Abstract
Transformed hairy root cultures have become an alternative for the biosynthesis of plant secondary metabolites with biological activities. In this present work, the effects of liquid Murashige and Skoog (MS) and Gamborg B5 (B5) medium on kinetic behavior, biomass and phenolic metabolite production were analyzed in Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. hairy root cultures. Liquid MS medium showed the highest biomass production (13.67 g L−1 dry weight) after 77 d of culture. For B5 medium, highest biomass was achieved sooner, at day 56, but with lower total biomass (8.10 g L−1 dry weight). After isolation, structural elucidation of the major compound present in T. lophophoroides hairy roots was determined by nuclear magnetic resonance and mass spectral analysis. As a result, a ferulic acid derivative (feruloyl-glucoside) was isolated from T. lophophoroides hairy roots and reported for the first time. Quantitative analysis indicated that feruloyl-glucoside was the major phenolic metabolite at 56 d of growth in MS medium (2.7267 ± 0.041 mg g−1 dry weight L−1) and at 7 and 35 d in B5 medium (2.6328 ± 0.108 and 2.4372 ± 0.026 mg g−1 dry weight L−1, respectively). The feruloyl-glucoside was not detected in untransformed roots (control). The present results suggested the potential of T. lophophoroides hairy roots culture for the production of this phenolic glycoside.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant secondary metabolites play an important role in plant defense mechanisms (Madala et al.2014) and represent a source of bioactive molecules (Ludwig-Muller et al. 2014). Phenolic glycosides, such as feruloyl-glucoside or its derivates, are constituents of commonly consumed fruits (Lucini et al. 2017) and their occurrence has been reported in different sources, such as Malus spp. (apple; Pérez-Ilzarbe et al. 1991), Cucumis sativus L. (cucumber; Abu-Reidah et al. 2012), Opuntia ficus-indica (L.) Mill. fruits (prickly pear; Kim et al. 2016), Brassica spp. (Lin and Harnly 2010) and Vitis vinifera L. (grape vine; Kovács and Dinya 2000), with proposed functional properties as anti-diabetic, hepatoprotective (Tang et al. 2017) and cosmetic whitening agents (Tanimoto et al. 2006).
Different cactus species have proven to be valuable sources of plant secondary metabolites (Astello-García et al. 2015). Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. is a small globular cactus endemic to Mexico that contains alkaloids (Štarha et al. 1999) and has been overexploited mainly for ornamental purposes (Smith et al. 2013; Villaseñor 2016). Turbinicarpus lophophoroides is a slow-growing plant in its natural habitat and is subjected to special protection by the Mexican normativity (NOM-059-SEMARNAT-2010; Semarnat 2010). Thus, the development of in vitro cultures by asexual propagation of T. lophophoroides has enormous potential, since high-yielding biomass production of cacti may be achieved in shorter periods of time and with minimal space requirements (Pérez-Molphe-Balch et al. 2015). In vitro culture via the generation of transformed hairy roots is a suitable alternative for increased biomass accumulation and improved plant secondary metabolite production through biotechnological approaches and has advantages such as (a) hormone-free medium for root formation, since hairy roots synthesize their own growth factors; (b) faster growth rate compared with that of plants grown in situ; (c) biochemical and genetic stability; (d) facilitation of basic research on hairy root-mediated secondary metabolite production coupled with productivity enhancement strategies; and (e) transformed hairy roots-based functional research (Georgiev et al. 2007; Georgiev et al. 2012; Mehrotra et al. 2015).
As part of ongoing efforts towards the conservation and preservation of cactus genetic resources and sustainable management, investigations by the current authors have focused on several aspects of T. lophophoroides biotechnology, including in vitro conservation of germplasm, induction of transformed hairy roots (Palomeque-Carlín et al. 2015; Pérez-Molphe-Balch et al. 2015) and phytochemical characterization. The research presented here identified and quantitatively determined the main compound present in hairy root cultures compared with non-transformed roots derived from in vitro plants of T. lophophoroides and investigated the effect of medium composition on changes in biomass accumulation and feruloyl-glucoside levels during the growth cycle, in order to increase and deepen the knowledge of transformed hairy roots of cacti and to explore their potential applications.
Materials and Methods
Plant material
Transformed hairy root cultures of T. lophophoroides were previously established by Palomeque-Carlín et al. (2015). The root tip segments (1 cm in length) were cultured on Murashige and Skoog (MS; Murashige and Skoog 1962) basal medium (Sigma-Aldrich®, St. Louis, MO) supplemented with 30 g L−1 sucrose and 10 g L−1 agar (plant cell culture tested; Sigma-Aldrich®), with a final pH of 5.7 adjusted with 1 N NaOH (Thermo Fisher Scientific®, Waltham, MA) and then sterilized by autoclaving at 121°C for 15 min. The hairy root cultures were grown under fluorescent light (General Electric, Boston, MA) of 40 μmol m−2 s−1 light intensity with a 16-h photoperiod and a temperature of 25 ± 2°C. Non-transformed roots and shoots obtained from in vitro plants were grown under the same conditions until enough biomass was obtained (at 273 d) and used as control.
Biomass and phenolic compound accumulation in different culture media
For transformed hairy root culture optimization experiments, 0.05 or 0.30 g of root tip segments was transferred into 200-mL Erlenmeyer flasks (narrow neck; DWK Life Sciences GmbH, Mainz, Germany) containing 70 mL liquid MS or Gamborg B5 (B5; Gamborg et al. 1968) basal medium (Sigma-Aldrich®), supplemented with 30 g L−1 or 20 g L−1 sucrose, respectively, and covered with aluminum foil. Each flask was maintained on a rotary shaker at 80 rpm (Sev-Prendo, Puebla City, México) at 25 ± 2°C in dark conditions for 13 wk. For kinetic evaluation, three random samples of independent experiments were collected each week to measure biomass fresh weight (FW) and dry weight (DW). Collected biomass was freeze-dried (FreeZone 4.5; Labconco Corporation, Kansas City, MO) and stored at − 20°C for further analysis.
Nutrient uptake evaluation
Nutrient consumption in the medium was evaluated by means of total sucrose concentration determination by the phenol-sulfuric spectrophotometric method proposed by Dubois et al. (1956). A standard curve was prepared with sucrose (0, 10, 20, 30, 40, 50, 60 and 70 μg mL-1). Aliquots of 2 mL were taken, and then, 0.5 mL of 5% (v/v) phenol solution (Sigma-Aldrich®) and 2.5 mL of concentrated sulfuric acid (Thermo Fisher Scientific®) were added. After 30 min of reaction, the absorbance of each sample (n = 3) was monitored at 490 nm with a Jenway Genova spectrophotometer (Cole-Parmer Instrument Co., Vernon Hills, IL). Additionally, the electric conductivity and pH (Waterproof tester; Hanna® Instruments, Smithfield, RI) of the medium were measured.
Determination of kinetic parameters of T. lophophoroides hairy root cultures and phenolic compound accumulation pattern
The growth curve of T. lophophoroides hairy root cultures was established according to the change in FW and DW each week during the 13-wk growth cycle as described above. Kinetic parameters such as growth rate (μ), duplication time (td) and growth index (GI) were calculated as proposed by Gómez-Aguirre et al. (2012). The growth rate equation was as follows: μ = ln (XE ÷ X0) ÷ Δt, where X0 and XE are the amounts of root biomass at the beginning and the end of the culture period interval (g L−1), Δt is the culture time interval (days) and μ is the specific growth rate (day−1); duplication time was calculated as follows: td = ln 2 ÷ μ, where td is the doubling time (d). The growth index was calculated as follows: GI = (XF − X0) ÷ X0, where XF and X0 are the final and initial root biomass, respectively.
For phenolic compound accumulation analysis, intermediate points of each growth phase were selected. Methanolic extracts were prepared with root samples collected at 21, 56 and 91 d for liquid MS medium and at 7, 35 and 84 d for liquid B5 medium and then, the total content of the major phenolic metabolite was purified and analyzed using chromatographic and spectroscopic approaches, and quantified by high-performance liquid chromatography (HPLC) analysis (see below).
Sample preparation, isolation, and identification of feruloyl-glucoside
For phytochemical analysis, T. lophophoroides hairy roots growing in MS medium were propagated for 56 d, when the exponential growth phase was achieved (45.25 g DW). Otherwise, non-transformed roots and shoots growing for 273 d were harvested (70 mg DW and 186 mg DW, respectively). All these samples were freeze-dried and then extracted three times with methanol (Thermo Fisher Scientific®), 5 mL of MeOH to 1 g of dried tissue at 60°C for 10 min according to Wagner and Bladt (1996). The resultant extract was concentrated to dryness by rotary evaporation (Heidolph Instruments GmbH & Co. KG., Schwabach, Germany) under reduced pressure (0.3 to 0.5 kPa) at 45°C (Trejo-Moreno et al. 2018) and then, the obtained methanolic crude extract (11.2 g DW) was fractionated by preparative Silica Gel 60, Kieselgel 0.063 to 0.200 mm, 70 to 230 mesh ASTM column chromatography (Merck KGaA, Darmstadt, Germany) using a gradient elution system composed of dichloromethane:methanol (100:00 ➔ 00:100). Collected fractions were analyzed by thin-layer chromatography (TLC). Aliquots of each fraction were spotted onto silica gel 60 F254 and silica gel 60 RP18 F254S aluminum plates (Merck) and eluted with dichloromethane:methanol (9:1) and water:acetonitrile (7:3), respectively. Plates were examined by ultraviolet (UV) fluorescence (365 nm) and then sprayed with cerium (IV) sulfate solution 0.1 N (Merck) and heated at 105°C for 1 to 2 min (Wagner and Bladt, 1996). The main collected fraction (fraction 22; 35.6 mg DW) was further analyzed using nuclear magnetic resonance (NMR) and mass spectrometry for qualitative analysis of the main chemical entity.
Qualitative analysis of feruloyl-glucoside
The main fraction (fraction 22) obtained after preparative chromatography was identified by means of mass spectrometry and NMR analyses. Mass spectroscopic analysis was carried out using an ultra-performance liquid chromatography-electrospray ionization-mass spectrometry (UPLC-ESI-MS) system (UPLC™ ACQUITY-Z-spray™ ESI-APCI-ESCi®; Waters Corp., Milford, MA) equipped with an Acquity UPLC™ BEH C18 column (2.1 mm × 50 mm i.d., 1.7-μm particle size; Waters Corp.) and MassLynx™ (Waters Corp.) software. The mobile phase consisted of 0.05% (v/v) trifluoroacetic acid (Sigma-Aldrich®) aqueous solution (solvent A) and acetonitrile (Merck) (solvent B). The gradient system was as follows: 0 to 4 min, 0% B; 4 to 5 min, 30% B; 5 to 6 min, 50% B; 6 to 8 min, 100% B. The flow rate was maintained at 0.3 mL min−1 and the injection volume was 5 μL. The UPLC-ESI-MS analysis was performed in negative ion mode. Finally, the major compound was identified by NMR analysis.
The conformational structure of the main detected metabolite was further elucidated by 1H NMR (400 MHz), 13C NMR (100 MHz), and two-dimensional hetero-nuclear multiple bond correlation (HMBC) spectroscopy (400 MHz) using a Varian Mercury Plus 400 MHz—ID3 spectrometer (Varian Inc., Palo Alto, CA) and acetone d6:dimethyl sulfoxide-d6 (Sigma-Aldrich®) (95:05) as solvent. Chemical shifts were described in parts per million (ppm) relative to tetramethylsilane (TMS; Sigma-Aldrich®) as internal standard. The resulting spectra were compared with reported data (Kim et al. 2016).
Quantitative HPLC analysis of feruloyl-glucoside at different growth phases of T. lophophoroides hairy roots
For quantitative analysis of feruloyl-glucoside at intermediate points of each growth phase of T. lophophoroides hairy roots cultures and in vitro non-transformed shoot and root tissues, a high-performance liquid chromatography (HPLC) method using a Waters 2695 separation module equipped with a Waters 996 photodiode array detector and Empower™ Pro software (Waters Corp.) was developed. A Supelcosil LC-F column (4.6 mm × 250 mm i.d., 5-μm particle size) (Sigma-Aldrich®) was used and the mobile phase consisted of 0.5% (v/v) trifluoroacetic acid aqueous solution (solvent A) and acetonitrile (solvent B). The gradient system was as follows: 0 to 1 min, 0% B; 2 to 3 min, 5% B; 4 to 20 min, 30% B; 21 to 23 min, 50% B; 24 to 25 min, 80% B; 26 to 27 100% B; 28 to 30 min, 0% B. The flow rate was maintained at 0.9 mL min−1 and the injection volume was 10 μL. The absorbance was measured at 330 nm. The retention time for the most abundant peak (peak 1) was 8.968 min (λ = 242, and 323 nm) and the concentration of this polyphenolic compound was estimated by interpolation of the peak areas and comparison with a calibration curve prepared with feruloyl-glucoside isolated of T. lophophoroides as described above. The calibration curve was linear in the range of 31.25 to 250 μg mL−1 feruloyl-glucoside in methanol (y = 35944× – 278037; R2 = 0.9971). Feruloyl-glucoside was identified by comparison of the retention times and UV spectra in each sample. All analyses were performed in triplicate and the data were expressed as mean values in milligram per gram of DW of sample.
Statistical analyses
Data are expressed as the mean ± standard deviation of three independent experiments. One-way analysis of variance (ANOVA) and Brown-Forsythe tests were used for multiple comparisons among means. The significance level for all statistical tests was 5%. Statistical analyses were performed with Prism 7 for Mac OS X (GraphPad Software Inc., San Diego, CA).
Results
Growth kinetics of T. lophophoroides hairy roots
The kinetic behavior of T. lophophoroides hairy root cultures growing in MS and B5 medium (Figs. 1 and 2, respectively) was determined. For MS medium, the lag, exponential, and stationary growth phases (0 to 35, 36 to 77, and 78 to 91 d, respectively) were observed (Fig. 1a). The specific growth rate corresponded to 0.06 d−1, which indicates the increase in the biomass of the root per unit of biomass concentration over time; the growth index was 307.78, and the duplication time was 12.14 d. Changes in total sucrose concentration (Fig. 1a), in pH, and electrical conductivity (Fig. 1b) in the medium were analyzed as a function of culture time and biomass growth in MS medium. The increase in biomass accumulation and the decrease of electrical conductivity in MS medium was in agreement with the consumption of the carbon source. The pH of the medium was constant along growth kinetics (Fig. 1B).
Growth curve of hairy roots of Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. in liquid Murashige and Skoog medium (Murashige and Skoog 1962). (a) Biomass of the root tissue (in dry weight g L−1) and total sugars (μg mL−1) in remaining liquid medium over cultivation time (d); (b) pH and electric conductivity (μS cm−1) values of the medium over cultivation time (d). Each value represents the mean of three replicates ± standard error.
Growth curve of hairy roots of Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. in liquid Gamborg B5 medium (Gamborg et al.1968). (a) Biomass of the root tissue (in dry weight g L−1) and total sugars (μg mL−1) in remaining liquid medium over cultivation time (d); (b) pH and electric conductivity (μS cm−1) values of the medium over cultivation time (d). Each value represents the mean of three replicates ± standard error.
On the other hand, the hairy roots of T. lophophoroides growing in B5 medium (Fig. 2a) showed shorter growth phases, but lower biomass yield, when compared with those parameters for MS medium: lag phase (0 to 14 d), exponential phase (15 to 49 d), stationary phase (50 to 55 d) and death phase (day 56 to 91). Kinetic parameters as given by growth index, duplication time and growth rate (33.00 d, 15.00 d and 0.05 d−1, respectively) were calculated. The total sugar concentration was measured to assess the agreement between biomass production and the decrease of the carbon source concentration in B5 liquid medium (Fig. 2a). Conductivity and pH (Fig. 2b) showed similar behaviors as observed in MS cultured hairy roots.
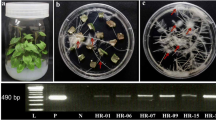
The morphological characteristics of T. lophophoroides hairy roots growing in MS medium showed lateral roots developed preferentially (Fig. 3c, d) from inoculum (Fig. 3a) with less pronounced tissue browning (Fig. 3d), whereas in B5 medium after inoculation, the hairy roots proliferated mainly by elongation (Fig. 3f, g) and the biomass turned brown at 84 d (Fig. 3H).
Phenotype of Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. hairy root cultures. (a–d) Hairy roots cultivated in Murashige and Skoog medium (Murashige and Skoog 1962) through time; (a) 0 d; (b) 21 d; (c) 56 d; (d) 91 d. (e–h) Hairy roots cultivated in Gamborg B5 medium (Gamborg et al.1968) through time; (e) 0 d; (f) 7 d; (g) 35 d; (h) 84 d. Scale bars 1 cm.
Chemical identification of the major compounds: identification of feruloyl-glucoside
After HPLC analysis, two peaks were detected (peak 1 at retention time (Rt) 8.979 min and peak 2 at Rt 10.225 min) from extracts of tissues in the exponential growth phase of T. lophophoroides hairy roots. The main peak (peak 1) was isolated and tentatively identified by means of mass spectrometry and then, the conformational structure was elucidated by NMR analysis. Adduct ion of peak 1 ([M-H]− at mass/charge number of ions (m/z 355.1047)) (Fig. S1) yielded fragments at m/z 175, 193 and 295, suggesting the presence of a feruloyl-glucoside derivative as proposed by Chougui et al. (2015). The mass accuracy between theoretical and measured mass was 3 mg L−1. This structure was corroborated by NMR analysis. The 1H and 13C NMR chemical shifts and the coupling constants of this compound, which displayed very similar data to those previously described for feruloyl-glucoside (Table 1; Kim et al. 2016). The 3J correlation between the anomeric proton of glucose (δ 6.33 d, J = 8.0) and the acetate carbonyl group at 167.41 in the HMBC spectrum indicated the position of the sugar in the ferulic acid skeleton. This is the first report of the presence of feruloyl-glucoside in T. lophophoroides hairy roots.
Quantitative HPLC analysis
The accumulation pattern of feruloyl-glucoside in T. lophophoroides hairy root cultures was determined using HPLC analysis. The present results suggested that the induction of T. lophophoroides hairy roots increased the accumulation of feruloyl-glucoside (peak 1, Rt 8.979 min, λ = 242 and 323 nm; Fig. 5e) at different growth stages in both MS and B5 culture medium (Fig. 5). The detected signals for non-transformed roots and shoots harvested at 273 d were too low for quantification (Fig. 5a, Table 2).
Quantitative high-performance liquid chromatography (HPLC) and ultraviolet absorption spectrum (inserts) analyses of feruloyl-glucoside detected in Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. hairy root cultivated in Murashige and Skoog (MS; Murashige and Skoog 1962) or Gamborg B5 (B5; Gamborg et al.1968) medium. (a) Untransformed roots (control) at 273 d; (b–d) hairy roots in MS medium at 21, 56, and 91 d, respectively; (e) feruloyl-glucoside isolated; (f–h) hairy roots in B5 medium at 7, 35, and 84 d, respectively. Peaks (1) feruloyl-glucoside and (2) unidentified compound.
The highest accumulation of feruloyl-glucoside in T. lophophoroides hairy roots growing in MS medium was achieved at the exponential growth phase (2.7267 ± 0.041 mg g−1 DW L−1; Fig. 5c), followed by the stationary (2.0498 ± 0.010 mg g−1 DW L−1; Fig. 5d) and the lag phase (1.2900 ± 0.054 mg g−1 DW L−1; Fig. 5b) (Table 2). For B5 medium, the content of feruloyl-glucoside was slightly higher at the lag phase (2.6328 ± 0.108 mg g−1 DW L−1; Fig. 5f), followed by the exponential phase (2.4372 ± 0.026 mg g−1 DW L−1; Fig. 5G); the stationary growth phase showed the lowest feruloyl-glucoside values with 0.3498 ± 0.0004 mg g−1 DW L−1 (Fig. 5H; Table 2).
Discussion
In recent years, the production of therapeutic bioactive molecules in biotechnological systems has gained attention, due to the capacity for enhanced biomass and metabolite yields (Matkowski 2008). One such biotechnological approach, transformed hairy roots, provides a plant-based method for the biosynthesis of bioactive molecules (Ludwig-Muller et al. 2014) such as phenolics.
Different species of cacti have been recognized as sources of bioactive metabolites (Jiménez-Aspee et al. 2014; Kim et al. 2016); nevertheless, reports dealing with the organ culture of cacti are scarce (Pérez-Molphe-Balch et al.2015). In the current study, the main compound present in transformed hairy root cultures of T. lophophoroides was identified and quantified for the first time; in contrast to the non-transformed roots and shoots derived from in vitro plants where feruloyl-glucoside was not detected. The effect of MS and B5 culture medium on biomass accumulation and feruloyl-glucoside levels during the growth cycle was investigated.
The biomass production in T. lophophoroides hairy roots was influenced by the use of different culture medium. The maximum biomass production was achieved at 77 d (13.7 g L−1; Fig. 1a) using MS liquid medium and at 56 d (8.1 g L−1; Fig. 2a) for liquid B5 medium. For other plant systems, it has been proposed that biomass growth can be influenced by different factors, such as inoculum size, carbon source availability, ammonium/nitrate ratio or pH (Wu et al.2006). According to the kinetic parameters calculated for T. lophophoroides hairy roots growing in MS or B5 medium, the MS medium (DT = 12.14 and μ = 0.06 d−1) generated a 307.78-fold increase in 77 d, compared with inoculum. On the other hand, inoculum of hairy roots growing in B5 medium (DT = 15.00 and μ = 0.05 d−1) yielded a 33.00-fold increase in 56 d; thus, MS medium was proposed as the best basal salt mixture for T. lophophoroides hairy roots biomass production.
After the isolation of the major metabolite of T. lophophoroides hairy root cultures, the identification was carried out. Mass spectral (see electronic supplementary material Fig. S1) and NMR analyses indicated the presence of feruloyl-glucoside (Table 1). Feruloyl-glucoside is a glycosylated hydroxycinnamic acid found in natural sources and such functional properties as whitening (Tanimoto et al. 2006), antioxidant, hypolipidemic, and anti-inflammatory activities (Tang et al. 2017) have been proposed. Quantitative analysis indicated that B5 medium yielded the highest accumulation of feruloyl-glucoside after 7 d of culture (2.6328 ± 0.108 mg g−1 DW L−1), when compared with that of MS medium (2.7267 ± 0.041 mg g−1 DW L−1) after 56 d of culture (Table 2); thus, B5 medium was proposed as optimal for the production of this phenolic glycoside in the lag phase of the culture (day 7). The observed differences in biomass and metabolite accumulation found in the current study for MS and B5 medium (see Table 2) might be attributed to the differences in medium composition, since it has been proposed that the ammonium/nitrate ratio in the culture medium affects phenolics and biomass production (Russowski et al.2006; Wu et al. 2006). The present results suggested that the accumulation pattern of feruloyl-glucoside in MS and B5 medium was independent of biomass accumulation (see Fig. 5 and Table 2), similar to that found for rosmarinic acid production in Dracocephalum moldavica L. hairy roots (Weremczuk-Jeżyna et al. 2013). In contrast, the detected signals for non-transformed roots and shoots harvested after 273 d were too low for quantification (see Fig. 5a, Table 2). It has been proposed that ferulic acid may participate in the lignin biosynthesis (Hamada et al.2003); thus, based in these findings, the feruloyl-glucoside may participate in the lignin formation in non-transformed roots and shoots of T. lophophoroides obtained from in vitro cultures. Further studies are required to asses this hypothesis. Information regarding the accumulation pattern of feruloyl-glucoside in biotechnological systems is scarce and limited to cell suspension cultures of Chenopodium rubrum L. (Bokern et al. 1991), where nevertheless information related to feruloyl-glucoside concentration is not given; thus, the current research substantiated the potential applications of T. lophophoroides hairy roots for the production of biomass and for the biosynthesis and study of this phenolic glycoside (or related compounds) after 7 d of culture in B5 medium (Fig. 5).
Conclusions
The present study demonstrated the capacity of T. lophophoroides hairy roots for the biosynthesis of feruloyl-glucoside, a ferulic acid derivative with functional properties. Among evaluated conditions, B5 medium proved to be suitable for feruloyl-glucoside biosynthesis after 7 d of culture; meanwhile, MS medium was better for biomass production under the given conditions. The results reported herein should contribute to the overall knowledge of cactus species and hairy root culture conditions. The identified metabolite feruloyl-glucoside was reported here for the first time in T. lophophoroides.
References
Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R, Fernández-Arroyo S, Segura-Carretero A, Fernández-Gutiérrez A (2012) HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res Int 46:108–117
Astello-García MG, Cervantes I, Nair V, Santos-Díaz MS, Reyes-Agüero A, Guéraud F, Negre-Salvayre A, Rossignol M, Cisneros-Zevallos L, Barba de la Rosa AP (2015) Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J Food Compos Anal 43:119–130
Bokern M, Wray V, Strack D (1991) Accumulation of phenolic acid conjugates and betacyanins, and changes in the activities of enzymes involved in feruloylglucose metabolism in cell-suspension cultures of Chenopodium rubrum L. Planta 184:261–270
Chougui N, Djerroud N, Naraoui F, Hadjal S, Aliane K, Zeroual B, Larbat R (2015) Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem 173:382–390
Dubois M, Gilles A, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substantes. Anal Chem 28:350–356
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Georgiev MI, Agostini E, Ludwig-Muller J, Xu J (2012) Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol 30:528–537
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175
Gómez-Aguirre YA, Zamilpa A, González-Cortazar M, Trejo-Tapia G (2012) Adventitious root cultures of Castilleja tenuiflora Benth. as a source of phenylethanoid glycosides. Ind Crop Prod 36:188–195
Hamada K, Tsutsumi Y, Yamauchi K, Fukushima K, Nishida T (2003) Treatment of poplar callus with ferulic and sinapic acids I: incorporation and enhancement of lignin biosynthesis. J Wood Sci 49:333–338
Jiménez-Aspee F, Quispe C, Soriano MPC, Fuentes Gonzalez J, Hüneke E, Theoduloz C, Schmeda-Hirschmann G (2014) Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res Int 62:286–298
Kim JW, Kim TB, Yang H, Sung SH (2016) Phenolic compounds isolated from Opuntia ficus-indica fruits. Nat Prod Sci 22:117–121
Kovács Z, Dinya Z (2000) Examination of non-volatile organic compounds in red wines made in Eger. Microchem J 67:57–62
Lin LZ, Harnly JM (2010) Phenolic component profiles of mustard greens, yu choy, and 15 other Brassica vegetables. J Agric Food Chem 58:6850–6857
Lucini L, Rocchetti G, Kane D, Trevisan M (2017) Phenolic fingerprint allows discriminating processed tomato products and tracing different processing sites. Food Control 73:696–703
Ludwig-Muller J, Jahn L, Lippert A, Puschel J, Walter A (2014) Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: strategies and applications. Biotechnol Adv 32:1168–1179
Madala NE, Steenkamp PA, Piater LA, Dubery IA (2014) Metabolomic insights into the bioconversion of isonitrosoacetophenone in Arabidopsis thaliana and its effects on defense-related pathways. Plant Physiol Biochem 84:87–95
Matkowski A (2008) Plant in vitro culture for the production of antioxidants - a review. Biotechnol Adv 26:548–560
Mehrotra S, Srivastava V, Ur Rahman L, Kukreja AK (2015) Hairy root biotechnology--indicative timeline to understand missing links and future outlook. Protoplasma 252:1189–1201
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Palomeque-Carlín A, Tafoya F, Alpuche Solís AG, Pérez-Molphe-Balch E (2015) Effects of different culture media and conditions on biomass production of hairy root cultures in six Mexican cactus species. In Vitro Cell Dev Biol–Plant 51:332–339
Pérez-Ilzarbe J, Hernández T, Estrella I (1991) Phenolic compounds in apples: varietal differences. Z Lebensm Unters Forsch 192:551–554
Pérez-Molphe-Balch E, Santos-Díaz MS, Ramírez-Malagón R, Ochoa-Alejo N (2015) Tissue culture of ornamental cacti. Sci Agric 72:540–561
Russowski D, Maurmann N, Rech SB, Fett-Neto AG (2006) Role of light and medium composition on growth and valepotriate contents in Valeriana glechomifolia whole plant liquid cultures. Plant Cell Tissue Organ Cult 86:211–218
Semarnat (2010) Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. NOM-059-SEMARNAT-2010. Diario oficial de la federación, México, pp. 1–78
Smith M, Fitz Maurice WA, Fitz Muarice B, Sotomayor M (2013) Turbinicarpus lophophoroides, Biznaguita. The IUCN red list of threatned species, 10.2305/IUCN.UK.2013-1.RLTS.T40981A2949100.en. Accessed 6/29/18
Štarha R, Chybidziurová A, Lacný Z (1999) Alkaloids of the genus Turbinicarpus (Cactaceae). Biochem Syst Ecol 27:839–841
Tang X, Olatunji OJ, Zhou Y, Hou X (2017) Allium tuberosum: antidiabetic and hepatoprotective activities. Food Res Int 102:681–689
Tanimoto S, Tominaga H, Okada Y, Nomura K (2006) Synthesis and cosmetic whitening effect of glycosides derived from several phenylpropanoids. Yakugaku Zasshi 126:173–177
Trejo-Moreno C, Méndez-Martínez M, Zamilpa A, Jimenez-Ferrer E, Perez-Garcia MD, Medina-Campos ON, Pedraza-Chaverri J, Santana MA, Esquivel-Guadarrama FR, Castillo A, Cervantes-Torres J, Fragoso G, Rosas-Salgado G (2018) Cucumis sativus aqueous fraction inhibits angiotensin II-induced inflammation and oxidative stress in vitro. Nutrients 10:1–14
Villaseñor JL (2016) Checklist of the native vascular plants of Mexico. Rev Mex Biodivers 87:559–902
Wagner H, Bladt S (1996) Plant drug analysis: a thin layer cromatography atlas. Springer, Berlin
Weremczuk-Jeżyna I, Grzegorczyk-Karolak I, Frydrych B, Krolicka A, Wysokińska H (2013) Hairy roots of Dracocephalum moldavica: rosmarinic acid content and antioxidant potential. Acta Physiol Plant 35:2095–2103
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. Journal of Plant Biology 49:193–199
Funding
This work was supported by the Departamento de Apoyo a la Investigación de la Universidad Autónoma de Aguascalientes (UAA) (Grants PIBT16-16). G.J. Solis-Castañeda is indebted to Consejo Nacional de Ciencia y Tecnología (CONACyT-México) for the doctoral fellowship awarded. Y.A. Gómez-Aguirre is grateful to Cátedras-CONACyT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Praveen Saxena
Electronic supplementary material
Figure S1.
Full mass spectrometry scan data in negative ion mode of Peak 1 (m/z 355.1) of Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. hairy roots cultures grown in liquid Murashige and Skoog (MS; Murashige and Skoog 1962) at 56 d (exponential growth phase) (PNG 111 kb)
Rights and permissions
About this article
Cite this article
Solis-Castañeda, G.J., Zamilpa, A., Cabañas-García, E. et al. Identification and quantitative determination of feruloyl-glucoside from hairy root cultures of Turbinicarpus lophophoroides (Werderm.) Buxb. & Backeb. (Cactaceae). In Vitro Cell.Dev.Biol.-Plant 56, 8–17 (2020). https://doi.org/10.1007/s11627-019-10029-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10029-z