urease activity nearly doubled after the addition of CF (Fig. 2d). For the impact of MOF on urease activity, although there was no difference between 25F and 37.5F, the result implied that MOF can improve urease activity significantly.
The results of application of fertilizer on available potassium were very similar with the trend of pH. CF did not provide much available potassium. MOF had an ability to release potassium ion according to Fig. 2e, except that there was no statistical difference between WF and 37.5F. However, excessive addition of MOF did not significantly increase the available potassium content.
3.3 SIW parameters
3.3.1 pH
Both CF and MOF significantly affected pH of SIW (Fig. 3a). Nevertheless, the effects were distinctly different. CF promoted an obvious decrease in pH value, which was always lower than 7.0 throughout the course of the experiment. The amount of MOF added affected pH value significantly at the first stage of this experiment. The pH value of SIW increased when more MOF was added, and it was alkaline in the presence of MOF.
3.3.2 The concentration of HCO3 −
Application of MOF had a great effect on HCO3 – concentration (Fig. 3b). Similar to pH, the application of CF made HCO3 − concentration reduce clearly, i.e. the concentrations of HCO3 − in the groups treated by MOF were much higher than those in the groups of CF and WF. Adding more MOF caused more HCO3 – producing.
3.3.3 K+ concentration
K+ concentration of the application of CF was highest during the whole experimental process (Fig. 3c). Overall, the K+ concentration was positive correlation to the amount of added MOF. Minimum addition of MOF (12.5F) did not seem to provide an extra K+ (see Fig. 3c). However, others treatments (25F, 37.5F, and 50F) had an ability to release relative more amounts of K+. The average K+ concentration in the 25F, 37.5F, and 50F treatments always exceeded that in WF.
3.3.4 Saturation index calculation
MINTEQ software was used to calculate the saturation index of calcite (SIC) using the following parameters: water temperature, pH, and the concentrations of K+, Na+, Ca2+, Mg2+, Cl–, SO4 2–, and HCO3 – ions. The application of CF made SIC decrease obviously (Fig. 3d). Overall, compared with WF, the addition of MOF can increase SIC. MOF was benefited to form saturation state of calcite.
4 Discussion
Excessive application of CF caused soil acidification. Here, our results showed that both soil and SIW pH decreased sharply with the addition of CF. Clearly, it was the nonnegligible stain for agriculture practice. Conversely, MOF was an environmentally friendly fertilizer, which can even mitigate the effect of acid rain (Allen and Brent 2010) and/or improve soil that has been acidified. Su et al. (2006) studied long-term effect of fertilizer and manure application on soil-carbon sequestration and soil fertility under the wheat–wheat–maize cropping system in northwest China. The results showed that application of inorganic fertilizer alone did not increase SOC concentrations compared with no application of fertilizers. Our finding that there was no difference between WF and CF on SOC (Fig. 2b) was consistent with above report. The MOF fertilizer indeed increased the content of SOC significantly. Our results showed that urease activity was significantly affected by different fertilization regimes. Specially, the activity of urease was positive correlation with the usage amount of MOF. It was noticed that urease activity had a positive correlation with organic carbon and total nitrogen (Zantua et al. 1977). So soil biogeochemical cycles, especially carbon and nitrogen cycles, was improved by MOF accordingly. Clearly, CF can not provide potassium persistently (Fig. 2e). In contrast, the content of available potassium was significantly increased after the adding of MOF. It implies that this kind of fertilizer style is favorable for long-term supply of potassium. Potassium is one of the most abundant cation in plants, comprising up to 10% of a plant’s dry weight (Leigh and Wynjones, 1984). The application of MOF is an undoubtedly attemptable way to improve soil potassium content to alleviate the lack of soluble potassium. It can avoided to overuse CF. The content of N, P, and K in 50 g MOF was equal to that in 7.476 g CF. For all soil parameters measured (Fig. 2), most importance of all, the addition of 25 g MOF was more significant than 7.476 g CF for improving soil quality.
From the potential effect of application of MOF on carbon fixation, previous research showed that secondary carbonate preferred to accumulate in pH range of 7.3–8.5 (Lal 2008). Our results here demonstrated that soil pH was just in this interval when MOF was used. Carbonate mineral precipitation was controlled by the saturation state of the soil solution and depended on activities of dissolved species (cation and (bi)carbonate). HCO3 – concentrations were positive correlation to the amount of MOF used (Fig. 3b). The more positive SIC meant that these HCO3 – was easier to form a stable form of solid carbon, e.g. CaCO3. Our recent study also suggests that adding dolomite and K-feldspar into soil is a feasible way to increase carbon sequestration (Xiao et al. 2016). In view of this, the application of MOF may be significant for accelerating atmospheric CO2 sequestration and/or slowing down soil CO2 emission.
5 Conclusion
The application of MOF not only improved plant growth and some indicators of soil quality, e.g. SOC content, microbial biomass, available potassium but also provided a possible method of accelerating carbon fixation. Despite the limitations of the work presented here, it still showed a promising way to improve soil quality in the long-term sustainable natural farming.
References
Allen DJ, Brent GF (2010) Sequestering CO2 by mineral carbonation: stability against acid rain exposure. Environ Sci Technol 44:2735–2739
Coutinho HLC, Noellemeyer E, de Carvalho-Balieiro F, Pineiro G, Fidalgo ECC, Martius C, da Silva CF (2015) Impacts of land-use change on carbon stocks and dynamics in central-southern South American biomes: Cerrado, Atlantic Forest and Southern Grasslands. Water Resour Manag 10(2):107–127
Dhadli HS, Brar BS (2016) Effect of long-term differential application of inorganic fertilizers and manure on soil CO2 emissions. Plant Soil Environ 62(5):195–201
Fan JL, Ding WX, Xiang J, Qin SW, Zhang JB, Ziadi N (2014) Carbon sequestration in an intensively cultivated sandy loam soil in the North China Plain as affected by compost and inorganic fertilizer application. Geoderma 230:22–28
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Kandeler E, Gerber H (1998) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kang Y, Hao Y, Shen M, Zhao Q, Li Q, Hu J (2016) Impacts of supplementing chemical fertilizers with organic fertilizers manufactured using pig manure as a substrate on the spread of tetracycline resistance genes in soil. Ecotoxicol Environ Saf 130:279–288
Lal R (2004a) Carbon sequestration in dry land ecosystems. Environ Manag 33:528–544
Lal R (2004b) Soil carbon sequestration to mitigate climate change. Geoderma 123:1–22
Lal R (2008) Sequestration of atmospheric CO2 in global carbon pools. Energy Environ Sci 1:86–100
Leigh RA, Wynjones RG (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. N Phytol 97:1–13
Li T, Ma G, Zhang X (2006) Root exudates of potassium-enrichment genotype grain amaranth and their activation on soil mineral potassium. J Appl Ecol 17:368–372
Liping G, Erda L (2001) Carbon sink in cropland soils and the emission of greenhouse gases from paddy soils: a review of work in China. Chemosphere Glob Chang Sci 3:413–418
Mahmoodabadi M, Heydarpour E (2014) Sequestration of organic carbon influenced by the application of straw residue and farmyard manure in two different soils. Int Agrophys 28:169–176
Metz B (2001) Climate change 2001 mitigation: contribution of Working Group III to the third assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Page AL (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy Inc, Soil Science Society of America Inc, Madison
Renforth P, Manning DAC, Lopez-Capel E (2009) Carbonate precipitation in artificial soils as a sink for atmospheric carbon dioxide. Appl Geochem 24:1757–1764
Rochette P, Gregorich EG (1998) Dynamics of soil microbial biomass C, soluble organic C and CO2 evolution after three years of manure application. Can J Soil Sci 78(2):283–290
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52:527–560
Schlesinger WH (1999) Carbon and agriculture: carbon sequestration in soils. Science 284:2095
Schuiling R (2014) Climate change and CO2 removal from the atmosphere. Nat Sci 6:659–663
Schuiling R, Andrade A (1999) Recovery of struvite from calf manure. Environ Technol 20:765–768
Su YZ, Wang F, Suo DR, Zhang ZH, Du MW (2006) Long-term effect of fertilizer and manure application on soil-carbon sequestration and soil fertility under the wheat–wheat–maize cropping system in northwest China. Nutr Cycl Agroecosyst 75:285–295
Sun L, Xiao L, Xiao B, Wang W, Pan C, Wang S, Lian B (2013) Differences in the gene expressive quantities of carbonic anhydrase and cysteine synthase in the weathering of potassium-bearing minerals by Aspergillusniger. Sci China Earth Sci 56:2135–2140
Triberti L, Nastri A, Giordani G, Comellini F, Baldoni G, Toderi G (2008) Can mineral and organic fertilization help sequestrate carbon dioxide in cropland? Eur J Agron 29:13–20
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Verma MP (2004) A revised analytical method for HCO3 − and CO3 2− determinations in geothermal waters: an assessment of IAGC and IAEA interlaboratory comparisons. Geostand Geoanal Res 28:391–409
Wu J, Joergensen R, Pommerening B, Chaussod R, Brookes P (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Xiao L, Hao J, Wang W, Lian B, Shang G, Yang Y, Wang S (2014) The up-regulation of carbonic anhydrase genes of Bacillus mucilaginosus under soluble Ca2+ deficiency and the heterologously expressed enzyme promotes calcite dissolution. Geomicrobiol J 31:632–641
Xiao L, Lian B, Hao J, Liu C, Wang S (2015) Effect of carbonic anhydrase on silicate weathering and carbonate formation at present day CO2 concentrations compared to primordial values. Sci Rep 5:7733. doi:10.1038/srep07733
Xiao LL, Sun QB, Li XX, Chu Y, Yuan HT, Ruan YL, Lu CM, Lian B (2016) A feasible way to increase carbon sequestration by adding dolomite and K-feldspar to soil. Cogent Geosci 2:1205324
Xie JC (1998) Present situation and prospects for the world’s fertilizer use. Plant Nutr Fertil Sci 4:321–330
Young LM (2003) Carbon sequestration in agriculture: the US policy context. Am J Agric Econ 85:1164–1170
Zantua MI, Dumenil LC, Bremner JM (1977) Relationships between soil urease activity and other soil properties. Soil Sci Soc Am J 41:350–352
Zhu X, Lian B, Yang X, Liu C, Zhu L (2013) Biotransformation of earthworm activity on potassium-bearing mineral powder. J Earth Sci 24:65–74
Acknowledgements
Funding was provided by the National Natural Science Foundation of China (Grant No. 41373078), the National Key Basic Research Program of China (Grant No. 2013CB956702), the Key Project of Natural Science Research in Colleges and Universities in Jiangsu Province (Grant No. 16KJA180003), and the Natural Science Foundation of Shandong Province (Grant No. ZR2016DQ12).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, L., Sun, Q., Yuan, H. et al. A practical soil management to improve soil quality by applying mineral organic fertilizer. Acta Geochim 36, 198–204 (2017). https://doi.org/10.1007/s11631-017-0139-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0139-5