Abstract
Anthranilate synthase (AS), a tetramer consisting of two α subunits and two β subunits, is the key control enzyme in the tryptophan (Trp) biosynthesis pathway. A naturally occurring α subunit of AS called ASA2 that is insensitive to Trp feedback inhibition was isolated from a tobacco suspension cell culture and has been extensively studied and used for both nuclear and plastid transformation. However, the obligate β subunit of AS had not been studied in tobacco. Therefore, the tobacco AS β subunit-encoding cDNA was cloned and its encoded protein was verified. Agrobacterium-mediated transformation of tobacco plants was performed, under the selection of the toxic Trp analogs, 7-methyltryptophan or α-methyltryptophan, with a construct containing both ASA2 and AS β subunit genes. Many transgenic plants overexpressing both subunits were identified and examined. Compared to the wild-type plants, the transgenic plants had higher levels of enzymatic activities for both holoenzyme and α subunit. The transgenic plants had 9 to 68 times the amount of free Trp as the wild-type plants, which was more pronounced than plants overexpressing ASA2 alone. This study demonstrates the potential of co-expressing AS α and β subunits as a robust plant transformation system as well as overcoming feedback inhibition to obtain high levels of Trp biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthranilate synthase (AS) is the first and the rate-limiting enzyme in the five-enzyme pathway for tryptophan (Trp) biosynthesis (Widholm 1973; Radwanski and Last 1995). In plants and most bacteria AS consists of two large α subunits and two smaller β subunits (Poulsen et al. 1993). In plants, the nucleus-localized anthranilate synthase subunit genes encode the precursor peptides that are synthesized in the cytoplasm and then transported to the plastids where tryptophan biosynthesis occurs (Radwanski and Last 1995). The α subunits possess catalytic sites and can catalyze the formation of anthranilate, in the absence of β subunit, when high NH4 + concentrations are available in vitro. The α subunits also can bind the end product Trp, resulting in feedback inhibition. The β subunits have glutamine amidotransferase activity, and interact with the α subunits to form α-β heterodimers when assembled into α2-β2 heterotetrameric holoenzyme (Morollo and Eck 2001; Spraggon et al. 2001). In contrast to its partner α subunit, the β subunit is not affected by feedback inhibition. An AS β subunit (ASB) mutant in Arabidopsis exhibited less than 40% of the AS activity from the wild-type plant but was morphologically normal and grew without Trp. However, the double mutant of ASB and anthranilate phosphoribosyltransferase (the second enzyme in the Trp biosynthesis pathway) required Trp for growth (Niyogi et al. 1993). This suggests that the AS β subunit is important, but not essential, for AS activity and Trp biosynthesis in vivo. As a result, AS α subunit has been studied much more extensively than the β subunit.
Trp feedback-insensitive forms of the AS α subunit have been identified and studied in plants such as Datura (Ranch et al. 1983), rice (Wakasa and Widholm 1987; Tozawa et al. 2001), Arabidopsis (Kreps and Town 1992; Li and Last 1996), and tobacco (Widholm 1972; Song et al. 1998; Zhang et al. 2001; Tsai et al. 2005). The feedback-insensitive α subunit also confers tolerance to toxic Trp analogs that inhibit Trp biosynthesis by false feedback inhibition of AS and so has been used as a selection system for plant transformation. It is well accepted that the AS β subunit plays no role in Trp feedback sensitivity or Trp analog toxicity, but its importance for the formation of the heterotetrameric AS holoenzyme (Morollo and Eck 2001; Spraggon et al. 2001) and natural activity in vivo has not been well studied or appreciated.
The feedback-insensitive AS α subunit from tobacco (ASA2), along with Trp analogs, has been successfully used as a selection system for both nuclear and plastid transformations in tobacco (Barone and Widholm 2008; Barone et al. 2009) and Astragalus sinicus hairy roots (Cho et al. 2004). Overexpression of ASA2, in most cases, results in more tolerance to Trp analogs by seedlings and higher free Trp concentrations in leaves and seeds in tobacco (Song et al. 1998; Zhang et al. 2001; Tsai et al. 2005), Astragalus sinicus (Cho et al. 2000), and soybean (Inaba et al. 2007). Similar results were also reported for the feedback-insensitive α subunits in Arabidopsis (Li and Last 1996), Zea mays (Anderson et al. 1997), rice (Tozawa et al. 2001; Yamada et al. 2004), and potato (Yamada et al. 2004). However, AS β subunit has not been extensively studied and not been widely included in the AS transgenic system. In the only published report of the combination of the α and β subunits, the Arabidopsis feedback-insensitive α subunit, along with the β subunit and Trp decarboxylase, was expressed in Catharanthus roseus hairy roots. Greater resistance to Trp feedback inhibition and an increase in Trp and tryptamine levels were observed as compared to the roots with induced expression of α subunit alone (Hong et al. 2006). This study indicates that co-expression of both subunits can produce greater effects than expressing the α subunit alone.
We have previously studied the effect of overexpressing the tobacco feedback-insensitive AS α subunit (ASA2) in both the nucleus and chloroplast on plant transformation and Trp production (Song et al. 1998; Zhang et al. 2001; Tsai et al. 2005; Barone and Widholm 2008; Barone et al. 2009). To further study the α–β subunit interactions and holoenzyme assembly for AS, we first cloned the tobacco AS β subunit cDNA and then included it along with the feedback-insensitive α subunit coding sequence from ASA2 in the same transformation vector. Here, we report our experiments with co-expressing these two subunits in transgenic plants. We have observed a robust selection using Trp analogs for plant transformation. The resultant transgenic plants have free Trp levels much higher than the untransformed wild type, as well as the α subunit-only transgenic plants from our previous work. Our study demonstrates an improved, alternative selectable marker system that may also confer the beneficial trait of Trp over-production in plants.
Materials and Methods
Plant growth.
Plants of tobacco (Nicotiana tabacum L., cv. Xanthi) were grown in MS medium (Murashige and Skoog 1962) supplemented with 3% sucrose and 2.7 g l−1 Phytagel™ in a growth chamber at 24°C under 16 h of cool white light of 150 μmol photons m−2 s−1.
Cloning of β subunit of anthranilate synthase (ASB).
RNA was extracted from young tobacco leaves using Qiagen RNeasy mini kit (Qiagen, Valencia, CA, USA), and converted into first-stranded cDNA using SuperScript™ II RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and oligo dT15. Polymerase chain reaction (PCR) was carried out using the first-stranded cDNA as template, the high fidelity Pfx DNA polymerase (Invitrogen) and several pairs of degenerative primers for 30 cycles at 94°C for 45 s, 55°C for 45 s and 68°C for 50 s. The eventual primer pair used, L54 (5′-aaggagatatacCATGGCCAGCATTATCGTCC-3′; lower case is linker sequence; start codon is underlined) and L55 (5′-actcgagcccGGGTTTAGTCTTGAGCTTCTGCTTC-3′; stop codon is underlined), were the result of data base mining and bioinformatics analysis (see “Results and Discussion”). A ∼0.9-kb fragment was cloned into pCR-Blunt II®-TOPO cloning vector (Invitrogen) and several putative clones were sequenced (Northwestern University Biotechnology Center, Evanston, IL) to confirm its ASB identity.
Analysis of recombinant proteins in bacteria.
The putative ASB cDNA was cloned into pET-30(+) vector (Novagen, Gibbstown, NJ, USA) in E. coli BL21 (DE3) cells. Protein synthesis was induced by cultivating the bacterial cells with 1 mM IPTG according to the Novagen protocol. Protein extracts were analyzed by 0.1% (w/v) SDS-10% polyacrylamide gel electrophoresis (SDS-PAGE). As described previously (Zhang et al. 2011), the induced, prominent ∼32 kDa band was excised, washed with 100 mM NH4HCO3/CH3CN and treated with 10 mM dithiothreitol and 55 mM iodoacetamide. The gel pieces were then digested with 20 μl of 10 μg/ml trypsin (Roche, Indianapolis, IN, USA) for 30 min, followed by incubating in 50 mM NH4HCO3 at 37°C overnight. The digest solutions were mixed with 1% (v/v) formic acid/ 2% (v/v) CH3CN, incubated at 30°C for 30 min and extracted with CH3CN, then subjected to matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF) mass spectrometer (University of Illinois Biotechnology Center, Urbana, IL) for peptide identification.
ASA2/ASB vector for plant transformation.
The tobacco ASA2 coding region including the transit sequence and termination sequence was obtained by BamH I-EcoR I cut of the ASA2 clone available in this lab (Song et al. 1998). The ASA2 fragment was ligated to the BamH I-EcoR I digested binary vector pBIN gusA-mGFP5. Then, both the CaMV35/ASA2/NOS-T and NOS-P/ASB/NOS-T gene cassettes were inserted into the plasmid pBIN gusA-mGFP5 to complete the transformation vector pASA2-ASB (Fig. 1). Overall, the ASA2 gene was driven by cauliflower mosaic virus 35S promoter (CaMV 35S) and ended with nopaline synthase terminator (NOS-T). The ASB gene was driven by nopaline synthase promoter (NOS-P) and terminated with NOS-T. This pASA2-ASB plasmid was then introduced into Agrobacterium tumefaciens strain LBA4404 for plant transformation. Although the NPTII gene for kanamycin resistance was present in the vector, the eventual selection for transgenic cell lines was done using 200 μM of the Trp analog 7-methyltryptophan (7MT) or 20 to 40 μM α-methyltryptophan (αMT).
Structure of the vector pASA2-ASB used for tobacco transformation. Relevant genes and their orientation are presented. The locations of the restriction enzyme sites for BamH I used to release a 3.2-kb fragment for Southern hybridization analysis are shown. Location and orientation of PCR primers (L49, L50, L54 and L55) are indicated by arrows. 35S-P: cauliflower mosaic virus 35S promoter. 35S-T: cauliflower mosaic virus 35S polyA region as terminator. NOS-P and NOS-T: nopaline synthase promoter and terminator. NPTII: neomycin phosphotransferase II conferring kanamycin resistance. More detailed description is in “Materials and Methods”. The figure size is not to scale.
Plant genetic transformation.
Agrobacterium-mediated transformation of leaf discs and subsequent selection were carried out as described in Tsai et al. (2010) using 7MT or αMT for selection. The primary resistant plantlets were cultivated on MS rooting medium containing 200 μM 7MT or 20 to 40 μM αMT. After initial screening with Southern blot hybridization and PCR, the rooted plants were transferred to soil in pots and grown to maturity in the greenhouse. The T1 seeds were germinated on MS medium containing 200 μM 7MT and the seedlings exhibited segregation with respect to sensitivity to 7MT. Genomic DNA extracted from leaves of normally-growing plants (T1) from each of the seven independent T0 lines were used for PCR using BioMix™ Red (Bioline, Springfield, NJ, USA), primers L49 (5′-TTGTATTAATGCAGTCGTTACCTAT C-3′) and L50 (5′-CCTCTGCAGCCCTTAGGAATGGCAAC-3′) for ASA2, and primers L54 and L55 for ASB. PCR was initiated with 2 min at 95°C followed by 30 cycles at 95°C for 20 s, 52°C for 30 s and 72°C for 15 s. The plants that were PCR positive for both subunits were chosen for enzyme and Trp analyses.
DNA and RNA analysis.
DNA and RNA were extracted from leaves of the untransformed wild-type and putative transformed plants using Qiagen Plant DNeasy and RNeasy mini kits. Southern and northern blot hybridizations were performed as described before (Zhang et al. 2001; Tsai et al. 2010).
Anthranilate synthase (AS) enzyme assay.
Protein extraction from young fully expanded leaves and AS enzyme activity assays were performed as described in Zhang et al. (2001) and Tsai et al. (2005). To determine the α-subunit activity or AS holoenzyme activity, either 100 mM NH4Cl or 10 mM Gln was used as a substrate, respectively, to measure the conversion rate of chorismate to anthranilate. Different concentrations of Trp were used to assess the sensitivity of AS to feedback inhibition.
Free Trp measurement.
The young expanded leaves of shoot cultured plants were lyophilized and used for measurement of free Trp concentrations according to our previous reports (Cho et al. 2000; Zhang et al. 2001; Tsai et al. 2005; Barone et al. 2009). Two independent extractions from each plant were analyzed. Coarsely ground tissue, 10–15 mg, was extracted in 1 ml 0.1 N HCL using a 1.5-ml-glass–glass homogenizer (Bellco Glass, Vineland, NJ, USA) and microcentrifuged to remove debris. A portion of the supernatant was deproteinated using Amicon Ultra-0.5 ml 10K filter units (Millipore, Billerica, MA, USA). Filtrates, 1–20 μl, were each analyzed twice by HPLC using a Kinetex 5u C18 100A column (150 × 4.6 mM; Phenomenex, Torrance, CA, USA). The mobile phase solvents were A: 40 mM sodium acetate, pH 4.0 and B: acetonitrile. The flow rate was 1 ml min−1 and the column eluted with a gradient from 90% A 0–4 min, decreasing to 30% A by 8 min, constant at 30% A till 10 min, and increasing to 90% A by 11 min with a total run time of 13 min. Detection was done with a Shimadzu RF-20A XS fluorescence detector (Ex: 275 nm; Em: 350 nm). Trp was eluted at 3.2 min. Trp peak area versus Trp amount was linear (R 2 = 0.9999) from 1 to 100 pmol (1–100 μl injected) with a slope of 1.115 × 105 peak area/pmol.
Results and Discussion
Cloning of the tobacco anthranilate synthase β-subunit (ASB).
In order to clone the anthranilate synthase β-subunit (ASB) from tobacco, we first broadly searched the expressed sequence tag (EST) data bases in the public domain. We initially identified the unannotated EST clones CK297322, CK284349, CK286317, CK289119, and CK284811 of Nicotiana benthamiana that were similar to the putative ASB homologues of soybean, rice, and Arabidopsis. From these ESTs, we were able to manually assemble a hypothetical 1,289 bp composite sequence that coded for a 286-amino acid open reading frame. Then we designed numerous degenerative primers to perform RT-PCR using tobacco leaf cDNA. Multiple amplified DNA fragments were cloned and sequenced. Several clones shared similar sequences with the ASB from other species. The 888 bp cDNA was determined to be likely a tobacco ASB [Electronic supplementary material (ESM) Fig. S1A ] .
In order to verify that the putative tobacco ASB cDNA indeed encoded the ASB protein, we overexpressed the ASB cDNA in E. coli to acquire a purified protein with an observed molecular weight of ∼32 kDa (ESM Fig. S1B ). MALDI-TOF mass spectrometer analysis revealed peptide fragments that were ASB sequences (data not shown), proving that the cDNA indeed codes for the tobacco anthranilate synthase β-subunit.
Sequence analysis revealed that all the plant ASB proteins examined possess a divergent, 60∼80 amino acid plastid-targeting transit peptide at the N-terminus (ESM Fig. S1C ) which is absent in the bacterial ASB (ESM Fig. S1D ). Considering that the AS α subunit also has a plastid transit peptide, the AS holoenzyme is probably assembled inside the plastid (chloroplast). It is worth noting that the ASB genes are highly conserved among plant species (ESM Fig. S1C ). For example, the mature proteins of ASB (without the transit peptide) from the dicot tobacco and monocot rice are more than 67% identical (Fig. S1C ). Similar to Arabidopsis and rice ASB, the tobacco ASB gene contains seven introns within its 861-bp coding region (ESM Fig. S1A ; intron sequences not shown).
Generation of ASA2-ASB transgenic plants.
We then introduced the gene cassette linking ASA2 and ASB genes both with transit sequences (pASA2-ASB, Fig. 1) into tobacco plants through Agrobacterium-mediated transformation, using 200 μM 7-methyltryptophan (7MT) or 20 to 40 μM α-methyltryptophan (αMT) as the selection agent. In order to verify the presence and transcription of the ASA2-ASB transgenes, many of the selected plants were variously screened by PCR (data not shown), Southern (Fig. 2) and northern blot hybridizations (Fig. 3 and ESM Fig. S2), confirming the integration of the transgenes. Comparatively high RNA expression levels for ASA2 and ASB were observed in the transformed plants, whereas the wild-type tobacco plants showed a residual level of expression (almost invisible in Fig. 3 and ESM Fig. S2) as similarly reported previously for ASA2 (Zhang et al. 2001; Tsai et al. 2005). These results further demonstrate the utility of the feedback-insensitive AS enzyme and Trp analogs such as 7MT and αMT as an effective selection system for plant transformation (Tsai et al. 2005; Barone and Widholm 2008; Barone et al. 2009), a useful addition to the toolbox for plant genetic transformation.
A representative initial screening by Southern blot hybridizations of various ASA2/ASB transgenic plants (T0 generation). Genomic DNA (20 μg) was digested with BamH I and hybridized with 32P-labeled ASA2 or ASB cDNA probes after electrophoresis and blotting. The name of AB2″α30 on top denotes transformation event series No. 2 using 30 μM α-methyltryptophan (αMT) for selection. The number on top indicates independent transgenic lines of AB2″α30. A and B show the same blot first probed with ASB cDNA, followed by reprobing with ASA2 cDNA. Wt, wild type. The size in kilobase is indicated by 1 kb DNA ladder (Stratagene, La Jolla, CA, USA). Note: not all lines reported in this paper are shown.
Representative northern blot hybridizations of various ASA2/ASB transgenic plants (T0 generation). RNA (30 μg) blots were hybridized with 32P-labeled ASA2 or ASB cDNA probes. Ethidium bromide-stained gels at the bottom are shown to indicate RNA loading. Names and numbers on top indicate individual transgenic lines obtained from selection with either 35 μM αMT (ABα35 lines) or 200 μM 7-methyltryptophan (AB7MT lines). The same blots were used for two hybridizations. Note: T1 progeny of lines AB7MT#1, AB7MT#2, AB7MT#3 and AB7MT#8, ABα35#8 and ABα35#12, and AB2″α30#15 (ESM Fig. S2) were used for further analyses by PCR (Fig. 4) and free Trp measurement (Fig. 6). More northern blot hybridizations are shown in “Electronic supplementary material”.
In order to choose suitable lines of transgenic plants for further analysis, the seeds of some T0 plants that had shown comparatively high expression levels for both ASA2 and ASB (Fig. 3, ESM Fig. S2) were germinated on medium containing 200 μM 7MT. The normally growing plants from the segregating seedlings (T1 generation) were chosen for DNA extraction and PCR amplification for both ASA2 and ASB to verify the presence of the transgenes. Since both genes contain numerous introns within the primer flanking regions (three introns for ASA2 and 7 introns for ASB), PCR was designed in a way that amplifies only the cDNA sequence present in the transformation vector (Fig. 1), whereas the endogenous intron-containing ASA2 and ASB genes in the plant genome were prevented from being amplified. Seven independent T1 lines (10 plants) were found to have both ASA2 and ASB cDNAs (Fig. 4) and were used for further analysis. Among these seven lines, four lines (AB7MT#1, 2, 3 and 8) were acquired through 7MT selection, and three lines (ABα35#8 and 12 and AB2″α30#15) were through selection with 35 or 30 μM αMT (Fig. 4).
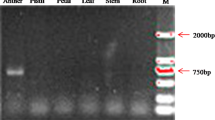
PCR verification of selective T1 plants that germinated and grew normally on medium containing 200 μM 7-methyltryptophan (7MT). Genomic DNA was PCR amplified for ASA2 (α subunit) and ASB (β subunit). Names and numbers on top indicate their parental (T0) individual plants originally obtained from selection with either 200 μM 7MT (AB7MT lines) or 35 μM αMT (ABα35 lines) or 30 μM αMT (AB2″α30 lines). Note: one plant from each independent line was assayed except for ABα35#8 (two plants, ABα35#8-1 and -2, were tested) and AB2″α30#15 (three plants, AB2″α30#15-1, -2 and -4, were tested).
Anthranilate synthase (AS) activity and free Trp in the ASA2-ASB expressing plants.
Compared to the wild-type plant, the ASA2-ASB transgenic plant AB2″α30#15 exhibited twice the total enzyme activity, as either α subunit or holoenzyme (Fig. 5). However, the degree of Trp feedback inhibition of AS appeared to be similar between the transgenic plants and the wild type. Nonetheless, as free Trp concentrations increased in the assays, the remaining AS activity was still much higher in the transgenic plants than in the wild type. For example, at 20 μM Trp, the wild-type plant lost almost all the AS activity, whereas the ASA2-ASB plants still had a specific activity of about 120 pmoles anthranilate/mg protein/min (Fig. 5b ). Interestingly, the pattern of AS enzyme activities as a function of free Trp concentration was similar to our previously reported transgenic tobacco plant expressing ASA2 alone using the same transformation vector (Tsai et al. 2005). This suggests that co-expressing β subunit plays an additive role in either total AS enzyme activity or Trp feedback inhibition.
Enzyme assay for specific activity of anthranilate synthase as either α subunit (a) or holoenzyme (b) from the wild type (Wt) and the transgenic plant AB2″α30#15 (its northern blot is shown in ESM Fig. S2).
Plants that expressed both subunits of AS had markedly higher leaf free Trp than the wild type, ranging from 9 times (for plant AB7MT#1-1) up to 68 times (for plant AB7MT#2-1) (Fig. 6). The free Trp of the wild type was 9.7 μmoles/g fw−1. These results reflect both a higher AS enzyme activity and less sensitivity to Trp feedback inhibition in the transgenic plants as compared to the wild-type plant. Remarkably, the free Trp in these plants was also much higher than the transgenic tobacco plants overexpressing the α subunit only in nuclear transformants (Tsai et al. 2005; Barone and Widholm 2008) or in plastid transformants (Zhang et al. 2001; Barone et al. 2009), suggesting that overexpression of the catalytic α subunit alone may encounter a shortage of β subunit which may prevent full assembly into active AS holoenzyme. Therefore, our study may demonstrate that co-expression of the cognate subunits facilitates AS formation and activity, leading to higher activity. As a result, higher threshold levels for Trp feedback inhibition and more tolerance to the toxicity of Trp analogs enable the plants to accumulate more Trp and to grow in the 7MT- or αMT-containing medium that is normally toxic to plants.
Furthermore, as compared to the wild-type plants, there were no visible phenotypic changes in transgenic plants grown in the greenhouse where they flowered normally and produced viable seeds. This suggests that high Trp accumulation did not lead to obvious negative effects on these plants, which is consistent with previous studies (e.g., Tozawa et al. 2001, Zhang et al. 2001, Tsai et al. 2005, Barone et al. 2009).
In conclusion, transgenic tobacco plants that co-express both subunits of AS exhibited higher anthranilate synthase activities, more resistance to Trp feedback inhibition and higher Trp levels, without obvious phenotypic changes, as compared to the untransformed wild type. Two-subunit expression system seems to be advantageous to α only system in terms of free Trp production and accumulation as well as serving as a selection regime for plant transformation. The availability of a range of transgenic tobacco plants, with ASA2 in the nucleus and plastids, and ASA2-ASB in the nucleus, should help further studies of the subunit interaction, enzyme assembly and functionality of anthranilate synthase, as well as the regulation of Trp biosynthetic pathway in plants.
References
Anderson PC, Chomet PS, Griffor MC, Kriz AL (1997) Anthranilate synthase gene and its use thereof. World Intellectual Property Organization, 97/26366
Barone P, Widholm JM (2008) Use of 4-methylindole or 7-methyl-DL-tryptophan in a transformant selection system based on the feedback-insensitive anthranilate synthase α-subunit of tobacco (ASA2). Plant Cell Rep 27:509–517
Barone P, Zhang X-H, Widholm JM (2009) Tobacco plastid transformation using the feedback insensitive anthranilate synthase [α]-subunit of tobacco (ASA2) as a new selectable marker. J Exp Bot 60:3195–3202
Cho H-J, Brotherton JE, Song H-S, Widholm JM (2000) Increasing tryptophan synthesis in a forage legume Astragalus sinicus by expressing the tobacco feedback-insensitive anthranilate synthase (ASA2) gene. Plant Physiol 123:1069–1076
Cho H-J, Brotherton JE, Widholm JM (2004) Use of the tobacco feedback-insensitive anthranilate synthase gene (ASA2) as a selectable marker for legume hairy root transformation. Plant Cell Rep 23:104–113
Hong S-B, Peebles CAM, Shanks JV, San K-Y, Gibson SI (2006) Expression of the Arabidopsis feedback-insensitive anthranilate synthase holoenzyme and tryptophan decarboxylase genes in Catharanthus roseus hairy roots. J Biotechnol 122:28–38
Inaba Y, Brotherton JE, Ulanov A, Widholm JM (2007) Expression of a feedback insensitive anthranilate synthase gene from tobacco increases free tryptophan in soybean plants. Plant Cell Rep 26:1763–1771
Kreps JA, Town CD (1992) Isolation and characterization of a mutant of Arabidopsis thaliana resistant to alpha methyltryptophan. Plant Physiol 99:269–275
Li J, Last RL (1996) The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110:51–59
Morollo AA, Eck MJ (2001) Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium. Nat Struct 8:243–247
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niyogi KK, Last RL, Fink GR, Keith B (1993) Suppressors of trp1 fluorescence identify a new arabidopsis gene, TRP4, encoding the anthranilate synthase beta subunit. Plant Cell 5:1011–1027
Poulsen C, Bongaerts RJM, Verpoorte R (1993) Purification and characterization of anthranilate synthase from Catharanthus roseus. Eur J Biochem 212:431–440
Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921–934
Ranch JP, Rick S, Brotherton JE, Widholm JM (1983) Expression of 5-methyltryptophan resistance in plants regenerated from resistant cell lines of Datura innoxia. Plant Physiol 71:136–140
Song H-S, Brotherton JE, Gonzales RA, Widholm JM (1998) Tissue culture specific expression of a feedback-insensitive Nicotiana tabacum anthranilate synthase. Plant Physiol 117:533–543
Spraggon G, Kim C, Xuong N-H, Yee M-C, Yanofsky C, Mills SE (2001) The structures of anthranilate synthase of Serratia marcescens crystallized in the presence of (i) its substrates, chorismate and glutamine, and a product, glutamate, and (ii) its end-product inhibitor, L-tryptophan. Proc Natl Acad Sci 98:6021–6026
Tozawa Y, Hasegawa H, Terakawa T, Kyo Wakasa K (2001) Characterization of rice anthranilate synthase α-subunit genes OASA1 and OASA2. Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant of OASA1. Plant Physiol 126:1493–1506
Tsai F-Y, Brotherton JE, Widholm JM (2005) Overexpression of the feedback-insensitive anthranilate synthase gene in tobacco causes tryptophan accumulation. Plant Cell Rep 23:548–556
Tsai F-Y, Zhang X-H, Ulanov A, Widholm JM (2010) The application of the yeast N-acetyltransferase MPR1 gene and the proline analogue L-azetidine-2-carboxylic acid as a selectable marker system for plant transformation. J Exp Bot 61:2561–2573
Wakasa K, Widholm JM (1987) A 5-methyltryptophan resistant rice mutant, MTR1, selected in tissue culture. Theor Appl Genet 74:49–54
Widholm JM (1972) Cultured Nicotiana tabacum cells with an altered anthranilate synthase which is less sensitive to feedback inhibition. Biochim Biophys Acta 261:52–58
Widholm JM (1973) Measurement of the five enzymes which convert chorismate to tryptophan in cultured Daucus carota cell extracts. Biochim Biophys Acta 320:217–226
Yamada T, Tozawa Y, Hasegawa H, Terakawa T, Ohkawa Y, Wakasa K (2004) Use of a feedback-insensitive a subunit of anthranilate synthase as a selectable marker for transformation of rice and potato. Mol Breed 14:363–373
Zhang X-H, Brotherton JE, Widholm JM, Portis AR (2001) Targeting a nuclear anthranilate synthase alpha-subunit gene to the tobacco plastid genome results in enhanced tryptophan biosynthesis. Return of a gene to its pre-endosymbiotic origin. Plant Physiol 127:131–141
Zhang X-H, Webb J, Huang Y-H, Lin L, Tang R-S, Liu A (2011) Hybrid Rubisco of tomato large subunits and tobacco small subunits is functional in tobacco plants. Plant Sci 180:480–488
Acknowledgments
We thank Fei-Yi Tsai for generation and initial analysis of transgenic plants, Pierluigi Barone for help with protein mass spectrometry, Paveena Vichyavichien for help with DNA extraction and PCR analysis, and Shannon O’Farrell for help with Trp measurement. The work was supported by funds from University of Illinois, Florida Atlantic University and Converse College.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1242 kb)
Rights and permissions
About this article
Cite this article
Zhang, XH., Brotherton, J.E. & Widholm, J.M. Co-expression of the tobacco anthranilate synthase β subunit with its feedback-insensitive α subunit as a selectable marker that also markedly increases the free tryptophan content. In Vitro Cell.Dev.Biol.-Plant 51, 564–570 (2015). https://doi.org/10.1007/s11627-015-9713-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9713-x