Abstract
Hildegardia populifolia (Roxb.) Schott & Endl. is a critically endangered medicinal tree species which also provide high-quality natural fibers. The present study was focused to develop reliable micropropagation protocol where nodal segment explants were inoculated on Murashige and Skoog medium (MS) and Woody Plant Medium (WPM) augmented with various cytokinins alone or with auxins. WPM was more effective producing up to 10.67 ± 0.88 shoots per explant with average shoot length of 4.20 ± 0.10 cm from nodal explants cultured for 6 wk on medium containing 5.0 μM 6-benzylaminopurine (BA) and 2.0 μM 1-naphthaleneacetic acid (NAA). Microshoots rooted efficiently with a 24-h pulse treatment ex vitro with 200 μM indole-3-butyric acid (IBA) producing 5.33 ± 0.33 roots per microshoot with mean root length of 4.77 ± 0.07 cm. The ex vitro rooting and simultaneous acclimatization resulted in a 98.33 ± 1.67% survival rate in soilrite. Genetic stability was confirmed by using DNA-based molecular markers. Various photosynthetic and biochemical parameter coupled with scanning electron microscopic analysis of leaves revealed the healthy adaptation of the plantlets to natural conditions. Gas chromatography and mass spectrometry analysis of leaf of mother and regenerated plantlets was performed to compare secondary metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hildegardia populifolia (Roxb.) Schott. & Endl. is a medium size deciduous tree, belonging to the family (Malvaceae) growing to a height of 15 to 20 m and easily recognizable by its smooth, pale green, fiber-rich stem bark (Lavanya et al. 2014; Raju et al. 2014). It is native to Eastern Ghats of Andhra Pradesh and Tamil Nadu in India (Ahmedullah and Nayar 1987; World Conservation Monitoring Centre 1998; Sarcar and Sarcar 2002). The distribution of the tree is very restricted in the forested eastern slopes of the Kalrayan Hills where approximately twenty trees survive (Ahmedullah 1990; Nayar and Sastry 1990; Oldfield et al. 1998). According to the IUCN Red List of Threatened Species (1998:e.T33656A9801072), this plant is in the critically endangered category (World Conservation Monitoring Centre 1998<www.iucnredlist.org>). This tree is utilized for its stem bark fibers, wood, and gum by local people leading to the habitat clearance and loss of this species.
H. populifolia has both economic and ethnic values. Leaves and stem bark of the plant are used for malaria and dog bite treatment in the traditional system of medicinal practice of Tamil Nadu and Andhra Pradesh (Varaprasad et al. 2009). Methanolic leaf extract and stem bark of this tree are reported to have pharmacologically significant compounds with antioxidant, anti-diabetic, antimalarial, anti-inflammatory, anti-cancer, antimicrobial activities and healing properties against a number of human pathogens (Lavanya et al. 2012; Saradha and Paulsamy 2012a and 2013; Gritto et al. 2015; Subbalakshmi and Pullaiah 2015). The fiber obtained from stem bark of H. populifolia is economically valuable because they are arranged in uniaxial direction, slightly inter-woven, and loosely bound together. It is used as a substitute for synthetic fibers, carbon, and glass for packaging and medical applications and is used as a substitute for plastic (Li et al. 2004; Rajulu et al. 2004 and 2005; Guduri et al. 2006; Jeevan Prasad Reddy et al. 2009; Dani et al. 2011). Previous investigations indicate that its fiber can be used as reinforcement in green composites as they are completely biodegradable (Li et al. 2004; Rajulu et al. 2005). These fibers are used for their strength, even at low density, to make natural fiber reinforced polymer composites (Rajulu et al. 2003). The fiber extracted from the stem bark is also used for domestic purposes for making ropes and baskets by the local population (Raju et al. 2014).
It is presumed that anthropogenic interferences, habitat loss, and intrinsic and extrinsic factors have resulted in poor H. populifolia regeneration and low seed viability (Nayar and Sastry 1990; Anuradha and Pullaiah 2001). Fungal infections during maturation stage may damage the cotyledons and embryos thus drastically reducing the number of viable seeds and is one of the reasons for the limited survival rate and reduced population size of seedlings in their natural habitat (Anuradha and Pullaiah 2004). Raju et al. (2014) reported that an attempt to germinate seeds in a forest nursery sown in polythene bags contains fertile soil and watered daily showed a germination rate of only 0.42%. The seeds germinated after 18 wk and the subsequent growth of seedling was very low. However, Saradha and Paulsamy (2012b) reported that H. populifolia could be clonal propagation through mature stem cuttings, although Lavanya et al. (2012) reported that this approach is not efficient to apply the mature stem cutting method under varied climatic conditions to repopulate this species to its habitat. Furthermore, H. populifolia seeds are heterozygous in nature and impossible to use for pure breeding lines. Because of these limitations, in vitro regeneration of H. populifolia was considered as a means to increase the numbers of this endangered species.
Development of H. populifolia in vitro cultures using nodal explants from a H. populifolia mother plant was initiated to regenerate true-t-type plants for large-scale production and conservation. Nutrient media (MS-Murashige and Skoog Medium and WPM-Woody Plant Medium) were compared to obtain the best morphogenic response. Considering the importance of acclimatization of in vitro raised plantlets, parameters such as pigment content, net photosynthetic rate, and antioxidant enzymes were also screened, in addition to ultra-structural studies of leaf texture from mother and in vitro raised plantlets. Gas chromatography and mass spectrometry were used to analyze the important metabolites in H. populifolia.
Materials and Methods
Collection of explants and establishment of aseptic cultures
Nodal segment (NS) explants of H. populifolia were excised from a 3-y-old established plant (mother plant) in the green house of the Botany Department, A.M.U., Aligarh, U.P., India. Before the inoculation, the NS were thoroughly rinsed in tap water for 30 min followed by a treatment with 1% (w/v) Bavistin solution (carbendazim powder, BASF India Ltd., Mumbai, India) for 30 min. The NS were also cleaned with 5% (v/v) Teepol (liquid detergent) for about 15 min, surface sterilized with 0.1% (w/v) freshly prepared HgCl2 (Qualigens, Mumbai, India) solution for 3 min, and rinsed with sterilize DDW (double distilled water) for 4 to 5 times in a laminar air flow hood.
Murashige and Skoog Medium (Murashige and Skoog 1962) and Woody Plant Medium (McCown and Lloyd 1981) were used in all the experiments. A single NS explant was inoculated per culture tube containing 20 mL nutrient medium augmented with 0 to 10.0 μM 6-benzyladenine (BA), kinetin (Kn), or thidiazuron (TDZ) for shoot induction and multiplication. For further growth and proliferation, the optimized cytokinin treatment was tested in combination with 0 to 3.0 μM indole-3-butyric acid (IBA), indole-3-acetic acid (IAA), or 1-naphthaleneacetic acid (NAA). Medium devoid of any plant growth regulators was the control. The media were also augmented with 3% (w/v) sucrose (Qualigens Fine Chemicals) and 0.8% (w/v) agar (Bacteriological grade, Hi-media, Mumbai, India). The pH of the medium was adjusted to 5.8 using 1 N HCl or 1 N NaOH, autoclaved for 15 min at 121°C and 15 psi pressure, and dispensed in 25 × 150 mm culture tubes (Borosil, Mumbai, India) and 100 cm3 Erlenmeyer flasks (Borosil). All the cultures were incubated under standard culture room conditions, i.e., 25 ± 2°C temperature, 55% ± 5% relative humidity, and 16/8 h photoperiod having 50 μmol m-2 s-1 PPFD (photosynthetic photon flux density) supplied by cool fluorescent lights (40 W, Philips, India).
Rooting and acclimatization
For ex vitro rooting, the basal cut end of regenerated healthy microshoots with well-expanded leaves was removed from culture and dipped in 0, 100, 150, 200, 250, or 300 μM of IAA, IBA, or NAA for 24 h and then planted in small thermocol cups (height 8.2 cm, top diameter 7.2 cm, bottom diameter 4.2 cm, purchased from Gulmarg Chemicals & Scientific Works, Aligarh, India) containing sterilized soilrite (Keltech Energies Pvt. Ltd., Bengaluru, India) and kept in the culture room for 4 wk at 25 ± 2°C and 16/8 h photoperiod. These thermocol cups were enclosed within transparent polythene bags, having small perforations for the purpose of gaseous exchange and to stabilize relative humidity after being placed in the culture room. After 4 wk, rooted regenerated plantlets were transferred to thermocol cups containing three different planting substrates: garden soil plus manure (3:1), soilrite, and vermicompost and kept in culture room for 4 wk. These regenerated plantlets were regularly irrigated with 1/4 strength MS salt solution (without vitamins) followed by tap water for 2 wk. Polythene bags were then removed and the plantlets were placed under direct exposure to the fluorescent light in culture room for another 2 wk, moved to the green house for 2 wk, and then transplanted to the clay pots containing garden soil followed by transfer from the green house to the field.
Genetic fidelity
To confirm the genetic fidelity, nine acclimatized regenerated plantlets were randomly selected and compared with the mother plant. Genomic DNA was isolated from fresh leaf tissues of H. populifolia using cetyltrimethyl ammonium bromide (CTAB) method according to Doyle and Doyle (1990). The purity (A260/280 ratio) of isolated DNA was performed on a UV-vis spectrophotometer. A total set of 10 random amplified polymorphic DNA (RAPD) (OPL Kit) and 10 inter simple sequence repeats (ISSR) (UBC, Vancouver, Canada) primers were used for PCR analysis on a thermocycler (Biometra, T Gradient, Thermoblock, Germany). The reaction mixture preparation, PCR amplification, and amplicons separation were performed by following the methods of Ahmad et al. (2018b).
Scanning electron microscopy analysis
For SEM analysis, leaf samples were taken from in vitro cultured plantlets and from the 4-wk-old acclimatized plants. The leaves were fixed in glutaraldehyde (Merck, Merck Specialties Pvt. Ltd., Mumbai, India) and kept at room temperature for 2 h followed by gradual dehydrated with an increasing alcohol step-wise series (30%, 50%, 70%, 90%, and 100%) with 15 min at each step. The samples were subjected to critical point drying and then abaxial surface of leaves coated with gold. Samples were mounted on aluminum stubs with double-sided adhesive tape (3M, Sumaré, Brazil) and examined by SEM (JSM-6510, LV-JEOL, Tokyo, Japan) at 15 kV where all images of leaf surface were processed digitally.
GC-MS analysis
Mature and healthy leaves were used from 4-wk-old successfully acclimatized plant as well as from a mother plant for GC-MS analysis. Ten replicates were used for the GC-MS study. Harvested leaves were washed and air dried for 2 to 3 d and then crushed to a fine powder using a mortar and pestle. One gram of powder was dissolved in 50 mL of methanol and contents were extracted for 24 h. The methanolic leaf extract was centrifuged at 5000 RCF for 5 min and supernatant filtered with an aminigen syringe filter (0.22 μm, Micro-por, Genetix Biotech Asia Pvt. Ltd., New Delhi, India) to remove any remaining residues. Finally, the total volume of each extraction was brought up to 10 mL with methanol solvent and used for phytochemical profiling. One microliter of methanolic leaf extract was used as sample and manually injected into RTX-5 column of GCMS (QP-2010 Ultra, Shimadzu, Kyoto, Japan) operating at 1000 eV ionization energy using helium as carrier gas at 173 kpa inlet pressure. Identification and confirmation of phytochemicals was performed using the database of the National Institute of Standards and Technology (NIST) and Wiley Library for mass spectra to determine their molecular weight (MW).
Physiological and biochemical studies
In vitro raised plantlets showing healthy growth were selected for physiological and biochemical analysis. A set of ten micropropagated plantlets were randomly selected and maintained separately from the other plants in the culture room. Leaf samples were taken at transplantation (0 d, control) and after 7, 14, 21, and 28 d of acclimatization.
Total chlorophyll content and net photosynthetic rate estimation
To estimate the total chlorophyll content of the leaves, 100 mg of fresh tissues was taken from interveinal areas of leaves and crushed in acetone (80%, 5 mL) using mortar and pestle and filtered using Whatman’s No.1 filter paper. The optical density (O.D.) of filtrate was read at wavelengths 645 and 663 nm for chlorophyll evaluation using a UV-visible spectrophotometer. The estimation of total leaf chlorophyll content was performed according to Mackinney (1941).
Expanded plant leaves were selected for the estimation of net photosynthetic rate (PN). This was evaluated by using a LI-COR 6400 infrared gas analyzer (IRGA; LI-COR Biosciences, Lincoln, NE) at 800 μmol/m2s photosynthetically active radiation (PAR) between 11:00 a.m. and 12:00 noon under the clear sunlight using a leaf chamber. The monitoring was based on the exchange of CO2 between leaf and atmosphere.
Biochemical enzymes evaluation
To evaluate superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX) antioxidant enzyme activity, 0.5 g of fresh leaf tissues was homogenized in 2.0 mL of extraction buffer composed of 1% (w/v) of polyvinylpyrrolidone (PVP), 1% (v/v) of Triton X-100, and 0.11 g of ethylene diamine tetra acetic acid (EDTA) in water using a pre-chilled mortar and pestle. Homogenate was filtered with Whatman’s No.1 filter paper and then centrifuged at 15000 RCF for 20 min using a high-speed centrifuge (Remi Instruments Ltd., Mumbai, India). The extraction was performed in darkness at 4°C and the supernatant was used as a crude extract for enzyme assays. The activity of SOD, CAT, GR, and APX was evaluated according to Dhindsa et al. (1981), Aebi (1984), Rao (1992), and Nakano and Asada (1981) respectively and quantified as enzyme units (EU) mg−1 protein.
Statistical analysis
To analyze regeneration percentage, data for number of shoots per explants and shoot length were recorded after 6 wk of culture while for rooting, experiment data were recorded after 4 wk of culture. Twenty replicates per treatment with three repetitions were taken to conduct the in vitro propagation experiments and the obtained data was analyzed by one-way analysis of variance (ANOVA) using SPSS Version 16 (SPSS Inc., Chicago, IL). Duncan’s multiple range test (DMRT) at P ≤ 0.05 was used to carry out the significance of difference among means and the results were denoted as mean ± SE. Graphically, the data were presented by using Sigma Plot ver. 10.0 (Systat Software, Inc., San Jose, California).
Results and Discussion
In vitro plant regeneration
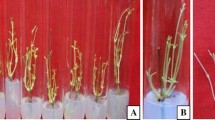
An improved micropropagation protocol has been developed for H. populifolia. Types and concentration of plant growth regulators and basal media had a significant effect on shoot and root formation. Comparative nutrient media comprising of MS and WPM basal salts were used to evaluate the best morphological response of the explants. In the present study, NS explants were inoculated on MS and WPM basal media with or without (control) PGRs (Fig. 1a). WPM was the superior nutrient medium compared with MS. BA, Kn, and TDZ (0, 0.5, 2.5, 5.0, 7.5, and 10.0 μM) were tested with both nutrient media. Initially, the explants swelled within 1 wk and, after 12 d, green protuberance appeared followed by organized shoot buds with leaf primordial after 18 d. Among the cytokinins tested, WPM supplemented with of 5.0 μM BA was found most significant with a maximum of 9.33 ± 0.33 shoots per explant; mean shoot length (SL) of 3.57 ± 0.09 cm from over 93% of the cultures was recorded after 6 wk (Table 1, Fig. 1c). Lowering or increasing the concentration of BA affected shoot number with 2.67 ± 0.88 and 4.00 ± 0.58 shoots per explant using 0.5 and 10.0 μM BA, respectively (Table 1). MS medium containing 5.0 μM BA gave rise to 7.67 ± 0.33 shoots per explant with a mean SL of 3.07 ± 0.07 cm from approximately 88%the cultures after 6-wk incubation (Table 1, Fig. 1b). BA was effective in bud breaking and promoted formation of multiple shoots due to enhanced permeability across the plasma membrane and its high cellular uptake (Malik et al. 2005). Superiority of BA among cytokinins has been also established by several workers such as in Trichosanthes dioica (Saurabh et al. 2017) and Rauwolfia serpentine (Zafar et al. 2019).
In vitro plant regeneration and establishment of Hildegardia populifolia (Roxb.) Schott. & Endl. (a) Nodal explant inoculated on Woody Plant Medium (WPM) basal medium. (b) Shoot induction and regeneration on Murashige and Skoog (MS) + 5.0 μM 6-benzyladenine (BA). (c) Shoot induction and regeneration on WPM + 5.0 μM BA. (d) Multiplication and proliferation of shoots on MS + 5.0 μM BA +2.0 μM 1-naphthaleneacetic acid (NAA). (e) Multiplication and proliferation of shoots on WPM + 5.0 μM BA +2.0 μM NAA.
The optimum concentration of BA (5.0 μM) was used in combination with 1.0, 2.0, and 3.0 μM NAA, IAA, or IBA in WPM and MS media (Table 2). Between the two basal media and cytokinin-auxin combinations tested, WPM supplemented with 5.0 μM BA and 2.0 μM NAA produced the maximum number of shoots per explant (10.67 ± 0.88) and greatest average SL (4.20 ± 0.10 cm) from over 98% of the cultures after 6 wk of incubation (Table 2, Fig. 1e). This is supported by Nakagawa et al. (2005) and Samantaray et al. (2013) where the combined effect of cytokinin and auxin was effective in regulation of apical dominance and morphogenesis in Petunia hybrida and Vitex trifolia, respectively. In our study, MS medium augmented with 5.0 μM BA and 2.0 μM NAA showed improved shoot proliferation but less effective in comparison with WPM medium as only 8.33 ± 0.33 shoots per explant were produced with an average SL of 3.37 ± 0.03 cm from over 93% of the cultures after 6 wk of incubation (Table 2, Fig. 1d). There are reports demonstrating the synergism of cytokinin and auxin in promoting in vitro culture from medicinal plants such as Decalepis salicifolia (Ahmad et al. 2018b) and Magnolia sirindhorniae (Cui et al. 2019).
Rooting and acclimatization
For ex vitro rooting, 4 to 6-cm-long microshoots were excised from WPM supplemented with 5.0 μM BA and 2.0 μM NAA. These microshoots were treated with 100, 150, 200, 250, and 300 μM IAA, IBA, or NAA for 24 h under sterile conditions and transferred directly to the thermocol cups containing sterilize soilrite (Fig. 2a). The optimal treatment was 200 μM IBA which produced 5.33 ± 0.33 roots per microshoot with an average root length of 4.77 ± 0.07 cm after 4 wk following a 24-h pulse treatment (Table 3, Fig. 2b, c). The lower concentrations of the auxins tested failed to induce rooting in the microshoots. The IBA-treated roots were healthy in nature, thick, and profusely branched, while short, fragile, fibrous roots having lesser number of secondary branching were observed on IAA and NAA at similar concentrations. Increased concentration of the auxin PGRs beyond the optimal level resulted in reduced root production after similar incubation periods (Table 3). A critical step in micropropagation is the transfer of regenerated plants from in vitro to greenhouse conditions. Regenerated plantlets with fully expanded leaves and well-developed root system were again hardened in thermocol cups, now containing three different planting substrates: garden soil and manure (3:1), soilrite, and vermicompost. Among these potting substrates, soilrite was the optimal substrate for acclimatization of regenerated plantlets with over 98% survival (Fig. 3). Soilrite gave optimal rooting because it is porous in nature and has good water holding capacity because of the presence of peat moss and vermiculite to support a healthy root system. After acclimatization in soilrite, the regenerated plantlets were transferred to garden soil and exhibited nearly 80% survival in greenhouse conditions. After 4 mo in soil, the plants had normal growth and morphology similar to those in nature (Fig. 2d, e).
Ex vitro rooting in Hildegardia populifolia (Roxb.) Schott. & Endl. (a) Microshoot treated with 200 μM indole-3-butyric acid transplanted in soilrite potting substrate. (b) Ex vitro rooted microshoot after 4 wk of culture. (c) Exposed view of above culture. (d) Regenerated plantlet hardened in earthen pots containing garden soil. (e) Successfully established an acclimatized plant.
Genetic fidelity
Uniformity in the genetic makeup of the regenerants as compared with the ex vitro grown mother plant is of immense practical importance in clonal propagation. Genetic fidelity of regenerants has been used to identify and remove somaclonal variants to maintain the genetic uniformity of the plants. Genetic fidelity of the regenerated plantlets was assessed using RAPD and ISSR DNA molecular markers. Mother plant and nine in vitro raised plantlets were randomly selected from the population of healthy growing plantlets and were subjected to molecular analysis. Among the set of ten RAPD primers screened from Kit OPL, nine primers produced clear, distinct, and reproducible bands (Table 4). Out of these, OPL − 5 produced a maximum of three monomorphic bands. All the amplified bands with respect to the molecular markers were scored between 100 and 1000 bp (Fig. 4a). Molecular profiling of regenerated plantlets were screened using ten UBC primers for ISSR markers, out of which nine primers produced clear and distinct bands (Table 5). UBC − 848 scored the maximum number of five monomorphic bands. In this case, more bands were depicted as compared with RAPD primers which were scored between 100 and 1500 bp of the molecular markers (Fig. 4b). The monomorphic banding pattern obtained from both molecular markers clearly depicted uniformity in the genetic makeup of the regenerated plants and the H. populifolia mother plant. Comparable results were obtained from Inula royleana (Amin et al. 2018), Decalepis salicifolia (Ahmad et al. 2018b), Prunus cerasifera (Nasri et al. 2019), and Hemidesmus indicus (Yadav et al. 2019) using RAPD and ISSR markers to confirm the genetic homogeneity.
DNA fingerprinting of mother plant and micropropagated plants of Hildegardia populifolia (Roxb.) Schott. & Endl. Obtained through (a) random amplified polymorphic DNA (RAPD) primer (OPL - 5) and (b) inter simple sequence repeats (ISSR) primer (UBC - 848) showing monomorphic banding pattern. 1 to 9 lanes show the DNA sample from regenerated plants and M shows the DNA from mother plant while L represent DNA ladder.
Ultra-structural study of in vitro and acclimated leaves
The anatomy of in vitro and acclimated leaves of H. populifolia were compared using SEM. The control conditions during in vitro culture resulted in abnormal anatomy associated with low light irradiance, gaseous exchange, and nutrients availability in culture vessels. A remarkable change was observed regarding the plant’s anatomy when transferred to greenhouse conditions. Electron micrographs of the abaxial surface of in vitro derived leaves showed highly constricted surface bearing few stomata which were deep seated and mostly closed (Fig. 5.1a), having unhealthy guard cells and uneven stomatal aperture (Fig. 5.1b). During acclimatization, regenerated plantlets slowly stabilized and achieved normal growth. Leaf characterization at this stage is a good indicator of plant health. SEM analysis of abaxial leaf surface of ex vitro acclimatized plantlets showed relaxed surface with numerous well-defined stomata (Fig. 5.2a), having clear aperture and functional guard cells as they possess both closed and open type of stomata (Fig. 5.2b). Related findings were also reported for Ceratonia siliqua (Shahzad et al. 2017) and Leucospermum cultivars (Suarez et al. 2019).
Scanning electron microscope (SEM) examination of in vitro derived leaves of Hildegardia populifolia (Roxb.) Schott. & Endl. (1a) abaxial surface of leaf showing deep seated closed stomata and (1b) unhealthy guard cells and uneven stomatal aperture of deformed stomata. SEM examination of leaf taken from acclimatized regenerated H. populifolia plantlet (2a) abaxial surface of leaf showing numerous well-developed stomata and (2b) open stomata having clear aperture.
GC-MS analysis
Previous work regarding the phytochemical analysis using different chromatographic techniques of H. populifolia has been reported by Saradha and Paulsamy (2013) and Gritto et al. (2015). However, our study is the first report with comparative analysis of mother and in vitro raised plantlet to examine the chemical uniformity and exploring the pharmacologically important metabolites by using GC-MS. Several compounds having pharmacological value in major and minor concentrations have been reported (Huang et al. 2009; Reddy and Couvreur 2009; Güneş 2013; Zalkhani and Moazedi 2020). More than forty compounds have been identified when GC-MS was performed for both mother and in vitro regenerated plants (Supp. Table 1 & 2, Figs. 6 and 7). Methanol was found to be the optimal solvent for extraction. The names of specific compounds, their retention time (RT), concentration (area and area %), molecular formula, and molecular weight (MW) from mother and in vitro regenerated plant are shown in Tables 6 and 7 respectively. Compounds such as squalene, methyl commate D, vitamin E, and 9-octadecenamidewere found in higher percentage from regenerated plants in comparison with the mother plant. The higher quantity of compounds from regenerated plants might be due to stress associated with in vitro culture. The combination of chromatography and mass spectrometry has been widely used for the screening of metabolites in several medicinal plants. In Cassia angustifolia leaves, Parveen et al. (2016) reported GC-MS data which showed the presence of 45 different phytocomponents on the basis of comparison of the mass spectrum of each constituent with NIST and Wiley libraries. Ahmad et al. (2017) also reported that methanolic leaf extract of Decalepis arayalpathra was rich in phytochemicals in comparison with the other polar solvents. Additional reports described GC-MS results from medicinal plant species including Zhumeria majdae (Fallah et al. 2019) and Hemidesmus indicus (Yadav et al. 2019).
Physiological and biochemical studies - Total chlorophyll and photosynthetic rate evaluation
During the first week, acclimatization of regenerated plants, reduction in total chlorophyll content (1.03 ± 0.14 mg g−1 to 0.57 ± 0.09 mg g−1) was observed (Fig. 8A). A gradual increase occurred during 4 wk of acclimatization and reached 1.80 ± 0.06 mg g−1 which correspond to increased photosynthetic efficiency (Fig. 8A). The phenomenon of reduction in chlorophyll content during the initial d of acclimatization was attributed to the poorly developed chloroplast with disordered grana (Pospóšilová et al. 1999). When the days of acclimatization increased, the total chlorophyll content also increased in micropropagated plants. A similar observation was also reported in Decalepis hamiltonii (Sharma et al. 2014) and D. arayalpathra (Ahmad et al. 2018a).
Changes in total chlorophyll content (A) net photosynthetic rate; PN (B) and antioxidant enzymes activity (C-SOD, D-CAT, E-GR, F-APX) in in vitro raised plantlets of Hildegardia populifolia (Roxb.) Schott. & Endl. during acclimatization. The value of bars represents mean ± SE. The same letter above the bars are not significantly different at P ≤ 0.05, using Duncan’s multiple range test (DMRT).
In vitro grown plants experience low CO2 and PPFD (photosynthetic photon flux density) with high air humidity (Van Huylenbroeck et al. 1998). A sudden change in the environmental conditions during regenerated plant transfer to ex vitro conditions results in environmental stress during plant acclimatization (Van Huylenbroeck et al. 1998). During the initial days of acclimatization, plants’ primary challenges are water loss due to poor stomatal functioning and absence of a thick cuticle. Therefore, a decrease in PN was recorded during the first wk of acclimatization (Fig. 8B). However, as the days of acclimatization increased, PN increased to its maximum value of 6.57 ± 0.07 μmol CO2 m−2 s−1 after 4 wk of acclimatization. Similar variation in PN was observed in other medicinal plants (Pospóšilová et al. 1988; El-Mahrouk et al. 2016; Yadav et al. 2019).
Antioxidant enzymes analysis
When plantlets are transferred from in vitro to ex vitro conditions, various stresses occur which promote the production of reactive oxygen species (ROS; Batková et al. 2008). Plant cells induce the production of various antioxidant enzymes such as SOD, CAT, GR, and APX to overcome the harmful effects of ROS (Mitrović and Bogdanović 2008; Kayihan et al. 2012; Xu et al. 2012). Antioxidant enzymes could reduce the oxidative damage to the plant by ROS when acclimating to a new environment (Yan 2009).
H. populifolia SOD activity increased from 1.70 ± 0.11 to a maximum of 4.17 ± 0.03 unit mg−1 protein after 21 d and then decreased to 3.63 ± 0.09 unit mg−1 protein after 28 d of acclimatization (Fig. 8C). Superoxide is converted to H2O2 and O2 by the activity of SOD, which played a significant role in the prevention of membrane oxidation and damage to biological molecules. The process of mitigating ROS is carried out by a series of affected membrane and stomatal enzymes, including SOD and APX, at the acceptor site of photosystem I (Scalet et al. 1995). Ahmad et al. (2018a) reported similar results in Decalepis arayalpathra where SOD activity increased after 7 d of acclimatization and then decreased after 28 d of acclimatization.
The decreased H2O2 levels suggest successful acclimatization. CAT reduced the effect of H2O2 in peroxisomes by its conversion into H2O and O2. A steady and gradual increase in CAT activity was observed which peaked at 321.67 ± 1.67 unit mg−1 protein after acclimatizing for 28 d (Fig. 8D). H. populifolia CAT activity increased in accordance with the findings in Decalepis arayalpathra and D. salicifolia (Ahmad et al. 2018a, 2018b). The photorespiratory detoxification of H2O2 into O2 and H2O in mitochondrial electron system is represented by increase in both CAT and SOD activities (Scandalios 1990).
GR and APX are two important enzymes of the ascorbate glutathione cycle involved with chloroplast-based detoxification of ROS via the Mehler pathway (Foyer and Mullineaux 1998). H. populifolia GR and APX enzyme activity increased gradually with the increase in days of acclimatization and peaked after 28 d at 9.40 ± 0.06 and 6.60 ± 0.06 unit mg−1 protein, respectively, (Fig. 8E, F) functioning in the cytosol, chloroplast, vacuoles, and apoplast (Asada 1999). The elevation of GR and APX during plant acclimatization was previously reported in Cynara scolymus (Pérez-Jiménez et al. 2015) and Hemidesmus indicus (Yadav et al. 2019).
Conclusions
A comprehensive protocol for large-scale micropropagation of H. populifolia uses a comparative nutrient media study and ex vitro physio-chemical analysis of regenerants. The true-to-type nature of the micropropagated plant was verified using DNA-based molecular markers. An active rise and fall in photosynthetic pigments and biochemical enzymes revealed the significance of plant adjustment during acclimatization to soil and hence can be correlated with the maximum survivability of the regenerated plants.
References
Aebi H (1984) Catalase in vitro methods. Methods in enzymology. Academic Press Inc 105:121–126
Ahmad Z, Shahzad A, Sharma S (2017) Evaluation of in vitro antioxidant activity, HPLC and GC-MS analysis along with chemoprofiling of Decalepis arayalpathra: a critically endangered plant of Western Ghats, India. Rend Fis Acc Lincei 28:711–720
Ahmad Z, Shahzad A, Sharma S (2018a) Enhanced multiplication and improved ex vitro acclimatization of Decalepis arayalpathra. Biol Plantarum 62:1–10
Ahmad Z, Shahzad A, Sharma S, Parveen S (2018b) Ex vitro rescue, physiochemical evaluation, secondary metabolite production and assessment of genetic stability using DNA based molecular markers in regenerated plants of Decalepis salicifolia (Bedd. Ex Hook.f.) Venter. Plant Cell Tiss Org Cult 132:497–510
Ahmedullah M (1990) In: Nayar MP, Sastry ARK (eds) Hildegardia populifolia (Roxb.) Schott. & Endl. Sterculiaceae. Red data book of Indian plants, vol 3. Botanical survey of India, Calcutta, pp 251–254
Ahmedullah M, Nayar MP (1987) Endemic plants of the Indian region- volume 1. Botanical Survey of India, Calcutta
Amin S, WaniTA KZA, Singh S, John R, Majeed U, Shapoo GA (2018) Genetic stability using RAPD and ISSR markers in efficiently in vitro regenerated plants of Inula royleana DC. Meta Gene 18:100–106
Anuradha T, Pullaiah T (2001) Effect of hormones on the organogenesis and the somatic embryogenesis of an endangered tropical Forest tree - Hildegardia populifolia (Roxb.) Schott. & Endl. Taiwania 46:62–74
Anuradha T, Pullaiah T (2004) In vitro germination studies on Hildegardia populifolia (Roxb.) Schott. & Endl. - an endangered tree taxon. The Indian Forester 130:1432–1438
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Batková P, Pospíšilová J, Synková H (2008) Prduction of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol Plant 52:413–422
Cui Y, Deng Y, Zheng K, Hu X, Zhu M, Deng X, Xi R (2019) An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci Rep 9:1–10
Dani J, Jeevan Prasad Reddy D, Rajulu AV, Li R (2011) Green composites from wheat protein isolate and Hildegardia populifolia natural fabric. Polym Compos 32:398–406
Dhindsa PS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissues. Focus 12:13–15
El-Mahrouk ME, Dewir YH, Murthy HN, Rihan HZ, Al-Shmgani HS, Fuller MP (2016) Effect of photosynthetic photon flux density on growth, photosynthetic competence and antioxidant enzymes activity during ex vitro acclimatization of Dieffenbachia cultivars. Plant Growth Regul 79:29–37
Fallah M, Farzaneh M, Yousefzadi M, Ghorbanpour M, Mirjalili MH (2019) In vitro mass propagation and conservation of a rare medicinal plant, Zhumeria majdae Rech.f & Wendelbo (Lamiaceae). Biocatal Agric Biotechnol 17:318–325
Foyer CH, Mullineaux PM (1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425:528–529
Gritto MJ, Nanadagopalan V, Doss A (2015) GC-MS analysis of Hildegardia populifolia (Roxb.) Schott. & Endl.: an endangered potential medicinal plant. J Pharm Biomed Sci 5:312–316
Guduri BR, Rajulu AV, Luyt AS (2006) Effect of alkali treatment on the flexural properties of Hildegardia fabric composites. J Appl Polym Sci 102:1297–1302
Güneş FE (2013) Medicinal use of squalene as a natural antioxidant. MÜSBED 3:220–228
Huang ZR, Lin YK, Fang JY (2009) Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 14:540–554
Jeevan Prasad Reddy D, Rajulu AV, Arumugam V, Naresh MD, Muthukrishnan M (2009) Effects of resorcinol on the mechanical properties of soy protein isolate films. J Plast Film Sheet 25:221–233
Kayihan C, Eyidogan F, Afsar N, Oktem HA, Yucel M (2012) Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol Plantarum 56:693–698
Lavanya AR, Muthukrishan S, Kumaresan V, Benjamin JFH, Ra MV (2012) In vitro micropropagation of Hildegardia populifolia (Roxb.) Schott & Endl., an endangered tree species from Eastern Ghats of Tamil Nadu, India. J Agric Technol 8:1727–1744
Lavanya AR, Muthukrishnan S, Muthukumar M, Benjamin JHF, KumarTS KV, Rao MV (2014) Indirect organogenesis from various explants of Hildegardia populifolia (Roxb.) Schott & Endl. - a threatened tree species from Eastern Ghats of Tamil Nadu, India. J Genet Eng Biotechnol 12:95–101
Li XH, Meng YZ, Wang SJ, Rajulu AV, Tjong SC (2004) Completely biodegradable composites of poly (propylene carbonate) and short, lignocellulose fiber Hildegardia populifolia. J Polym Sci B Polym Phys 42:666–675
Mackinney G (1941) Absorption of light by chlorophyll solution. J Biol Chem 140:315–322
Malik SK, Chaudhury R, Kalia RK (2005) Rapid in vitro multiplication and conservation of Garcinia indica: a tropical medicinal tree species. Sci Hort 106:539–553
McCown BH, Lloyd G (1981) Woody Plant Medium (WPM) - a mineral nutrient formulation for microculture of woody plant species. Hort Sci 16:453
Mitrović A, Bogdanović J (2008) Activities of antioxidative enzymes during Chenopodium rubrum L. ontogenesis in vitro. Arch Biol Sci 60:223–231
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakagawa H, Jiang CJ, Sakakibara H, Kojima M, Honda I, Ajisaka H, Nishijima T, Koshioka M, Homma T, Mander LN, Takatsuji H (2005) Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. Plant J 41:512–523
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specificperoxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nasri A, Baklouti E, Romdhane AB, Maalej M, Schumacher HM, Drira N, Lotfi F (2019) Large-scale propagation of Myrobolan (Prunus cerasifera) in RITA bioreactors and ISSR-based assessment of genetic conformity. Sci Hort 245:144–153
Nayar MP, Sastry ARK (eds) (1990) Red data book of Indian plants. Botanical Survey of India, Calcutta
Oldfield S, Lusty C, MacKinven A (compilers) (1998) The world list of threatened trees. World Conservation Press, Cambridge
Parveen S, Shahzad A, Upadhyay A, Yadav V (2016) Gas chromatography-mass spectrometry analysis of methanolic leaf extract of Cassia angustifolia Vahl. Asian J Pharm Clin Res 9:111–116
Pérez-Jiménez M, López-Pérez AJ, Otálora-Alcón G, Marín-Nicolás D, Piñero Zapata MC, Amor FMD (2015) A regime of high CO2 concentration improves the acclimatization process and increase plant quality and survival. Plant Cell Tiss Org Cult 121:547–557
Pospóšilová J, Solarova J, Catsky J, Ondrej M, Opatrny Z (1988) The photosynthetic characteristics during micropropagation of tobacco and potato plants. Photosynthetica 22:205–213
Pospóšilová J, Tichá I, Kadleček P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plantarum 42:481–497
Raju AJS, Chandra PH, Krishna JR (2014) Monoecy, anemophily, Anemochory and regeneration ecology of Hildegardia populifolia (Roxb.) Schott. & Endl. (Malvaceae), an economically important endemic and endangered dry deciduous tree species of southern Eastern Ghats, India. J Threat Taxa 6:5434–5446
Rajulu AV, Meng YZ, Li XH, Rao GB, Devi LG, Raju KM, Reddy RR (2003) Effect of alkali treatment on properties of the lignocellulose fabric Hildegardia. J Appl Polym Sci 90:1604–1608
Rajulu AV, Rao GB, Devi LG (2004) Tensile properties of natural fabric Hildegardia populifolia/polycarbonate toughened epoxy composites. Polym Compos 25:563–568
Rajulu AV, Rao GB, Devi LG, Ramaiah S, Prada DS, Bhat KS, Shylashree R (2005) Mechanical properties of short, natural fiber Hildegardia populifolia-reinforced styrenated polyester composites. J Reinf Plast Comp 24:423–428
Rao MV (1992) Cellular detoxifying mechanism determines age-dependent injury in tropical plants exposed to SO2. J Plant Physiol 140:733–740
Reddy LH, Couvreur P (2009) Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev 61:1412–1426
Samantaray S, Bishoyi AK, Maiti S (2013) Plant regeneration from callus cultures of Vitex trifolia (Lamiales: Lamiaceae): a potential medicinal plant. Rev Biol Trop 61:1083–1094
Saradha M, Paulsamy S (2012a) Antibacterial activity of leaf and stem bark extracts of the endangered tree species, Hildegardia populifolia (Roxb.) Schott. & Endl. (Sterculiaceae). J Res Antimicrob 1:23–27
Saradha M, Paulsamy S (2012b) Effect of growth hormones on rooting attributes of stem cuttings of endangered plant species, Hildegardia populifolia (Roxb.) Schott. & Endl. (Sterculiaceae). Int J Biol Pharm Allied Sci 1:1145–2277
Saradha M, Paulsamy S (2013) GC-MS analysis for bioactive compounds from methanolic leaf and stem bark extracts of Hildegardia populifolia (Roxb.) Schott. & Endl. Int J Pharm Sci Rev Res 23:328–332
Sarcar MK, Sarcar AB (2002) Status, botanical description, natural distribution zone, propagation practices and conservation efforts of Hildegardia populifolia (Roxb.) Schott & Endl. - a threatened tree species of dry tropical forests in India. The Indian Forester 128:757–770
Saurabh S, Prasad D, Vidyarthi AS (2017) In vitro propagation of Trichosanthes dioica Roxb. for nutritional security. J Crop Sci Biotech 20:81–87
Scalet M, Federice R, Guido MC, Manes F (1995) Peroxidase activity and polyamine changes in response to ozone and simulated acid rain in Aleppo pine needles. Environ Exp Bot 35:417–425
Scandalios JG (1990) Response of plant antioxidant defence genes to environmental stress. Adv Genet 28:1–41
Shahzad A, Akhtar R, Bukhari NA, Perveen K (2017) High incidence regeneration system in Ceratonia siliqua L. articulated with SEM and biochemical analysis during developmental stages. Trees 31:1149–1163
Sharma S, Shahzad A, Ahmad A, Anjum L (2014) In vitro propagation and the acclimatization effect on the synthesis of 2-hydroxy-4-methoxy benzaldehyde in Decalepis hamiltonii Wight and Arn. Acta Physiol Plant 36:2331–2344
Suarez E, Alfayate C, Frances JFP, Perez JAR (2019) Structural and ultrastructural differences between field, micropropagated and acclimated leaves and stems of two Leucospermum cultivars (Proteaceae). Plant Cell Tiss Org Cult 136:15–27
Subbalakshmi C, Pullaiah T (2015) Phytochemical screening and antimicrobial activities of a medicinal plant Hildegardia populifolia. Int J Plant Anim Environ Sci 5:107–110
Van Huylenbroeck JM, Piqueras A, Debergh PC (1998) Photosynthesis and carbon metabolism in leaves formed prior and during ex vitro acclimatization of micropropagated plants. Plant Sci 134:21–30
Varaprasad B, Katikala PK, Naidu KC, Penumajji S (2009) Antifungal activity of selected plant extracts against phytopathogenic fungi Aspergillus niger. Indian J Sci Technol 2:87–90
World Conservation Monitoring Centre (1998) Hildegardia populifolia. The IUCN Red List of Threatened Species 1998: e.T33656A9801072. <www.iucnredlist.com>
Xu FJ, Li G, Jin CW, Liu WJ, Zhang SS, Zhang YS, Lin XY (2012) Aluminium induced changes in reactive oxygen species accumulation, lipid peroxidation and antioxidant capacity in wheat root tips. Biol Plant 56:89–96
Yadav V, Shahzad A, Ahmad Z, Sharma S, Parveen S (2019) Synthesis of nonembryogenic synseed in Hemidesmus indicus R. Br.: short term conservation, evaluation of phytochemicals and genetic fidelity of the regenerants. Plant Cell Tiss Org Cult 138:363–376
Yan L (2009) Physiological responses of tomato seedlings (Lycopersicon esculentum) to salt stress. Mod Appl Sci 3:171–176
Zafar N, Mujib A, Ali M, Tonk D, Gulzar B, Malik M, Sayeed R, Mamgain J (2019) Genome size analysis of field grown and tissue culture regenerated Rauvolfia serpentine (L.) by flow cytometry: histology and scanning electron microscopic study for in vitro morphogenesis. Ind Crop Prod 128:545–555
Zalkhani R, Moazedi AA (2020) Basic and clinical role of vitamins in epilepsy. J Res App Basic Med Sci 6:104–114
Acknowledgments
AIRF-Jawaharlal Nehru University, New Delhi, and USIF-Aligarh Muslim University, Aligarh, is highly acknowledged for GC-MS and SEM study respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Zhezhi Wang
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Upadhyay, A., Shahzad, A. & Ahmad, Z. In vitro propagation and assessment of genetic uniformity along with chemical characterization in Hildegardia populifolia (Roxb.) Schott & Endl.: a critically endangered medicinal tree. In Vitro Cell.Dev.Biol.-Plant 56, 803–816 (2020). https://doi.org/10.1007/s11627-020-10085-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10085-w