Abstract
This study focuses on the hydrochemical characteristics of 47 water samples collected from thermal and cold springs that emerge from the Hammam Righa geothermal field, located in north-central Algeria. The aquifer that feeds these springs is mainly situated in the deeply fractured Jurassic limestone and dolomite of the Zaccar Mount. Measured discharge temperatures of the cold waters range from 16.0 to 26.5 °C and the hot waters from 32.1 to 68.2 °C. All waters exhibited a near-neutral pH of 6.0–7.6. The thermal waters had a high total dissolved solids (TDS) content of up to 2527 mg/l, while the TDS for cold waters was 659.0–852.0 mg/l. Chemical analyses suggest that two main types of water exist: hot waters in the upflow area of the Ca–Na–SO4 type (Hammam Righa) and cold waters in the recharge zone of the Ca–Na–HCO3 type (Zaccar Mount). Reservoir temperatures were estimated using silica geothermometers and fluid/mineral equilibria at 78, 92, and 95 °C for HR4, HR2, and HR1, respectively. Stable isotopic analyses of the δ18O and δD composition of the waters suggest that the thermal waters of Hammam Righa are of meteoric origin. We conclude that meteoric recharge infiltrates through the fractured dolomitic limestones of the Zaccar Mount and is conductively heated at a depth of 2.1–2.2 km. The hot waters then interact at depth with Triassic evaporites located in the hydrothermal conduit (fault), giving rise to the Ca–Na–SO4 water type. As they ascend to the surface, the thermal waters mix with shallower Mg-rich groundwater, resulting in waters that plot in the immature water field in the Na–K–Mg diagram. The mixing trend between cold groundwaters from the recharge zone area (Zaccar Mount) and hot waters in the upflow area (Hammam Righa) is apparent via a chloride-enthalpy diagram that shows a mixing ratio of 22.6 < R < 29.2 %. We summarize these results with a geothermal conceptual model of the Hammam Righa geothermal field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Because of its geologic setting in the tectonically active Alpine-Magrebide belt, the northern part of Algeria has considerable geothermal potential of the low enthalpy type, generating a heat discharge of 240 MWt (Saibi 2015). Geothermal development and exploration activities are currently increasing in line with Algeria’s plan to diversify the sources of energy for electricity production, which currently is primarily derived from fossil-fuel resources (which account for 98 % of production). The country’s goal is to attain 40 % of consumed electricity generation from renewable energy resources by 2030 (Bouchareb-Haouchine 2012; Saibi 2015).
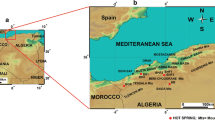
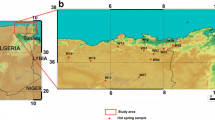
The study area is located in the north-central part of Algeria (Fig. 1), approximately 100 km southwest of the city of Algiers and at an altitude of 525 m above sea level (ASL). The thermal activity of the Hammam Righa area is characterized by artesian thermal springs, travertine deposition, sulfur gas seepage, and hydrothermal alteration zones. The thermal waters of Hammam Righa are used in some of the most famous Algerian hot spas. Referred to as “Aqua Calidea” during the Roman period, they have been used for balneology and bathing since that time (Belhai et al. 2013).
This study investigated the hydrochemical characteristics of samples from 44 hot springs and three cold springs from the study area collected in 1990, 2010, and 2013. The distribution of the three main hot springs in the upflow area (HR1, HR2, and HR4) roughly parallels the northeast-southwest fault network considered to be the main fault system of the Hammam Righa geothermal (HRG) manifestation (Bouchareb 1987; Issaadi 1992; Bouchareb-Haouchine 1993; Bouchareb-Haouchine et al. 2012). The discharge temperature of hot springs in the HRG manifestation ranges from 32.5 to 68.2 °C, and the springs have a mean discharge rate of 2.5 L/s. Two cold spring samples (HR5 and HR6) were collected from the Zaccar Mount, which is considered the recharge area for the HRG manifestation (southwest of the study area; Fig. 1b). Another, HR3, was sampled close to the upflow area (Hammam Righa).
Previous studies conducted at the HRG area have shown that the chemical characteristics and the location of hydrothermal discharge are strongly linked to the regional geology (Issaadi 1992; Fekraoui and Kedaid 2005; Bouchareb-Haouchine et al. 2012; Belhai et al. 2013). The geothermal reservoir is mainly hosted by the limestone and dolomite sequences of the Tellian zones. However, the thermal waters could also be related to adjacent magmatic intrusions of the post-thrust Miocene basins (Issaadi 1992). Stable isotopic analysis of the δ18 O and δD compositions of HR1 and HR2 (hot springs) as well as HR3 (a cold spring) and local meteoric water were carried out to determine the origin of the thermal waters.
This work aimed to comprehensively evaluate the geothermal potential of the HRG field for future development (electricity generation and/or heating) by using existing data (from 1990 and 2010) combined with new data (from 2013) from sampling campaigns in the HRG area. To construct a reliable geothermal conceptual model for the HRG field, we focused on water chemistry, using correlations between various chemical components to investigate the key controls on water chemistry and to highlight the heterogeneity in mineralization resulting from the complex geologic setting.
To this end, several cationic and silica geothermometers as well as fluid/mineral equilibria were applied to assess the geothermal reservoir temperatures for the HRG manifestation. These were applied to the datasets collected in campaigns conducted by Bouchareb-Haouchine in 1990 and 2010, as well as the dataset from the new 2013 campaign. A chloride mixing model is discussed in detail; it was used to estimate the rate of mixing with cold groundwater and the effects of the conductive cooling process as waters ascend, and also to confirm the estimated reservoir temperature obtained by chemical geothermometers. Finally, the isotopic data (δ18 O and δD values) of four samples were used to determine the origins of the thermal water and to ascertain the contributions of Miocene magmatic events.
2 Geologic setting
The northern part of Algeria belongs to the North African margin and forms part of the Alpine-Magrebide belt that extends from Gibraltar to Sicily-Calabria (Fig. 2a, inset). The Alpine-Magrebide belt is the result of collision between the African and European plates, which began in the late Permian at the beginning of the breakup of Pangea and progressed through the Cenozoic Alpine orogeny (Auboin and Durand-Delga 1971; Bouaziz et al. 2002; Domzig et al. 2006; Fourré et al. 2011). Two main structural domains exist in northern Algeria: the Tellian Atlas in the north and the Saharan Atlas further to the south.
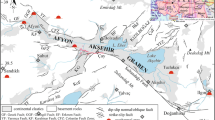
a Small-scale map of the main geologic domains of the Alpine-Magrebide belt (inset, modified from Belhai 1996). And a simplified geologic map of the HRG field. Geologic cross-sections: b oriented northeast-southwest from the upflow area (Hammam Righa) to the recharge zone (Zaccar Mount), indicating the stratigraphy and structure of the basin; and c lithologic cross-section oriented northwest-southeast in the Zaccar area showing the basement rock (modified from Bouchareb-Haouchine et al. 2012)
The HRG manifestation area lies in the external zones of the Tellian Atlas, or the so-called “Tellian zones” (Wildi 1983), which are mainly characterized by Miocene folds and nappes thrust over the Atlasic foreland (Saharan Atlas). The HRG field is located at the western edge of the post-thrust Miocene basin of Boumadfaa and is bounded by the Zaccar Massif (Mount), which consists of a relatively thick dolomitic and marine carbonate sequence of Lower Jurassic age (Fig. 2a).
The upflow area of the HRG field is characterized by the presence of N060-N075 and N135-N150 conjugated faults, which were initiated during the Helvetian stage of the upper Miocene. These major northeast-southwest structural lineaments, which border the springs, accommodated the closure of the Mediterranean Sea and the displacement of Tellian thrust-sheets southward during the late Miocene (Belhai 1996; Bouchareb-Haouchine et al. 2012).
The exposed Paleozoic age volcanic-sedimentary complex and metamorphic formations in the Zaccar Massif constitute the basement rock of the study area (Fig. 2a, c). They are overlain by a relatively thick and deformed “fractured” sequence of Jurassic dolomite and Mesozoic carbonates typical of the Tellian zones (Mattauer 1958). The deep-seated northeast-southwest trending faults and folds give rise to a limited extrusion of evaporites from the Triassic basement (Glangeaud 1932; Lepvrier and Velde 1976; Belhai 1996). These evaporites are mainly composed of gypsum and anhydrite, with interbedded clays and thin deformed dolomite layers outcropping as a discontinuous border that delineates an abnormal contact between the different sets (Fig. 2a, b). To the east, the Miocene formations outcrop unconformably with a carbonate formation of Cenomanian and Senonian age, consisting of marl and folded marl-limestone up to 1000 m thick. The presence of conglomerate and sandstone accompanied by marl and limestone from the Cartenian stage of the lower Miocene indicates a period of non-deposition due to tectonic uplift and subsidence in the basin. These formations are covered by an 80 m-thick upper sequence of marl and sandstones that is Helvetian in age (upper Miocene). Faults cutting these Miocene formations give rise to a graben structure in the southwestern part of the study area (Fig. 2b). Extensive hydrothermal deposits of travertine comprise the Quaternary formations. These outcrop in three main areas; thermal water rises through the largest one, which is up to 50 m thick.
Post-collisional calc-alkaline magmatic activity of the post thrust–Miocene basins has occurred periodically since the early Miocene following the opening of the Mediterranean Sea between 11 and 13 Ma (Bellon 1976; Lepvrier and Velde 1976). These volcanic products include rocks from the calc-alkaline series (from andesite to rhyolite to trachyte and dacite) and generally erupted in areas with east–west faults.
2.1 Hydrogeology
Hydrogeologic study has revealed the existence of several potential aquifers. The most important one is the highly fractured Jurassic limestone and dolomite of the Zaccar Massif, which extends to the upflow area and acts as a reservoir in the HRG field. These formations lie on the impermeable metamorphic complex of the Zaccar Mount. The Jurassic aquifer is primarily fed by rainwater infiltration; it has a precipitation rate of 7 Ls−1/km2 and a flow rate of 200 L/s to an area of 30 km2 mostly composed of limestone (INRH 1976). The rising Triassic formation is mainly composed of gypsum and anhydrite, and clays can act as a cap rock that contains heat and maintains water pressure.
In 1968, four wells were drilled by GEMP (Groupe d’Etudes sur les Milieux Poreux), to depths ranging from 35 to 65 m. These wells cross the Quaternary travertine and Miocene formations. Water pumped from the wells attains temperatures of 69.0 °C with discharge rates of 3.69 and 3.66 L/s and a transmissivity, T, of approximately 10−4 m2/s. After a long period of exploitation, the current flow rate is approximately 2.5 L/s for HR1 and only 1 L/s for both HR2 and HR4.
3 Sampling and analysis methods
The first sampling campaign for hydrochemical analyses was performed in 1990. Over 12 months, 12 samples were collected at each hot spring (HR1, HR2, and HR4). A second campaign took place in 2010 to complete the initial dataset. The first and second campaigns were conducted by Dr. Bouchareb-Haouchine. In January 2013, a third campaign was carried out for this study, including four samples from the upflow area of Hammam Righa (HR1, HR2, and HR4; hot spring samples) and one cold spring sample (HR3). Cold spring samples from the Zaccar Mount (HR5 and HR6) were also collected. Temperature, electrical conductivity (EC), and pH measurements were conducted on-site. Major ion analyses (for Na+, K+, Ca2+, Mg2+, and SiO2) were measured by atomic absorption spectrophotometry, while SO4 2− concentrations were determined by spectrophotometry and alkalinity was determined using standard titration techniques. Cl− was analyzed using the AgNO3 titration method at the National Agency of Hydraulic Resources (ANRH-Algiers).
For SiO2 analysis, samples were diluted tenfold using deionized water to prevent SiO2 precipitation in the water. SiO2 concentrations were determined by atomic absorption spectrophotometry. Ionic balance provides an indication of the accuracy and reliability of the analysis and is given by the following equation (Freeze and Cherry 1979):
Analyses with an ionic balance error exceeding 5 % were excluded from the dataset.
The δ18O and δD values of HR1, HR2, and HR3 were analyzed by mass spectrometry at the Department of Environment of Kyuden Sangyo Co., Inc. (Japan). Isotopic values are reported using standard notation relative to the NIST/IAEA reference material V-SMOW. The analytical precision is ±0.1 and ±1 ‰ for δ18O and δD, respectively.
4 Results
4.1 Water chemistry
Temperatures (in °C), pH values, electrical conductivity (EC, in µS/cm), and chemical analyses from the HRG field are listed in Table 1. The water samples show heterogeneity in their chemical-physical features. Both cold water (T < 25 °C) and hot water (T from 32.1 to 69.0 °C) samples had a near-neutral pH of 6.0–7.6. Thermal waters show a higher total dissolved solids (TDS) concentration of up to 2527 mg/L, while the TDS for cold water ranges from 659.0 to 852.0 mg/L. The higher TDS value in hot water is likely due to the interaction between infiltrated meteoric water and the host rock and probably reflects a longer circulation and residence time.
The cold and hot waters belong to two main chemical classification types, based on Piper and ternary diagrams (Fig. 3a, b). Thermal water facies from the upflow area of the HRG field are of the Ca–Na–SO4(Cl) type and the Ca–Na–SO4 (HCO3) type for HR1, HR2, HR4 and HR3, suggesting interaction with gypsum/anhydrite-bearing Triassic formations. Cold waters from the Zaccar Mount (HR5 and HR6) are of the Ca–Na–HCO3 type. The high HCO3 − content of these waters (317–366 mg/L) reflects the interaction between meteoric water and high permeability and CO2-rich karstic (fractured) limestone and dolomite aquifers, as shown in Eq. 2:
Chemical sources are more difficult to interpret in sedimentary environments such as the Tellian zones of the HRG manifestation, because the mineralogical and chemical properties of the host aquifers are inherently more heterogeneous. The heat flux, tectonic regime, and hydrologic conditions (e.g., infiltration depth and residence time in the reservoir) are specific to each field. In the context of this complexity, we discuss the key factors controlling the evolution of the Hammam Righa thermal waters.
A Na+ and Cl− scatter diagram shows a very good correlation with the halite dissolution line (Y = X). We argue that halite-bearing Triassic evaporites are likely the source of Na+ and Cl− in thermal waters of the Hammam Righa field. However, Cl-rich hot waters in the upflow area show Cl−/∑anions > 0.73, indicating a Na+/Cl− ratio of approximately 1 (the stoichiometric Na+/Cl− ratio of halite dissolution; Fig. 4a).
a Na+ versus Cl−, b Ca2+ versus SO4 2−, and c Ca2+ versus HCO3 − scatter diagrams of the Hammam Righa waters, showing dissolution of halite, gypsum, and calcite (red lines). d Na+/Cl− versus HCO3 −/(Ca2++ SO4 2−) plot summarizing the key source process controlling the chemistry of the thermal waters and e Ca2+ + Mg2+ versus HCO3 −/SO4 2− plot
All the thermal waters from the upflow area (HR1, HR2, and HR4) are located on the gypsum/anhydrite dissolution line (−0.06 < SI anhydrite <−0.3; Table 1) and have a higher Ca/SO4 ratio of approximately 1, while cold springs from the Zaccar Massif have a Ca/SO4 ratio lower than 1 (Fig. 4b). This suggests that the dissolution of gypsum/anhydrite minerals likely occurs in the Triassic evaporitic sequence in the local stratigraphy. According to Capaccioni et al. (2014), this process can be described as follows:
Carbonate formations are dominant in Tellian zones such as the Zaccar Mount region. Cold springs from the recharge area (Zaccar Mount; HR5 and HR6) have a Ca2+–HCO3 − composition and plot on the calcite dissolution line with Ca2+/HCO3 − ratios typically near 1 (Fig. 4c). In contrast, hot waters from the Hammam Righa (upflow area) display Ca2+/HCO3 − ratios greater than 1, which implies another source of Ca2+ besides the carbonates; this source could be sulfate dissolution and leaching of Ca2+ from Triassic evaporite minerals. The water samples are consistently in equilibrium with calcite (−0.47 < SI calcite < 0.74; Table 1). Generally, carbonate mineral dissolution can be written as follows (Garrels and Mackenzie 1971):
The key process controlling the chemistry of the thermal waters of the HRG field is summarized in Fig. 4d, e. The Na/Cl ratio of most samples is buffered at approximately 1, typical of halite dissolution in the Triassic formation. Most of the waters from the HRG field have an HCO3/(Ca + SO4) ratio between 1.0 and 10.0 (Fig. 4d). This shift towards gypsum dissolution is characteristic of Tellian zones (Fourré et al. 2011). Hot waters from the upflow area have HCO3/SO4 ratios between 0.1 and 1.0 (Fig. 4e), and higher Ca + Mg contents of approximately 20 meq/L. This suggests evaporitic (gypsum/anhydrite) influences in the upflow area, shifting to carbonate and dolomite in the recharge zone area, which has an HCO3/SO4 ratio between 1.0 and 10.0. The contribution of dolomite is implicated by a high Mg content in thermal waters of up to 62 mg/L, which is unlikely with high enthalpy geothermal fluids that have low Mg contents between 0.01 and 0.1 mg/L (Nicholson 1993). Ca2+, Mg2+, and HCO3 − can be derived from alteration/weathering of carbonates, as explained by the following reaction (Garrels and Mackenzie 1971):
4.2 Chemical geothermometry
Chemical geothermometers enable estimation of the temperature of the reservoir fluid. They are based on specific temperature-dependent chemical reactions that exist between water and the minerals within the rock at deep thermal reservoir temperatures (Pasvanoglu and Chandrasekharam 2011). In this study, the following geothermometers were applied to estimate the HRG field reservoir temperature for HR1, HR2, and HR4 samples from 1990, 2010, and 2013, as shown in Table 2 and Fig. 5: (A): Na–K–Ca (Fournier and Truesdell 1973); (B) Na/K (Nieva and Nieva 1987); (C): Na/K (Fournier 1979); (D): Na/K (Truesdell 1976); (E): Na/K (Tonani 1980); (F): Na/K (Giggenbach 1988); (G): K/Mg (Giggenbach 1988); (H): quartz (Verma 2000); (I): chalcedony (Fournier 1992); (J): silica (Giggenbach 1992); (K): silica no steam loss (Fournier 1977); (L): silica (Fournier and Potter 1982); and (M): silica max steam loss (Fournier 1977).
Reservoir temperatures estimated using several geothermometers for the Hammam Righa thermal waters; a HR1 cationic geothermometer; b HR2 cationic geothermometers; c HR4 cationic geothermometers; d silica geothermometers and e Na–K–Mg1/2 triangular plot for thermal waters from the HRG field, combining the fast-responding K/Mg geothermometer with non-equilibrating Na/K geothermometers (Giggenbach 1988)
Temperatures estimated by the Na/K geothermometers from (B) to (F) give high and widely ranging results from 96 to 185 °C for HR1, 96–184 °C for HR2, and 85–181 °C for HR4 (Table 2; Fig. 5). These wide ranges of temperatures may not be reliable, because they are mostly suitable for equilibrated chloride waters (Simmons 2002; Alacali and Savaşçın 2015). In contrast, temperatures estimated by the Na–K–Ca geothermometer (A) vary from 82 to 94 °C for HR1, 82–93 °C for HR2, and 72–90 °C for HR4. The R correction of Fournier and Potter (1979) ranged from 50 > R > 5, [i.e., R = (Mg2+ × 100)/(Mg2++ Ca2++ K+)]. However, the Mg correction is negative and cannot be applied because of the high Mg content of the hot waters. These results suggest that the Na–K–Ca temperatures in the HRG field are less affected by mixing with cold waters and precipitation of calcite causing the loss of Ca (that is, calcite and anhydrite are close to equilibrium in the upflow areas; Table 1).
Temperatures inferred by the K/Mg geothermometer (G) range from 35 to 39 °C for HR1 and HR2 and from 38 to 45 °C for HR4. The K/Mg geothermometer–estimated temperatures were much lower than the discharge temperatures due to rapid equilibration after mixing with Mg-rich groundwater (Giggenbach 1988; Pasvanoğlu and Chandrasekharam 2011). Therefore, conductive cooling and mixing with cooler Mg-rich waters have a strong influence on the temperature decrease of thermal water as it flows upward to discharge at springs (Han et al. 2010; Pasvanoğlu 2013).
The ternary diagram of Na/1000-K/100-Mg1/2 (Fig. 5e) proposed by Giggenbach (1988) was used to estimate the reservoir temperature and to select the waters most suitable for geothermometry. The diagram helps identify the fluid maturity of waters that have attained equilibrium with the host lithology and demonstrates the effects of mixing. All thermal waters from the HRG field plotted in the immature water field, close to the Mg1/2 corner. This pattern may be the result of mixing of fully equilibrated or partly equilibrated thermal waters with cooler, Mg-rich groundwater that comes from dissolution of the Jurassic dolomitic sequence of the Zaccar area and leaching of Mg into the water. However, the use of immature waters to evaluate geothermal reservoir temperatures is not robust and confers only weak reliability to cationic geothermometers (Giggenbach 1988; Tarcan 2005).
It is well known that silica geothermometers are more sensitive to mixing processes, which have a negative effect on the reliability of those geothermometers. At temperatures less than 180 °C, the solubility of silica is often controlled by chalcedony rather than quartz. Thus, in dolomite-limestone reservoirs such as the HRG field, it is important to apply quartz geothermometers cautiously (Fournier 1992). The chalcedony (I; Fournier 1992) and silica (J; Giggenbach 1992) geothermometers suggest temperatures varying between 70 and 76 °C for HR1, 67 and 72 °C for HR2, and 46 and 50 °C for HR4. These results are similar to the discharge temperatures measured on-site for each sampling point. Thus, the quartz geothermometer (H; Verma 2000) suggests similar temperatures to the Na–K–Ca geothermometer, of 94 and 87 °C for HR1-13 and HR2-13, respectively. Finally, the silica no steam loss geothermometer (K; Fournier 1977) gives a similar temperature to Na–K–Ca for HR4-13, estimated at 86 °C.
4.3 Fluid-mineral equilibria
The mineral saturation indices at the discharge temperatures of the thermal waters of the HRG field were calculated using the computer program PHREEQC (Parkhurst and Appelo 1999) and are reported in Table 3. Results show that the thermal springs are under-saturated with respect to gypsum, anhydrite, dolomite, halite, and amorphous silica. They are oversaturated or nearly in equilibrium with respect to quartz, chalcedony, calcite, and aragonite (except for HR4-13).
The SI CO2 (g) under-saturation state suggests that CO2 contributions in the thermal water are partly due to the precipitation of calcite related to the travertine deposits around HR1, HR2, and HR4. This causes a loss of Ca in the hot waters.
The saturation state results presented in Fig. 6 indicate that the thermal waters undergo a change of saturation indices with temperature. All waters are oversaturated with respect to calcite and aragonite at temperatures above 30–50 °C (Fig. 6b, c), quartz at temperatures below 75–100 °C (Fig. 6f), and chalcedony at temperatures below 60 °C (see Fig. 6g).
The fact that hot waters are undersaturated with respect to gypsum and anhydrite at temperatures lower than 150 and 90 °C (see Fig. 6e and a, respectively) suggests the dissolution of these minerals in thermal waters. This is further attested by the absence of sulfate deposits in the spring vent.
Assuming a temperature-dependent chemical equilibrium between minerals and the fluid in the deep reservoir, the geothermal reservoir temperature is graphically indicated by the intersection of the mineral curve with the line SI = 0.
For HR1-13, the anhydrite and quartz curves intersect near SI = 0 at a temperature of 95 °C (Fig. 7a), which corresponds to the temperatures estimated by the quartz geothermometer (H; Verma 2000). For HR2-13, the quartz and anhydrite curves intersect at the SI = 0 line at 92 °C (Fig. 7b), which is also similar to the temperature inferred by the quartz geothermometer (H; Verma 2000). In contrast, for HR4-13 the quartz and halite curves intersect at the SI = 0 equilibrium line at a temperature of 78 °C (Fig. 7c), similar to that inferred by the silica geothermometer (L).
Thus, a temperature range from 78 to 95 °C is the zone of overlap between the chemical geothermometers and fluid mineral equilibria. This range is considered as the most likely reservoir temperature for the HRG field. The lower temperature of HR4-13 suggests mixing with cold groundwater.
4.4 Evidence of mixing
4.4.1 Chloride mixing model
The enthalpy-chloride mixing model of Fournier (1979) is useful for characterizing the parent geothermal liquid for the HRG field thermal waters and delineating the upflow and cooling process. This can help elucidate the hydrologic complexities of various hydrothermal systems and estimate reservoir temperatures (Mutlu 1998; Guo et al. 2009; Guo and Wang 2012).
A principal trend can be inferred from Fig. 8a: mixing with cold groundwater that is less enriched in Cl− causes a decrease in the enthalpy and Cl− content. This process is apparent in the HR4 samples, which are likely mixed with cooler groundwater from the Zaccar Massif (recharge zone area), as represented by HR5 and HR6.
a Chloride-enthalpy mixing model for northwestern Algerian thermal waters showing reservoir temperatures (parent fluid) with mixing trends. b Plot of δ18O versus δD for thermal waters showing trends and shifts from different geothermal fields (from Giggenbach 1992). The Global Meteoric Water Line (GMWL) is also shown
To simplify mixing calculations, it is assumed that the sampled groundwater is a mixture of two end-members: thermal and non-thermal waters. Chloride is used to estimate the mixing ratio because it is considered a highly mobile component and does not precipitate in chemical reactions even at high concentrations and temperatures (Han et al. 2010). The equation for the mixing ratio is as follows:
where R is the mixing ratio, expressed as the percentage of non-thermal groundwater; [Cl −MIX ] is the chloride content in the mixed thermal water of HR4, which was approximately 292 mg/L; \(\left[ {{\text{Cl}}_{\text{T}}^{ - } } \right]\) is the chloride content in the thermal waters HR1 and HR2, which was approximately 355–381 mg/L; and \(\left[ {{\text{Cl}}_{\text{C}}^{ - } } \right]\) is the chloride content in cold groundwater of HR6 from the Zaccar Massif. The estimated mixing ratio is between 22.6 % and 29.2 %, and the results are reported in Table 4.
Mixing ratio values based on chloride contents are much lower than those obtained by the silica-enthalpy mixing model of Bouchareb-Haouchine et al. (2012) and give a mixing ratio of 60 %. The mixing proportion depends on the distance between the center of groundwater exploitation and the location near the fault zone. In the HRG field, HR4 samples are located along deep-seated northeast-southwest Miocene faults, which can cause a higher mixing ratio.
5 Stable isotope geochemistry
The isotopic signature of thermal waters can act as a tracer for the fluid origins and reservoir processes in geothermal systems (Craig et al. 1956; Craig 1963; White 1986). The δ18O and δD values are reported in Table 5, and they range from −7.7 to −8.2 ‰ and −47 to −49 ‰, respectively (Fig. 8b).
The thermal waters from the HRG field fall along the Global Meteoric Water Line (GMWL; Fig. 8b), the equation for which is δD = 8.13 × δ18O + 10.8 (Rozanski et al. 1993). This suggests a meteoric origin for the thermal waters.
The plot shows the absence of an oxygen shift towards positive values, which indicates that there is no interaction with the host rock and suggests that the thermal waters belong to a low-enthalpy resource. The downward shift of the δD values of the samples relative to local meteoric water reflects the high altitude of the recharge zone area (the Zaccar Massif, at 1200 m ASL). The meteoric water likely infiltrated through a deep-seated fault network and was heated during deep circulation. Recharge probably took place in the fractured Jurassic limestone and dolomite of the Zaccar Massif. The thermal waters preserved heat when rising up to the ground surface by interaction with the Triassic evaporite sequence bordering the hydrothermal conduit (fault). The negative shift in δ18O values from HR3-13 to HR1-13 and HR2-13 is likely due to interaction with CO2 from carbonated rocks.
The tritium (3H) value in the thermal waters varies between 3.3 TU for HR1, 6 TU for HR4, and 15.7 TU for HR5, while the tritium value of meteoric water from northern Algeria is approximately 50 TU (Issaadi 1992). The elevated 3H content in thermal waters of the HRG field is likely explained by mixing with surface waters, while the 3H increase from the upflow area represented by HR1 to the recharge zone area represented by HR5 indicates the flow direction. The groundwater component present in the mixed thermal waters can be deduced using the following simple equation:
where X stands for the groundwater component in mixed thermal waters given in %; Tsp represents the tritium value of HR1 and HR4; and Tgw indicates the tritium value of HR5 (the cold spring from the Zaccar Massif). Mixing in the Hammam Righa thermal waters varies between 21 % and 38 %.
6 Discussion and conclusions
The thermal waters from the HRG field are classified as two main types. The first is Ca–Na–SO4 (Cl) and Ca–Na–SO4 (HCO3), represented by HR1, HR2, HR4, and HR3 waters mainly located in the upflow area of the Hammam Righa, suggesting an interaction with gypsum/anhydrite-bearing Triassic formations. The second is cold springs located in the Zaccar Massif, represented by HR5 and HR6, indicating a Ca–Na–HCO3 water type. This mineralization heterogeneity in the waters results from the complex geological features of the Tellian sector. Dissolution of gypsum/anhydrite in HR1, HR2 and HR4 exerts an important control on the chemistry of hot water. Dissolution of calcite is a typical characteristic of cold water from the Zaccar area.
Na/K geothermometers give a wide range of results between 95 and 185 °C, because of the enrichment of Na from the dissolution of halite minerals in Triassic evaporites. The K/Mg geothermometer records lower temperatures than the thermal water discharge (35–45 °C) because of Mg leached from near-surface reactions with Jurassic dolomite and mixing with shallow groundwater. This effect is apparent from the position of the waters in the Na–K–Mg diagram, which fall close to the Mg corner in the immature water field. Na–K–Ca geothermometers without Mg correction give suitable reservoir temperatures that are similar to those estimated by the quartz geothermometer (Verma 2000). However, temperatures estimated by the silica geothermometers (Fournier and Potter 1982; Verma 2000) give the most suitable temperatures (95 °C for HR1, 92 °C for HR2, and 78 °C for HR4). The fluid mineral equilibria overlap in the same range, confirming the temperatures given by the silica geothermometers.
According to stable isotope data (δ18O and δD values), meteoric waters recharged at higher altitudes (the Zaccar Massif at 1200 m ASL) circulated through deeper fault networks that acted as conduits for ascending water and saline sediments that preserve heat and maintain pressure (Fig. 9). The high heat flow in northern Algeria is caused by the tectonic setting and supplies the required heat to the geothermal system. Ascending thermal water is conductively cooled and loses heat to surrounding host rocks in HR1 and HR2 or is mixed with cooler Mg-rich shallow groundwater that is mainly responsible for the temperature decrease (e.g., HR4, which has a mixing ratio 22.6 > R > 29.2 % in Table 4). Assuming a higher geothermal gradient in the Tellian zones of northern Algeria, which can reach 33 °C km−1 (Bouchareb-Haouchine 2012); \(T = T_{0} + \frac{\partial T}{\partial Z}Z\), where T 0 is a surface temperature of approximately 15 °C and \(\frac{\partial T}{\partial Z}\) represents the geothermal gradient of the HRG field. Therefore, the circulation depth Z is approximately 2.1–2.2 km at HR1 and HR2 and 1.9 km for HR4. According to the geologic cross-section (Fig. 9), these depths correspond to the Jurassic dolomite and limestone sequence, which acts as a reservoir. The possible contribution of the Miocene calc-alkaline intrusion of 16–10 Ma in the heating process should be rejected, as the geothermal system is a non-volcanogenetic system.
References
Alacalı M, Savaşçın MY (2015) Geothermometry and hydrothermal alteration at the Balçova geothermal field, Turkey. Geothermics 54:136–146
Auboin J, Durand-Delga M (1971) Aire mediterraneenne. Encyclopidia Universalis 10:743–745
Belhai D (1996) Evolution tectonique de la zone Ouest-algeroise (Tenes-Chenoua), Approche stratigraphique et structurale. These Doctorat d’ Etat., Univ. Sci. et Tech., Alger
Belhai M, Bouchareb-Haouchine FZ, Fujimitsu Y (2013) Geochemistry of the Hammam Righa Thermal Manifestation North-Central Part of Algeria. Symposium on Earth Sciences and Technology. Kyushu University, Fukuoka, Japan
Bellon H (1976) Séries magmatiques néogènes et quaternaires du pourtour méditerranéen occidental, comparées dans leur cadre géochronométriques. Implications géodynamiques, thèse d’État, université Paris-Sud–Orsay
Bouaziz S, Barrier E, Soussi M, Turki M, Zouari H (2002) Tectonic evolution of the northern african margin in Tunisia from paleostress data and sedimentary record. Tectonophysics 357:227–253
Bouchareb FZ (1987) Contribution à l’étude hydrogéologique des souces thermo-minérales de Hammam-Righa. Mémoire d’ingéniorat d’état, I.S.T, Univ. Sci. Tech. USTHB Alger
Bouchareb-Haouchine FZ (1993) Apports de la géothermométrie et des données de forages profonds à l’identification des réservoirs géothermiques de l’Algérie du Nord. Application à la région du Hodna. Thèse de Magister, Univ. Sci. Tech. USTHB Alger
Bouchareb-Haouchine FZ (2012) Etude Hydrochimique des Sources Thermales de l’Algérie du Nord- Potentialités Géothermiques. These Doctorat en Sciences, USTHB, Algiers
Bouchareb-Haouchine FZ, Boudoukha A, Haouchine A (2012) Hydrogéochimie et Géothermométrie: Apports à l’identification du réservoir thermal des sources de hammam Righa, Algérie. H.S.J. Hydrol Sci J 57(6):1184–1195
Capaccioni B, Tassi F, Renzulli A, Vaselli O, Menichetti M, Inguaggiato S (2014) Geochemistry of thermal fluids in NW Honduras: new perspectives for exploitation of geothermal areas in the southern Sula graben. J Volcanol Geoth Res 280:40–52
Craig H (1963) The isotopic geochemistry of water and carbon in geothermal area. In: Tongiori E (ed) Nuclear geology in geothermal areas. Consiglio Nazionale delle Ricerche, Laboratorio di Geologia Nucleare, Spoleto
Craig H, Boato G, White DE (1956) Isotopic geochemistry of thermal waters. Natl Acad Sci 400:29–38
Domzig A, Yelles AK, Le Roy C, Déverchère J, Bouillin JP, Bracene R, Mercier de Lépinay B, Le Roy P, Calais E, Kherroubi A, Gaullier V, Savoye B, Pauc H (2006) Searching for the Africa-Eurasia Miocène boundary offshore western Algeria (MARADJA’03 cruise). Comptes Rendus Geosci 338:80–91
Fekraoui A, Kedaid FZ (2005) Geothermal resources and uses in Algeria: a country update report proceedings world geothermal congress 2005 Antalya, Turkey, 24–29 April
Fournier RO (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:41–50
Fournier RO (1979) A revised equation for Na/K geothermometer. Geoth Res Council Trans 3:221–224
Fournier RO (1992) Water geothermometers applied to geothermal energy. In: D’Amore, F. (Coordinator), Application of geochemistry in geothermal reservoir development. UNITAR/UNDP, Vial del Corso, Italy, pp. 37–69
Fournier RO, Potter RW (1979) Magnesium correction to the Na–K–Ca chemical geothermometer. Geochim Cosmochim Acta 43:1543–1550
Fournier RO, Potter RW (1982) A revised and expanded silica (quartz) geothermometer. Geothermal Research Council Bulletin, 3–12 November
Fournier RO, Truesdell AH (1973) An empirical Na–K–Ca geothermometer for natural waters. Geochim Cosmochim Acta 37:1255–1275
Fourré E, Di Napoli R, Aiuppa A, Parello F, Gaubi E, Jean-Baptiste P, Allard P, Calabrese S, Ben Mamou A (2011) Regional variations in the chemical and helium–carbon isotope composition of geothermal fluids across Tunisia. Chem Geol 288:67–85
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc., Englewood Cliffs
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. Norton, New York
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765
Giggenbach WF (1992) Isotopic composition of geothermal water and steam discharges. In: D’Amore F. (coordinator) Application of geochemistry in Geothermal Reservoir development.UNITAR/UNDP, Vial del Corso, Italy, pp. 253–273
Glangeaud L (1932) In: Cadoret Y (ed) Etude géologique de la région littorale de la Province d’Alger. Impr. de l’Univ., Bordeaux
Guo Q, Wang Y (2012) Geochemistry of hot springs in the Tengchong hydrothermal areas, Southwestern China. J Volcanol Geotherm Res 215–216:61–73
Guo Q, Wang Y, Liu W (2009) Hydrogeochemistry and environmental impact of geothermal waters from Yangyi of Tibet, China. J Volcanol Geotherm Res 180:9–20
Han DM, Liang X, Jin MG, Currell MJ, Song XF, Liu CM (2010) Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou basin. J Volcanol Geotherm Res 189:92–104
INRH (Institut National des Ressources Hydraulique), (1976) Contribution à l’estimation des potentialités hydriques du massif du Zaccar Chergui. Institut National des Ressources Hydriques, Note interne, rapport de mission, Alger
Issaadi A (1992) Le Thermalisme dans son Cadre Geostructural, Apport a la connaissance de la structure profonde de l’Algérie et de ses Ressources Géothermales. These Doctorat d’Etat., Univ. Sci. et Tech., Alger
Lepvrier C, Velde D (1976) À propos des intrusions tertiaires de la marge nord-africaine entre Cherchel et Ténès (Algérie). Bull Soc Géol France 18(7):991–998
Mattauer M (1958) Carte géologique de l’Ouarsenis oriental, 2e éd. Editeur Serv. Cart. Géol. Algérie. 1/200000
Mutlu H (1998) Chemical geothermometry and fluid-mineral equilibria for the Omer-Gecek thermal waters, Afyon area, Turkey. J Volcanol Geotherm Res 80:303–321
Nicholson KN (1993) Geothermal fluids. Chemistry and exploration techniques. Springer, Berlin
Nieva D, Nieva R (1987) Development in geothermal energy in Mexico, part 12—a cationic composition geothermometer for prospection of geothermal resources. Heat Recovery Syst CHP 7:243–258
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey: Earth Science Information Center, Open-File Reports Section [distributor], Water-Resources Investigations Report 99-4259
Pasvanoğlu S (2013) Hydrogeochemistry of thermal and mineralized waters in the Diyadin (Ağri) area, Eastern Turkey. Appl Geochem 38:70–81
Pasvanoğlu S, Chandrasekharam D (2011) Hydrogeochemical and isotopic study of thermal and mineralized waters from the Nevşehir (Kozakli) area, Central Turkey. J Volcanol Geotherm Res 202:241–250
Rozanski K, Araguás-Araguás L, Gonfiantini R (1993) Isotopic patters in modern global precipitation. In: Swart PK et al (eds) Climate change in continental isotopic records. American Geophysical Union Monogr Ser, vol 78. American Geophysical Union, Washington, pp 1–36
Saibi H (2015) Geothermal resources in Algeria. In: Proceedings in World Geothermal Congress 2015, Melbourne, Australia, 19–24 April
Simmons SF (2002) Geochemistry Lecture Notes, Semester I, Geotherm 601, 602, 603, Geothermal Energy Technology Course Geothermal Institute, University of Auckland, New Zealand
Tarcan G (2005) Mineral saturation and scaling tendencies of waters discharged from wells (> 150°C) in geothermal areas of Turkey. J Volcanol Geotherm Res 142:263–283
Tonani F (1980) Some remarks on the application of geochemical techniques in geothermal exploration. In: Proc. Adv. Eur. Geoth. Res., Second Symposium, Strasbourg, pp. 428–443
Truesdell AH (1976) Summary of section III. Geochemical techniques in exploration. In: Proceeding 2nd UN symposium on the development and use of geothermal resources, San Francisco, 1975, 1, liii–lxxix
Verma MP (2000) Revised quartz solubility temperature dependence equation along the water–vapor saturation curve. In: Proceedings of the 2000. World Geothermal Congress, Kyushu and Tohoku, Japan, 28 May–19 June, pp. 1927–1932
White AF (1986) Chemical and isotopic characteristics of fluids within the baca geothermal reservoir, Valles Caldera, New Mexico. J Geophys Res 91:1855–1866
Wildi W (1983) La chaine tello-rifaine. Structure, stratigraphie et évolution du Trias au Miocène. Rev Geol Dyn et Geogr Phys 24:201–297
Acknowledgments
We would like to express our sincere thankful acknowledgement for the MEXT (Ministry of Education, Culture, Sports, Science and Techn ology, Japan) Ph.D. scholarship providing support for the first author during this study. We would also like to show our sincere gratitude and acknowledgement to the G-COE of Kyushu University for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belhai, M., Fujimitsu, Y., Bouchareb-Haouchine, F.Z. et al. A hydrochemical study of the Hammam Righa geothermal waters in north-central Algeria. Acta Geochim 35, 271–287 (2016). https://doi.org/10.1007/s11631-016-0092-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-016-0092-8