Abstract
Field performance evaluation and genetic integrity assessment were conducted in Argyranthemum plants derived from cryopreserved shoot tips. Some variations in root formation and vegetative growth were found in the plants following cryopreservation, but morphologies of the leaves and flowers, and color, number and size of the flowers remained unchanged in the plants recovered from cryopreservation, compared with the control. Assessments of genetic integrity by the two molecular markers: inter simple sequence repeat (ISSR) and amplified fragment length polymorphism (AFLP) did not detect any polymorphic bands across the plants tested following cryopreservation. These data indicate that cryopreservation reduces, to a certain degree, root formation and vegetative growth, but it does not alter morphologies of leaves and flowers, may not cause any genetic alternations, and has no adverse effects on quantity and quality of the flowers. Therefore, the droplet vitrification cryopreservation can be considered promising for long-term preservation of Argyranthemum germplasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Argyranthemum, commonly called Marguerite (Walter et al. 2008), is a perennial ornamental plant, native to the Canary Islands, Spain, and Madeira, Portugal (Brickell 1999). In Norway, Argyranthemum is an economically important ornamental crop, with approximately 4.5 million plants produced per year.
The demand for novel flower cultivars by the market is stronger than ever, and the global flower industry thrives on novelty (Tanaka et al. 2005). Conservation and availability of genetic resources provide basic supports for breeding novel ornamental cultivars by both traditional and genetic engineering programs (Wang and Perl 2006; Kulus and Zalewska 2014). Cryopreservation, with several distinct advantages such as the capability for long-term storage, minimal requirement for storage space, and maintenance of genetic integrity of stored materials, is considered an ideal means for long-term conservation of plant genetic resources (Benson 2014; Wang et al. 2014).
Shoot tips are a preferred tissue over seeds, embryos, cells, and callus for conservation of plant genetic resources, because they are genetically identical to the mother plants (Engelmann 1997). Genetic integrity in the plants recovered from cryopreserved shoot tips is still a critical concern (Kulus and Zalewska 2014; Wang et al. 2014). To date, there have been numerous studies on assessments of genetic integrity in the plants recovered from cryopreserved shoot tips (Harding 2004; Wang et al. 2014). Inter simple sequence repeat (ISSR) and amplified fragment length polymorphism (AFLP) were among the frequently used tools for this purpose (Harding 2004; Wang et al. 2014). For assessments of genetic integrity, most of the previous studies concentrated on tuber crops, forest species, and fruit plants (Harding 2004; Wang et al. 2014), while only a few used ornamental crops such as Chrysanthemum × morifolium (Martín and González-Benito 2005; Martín et al. 2011) and Paeonia lactiflora (Seo et al. 2007).
Evaluations of field performance in the plants regenerated from cryopreserved shoot tips are also important, but up to now, studies on this issue are quite limited, particularly in ornamental crops (Wang and Perl 2006; Kulus and Zalewska 2014). Furthermore, assessments of genetic integrity and evaluations of field performance in plants regenerated from cryogenic treatments were rarely included in the same study (Kaity et al. 2009; Ashmore et al. 2011).
We previously reported a droplet vitrification cryopreservation for shoot tips of Argyranthemum ‘Yellow Empire’ (Zhang et al. 2014). The current study focused on field performance evaluation and genetic integrity assessment in greenhouse-grown plants recovered from cryopreserved shoots tips of this plant.
Materials and Methods
Plant materials. Argyranthemum ‘Yellow Empire’, one of the most popular cultivars, in North European countries including Norway, was used in the present study. In vitro shoot cultures were established, according to Zhang et al. (2014), and maintained on a basic medium (BM) at 23°C under 18-h photoperiod with a light intensity of 50 μmol m−2 s−1 provided by cool-white fluorescent tubes (Philips TL-D Super 80, 58 W/840). BM was composed of MS (Murashige and Skoog 1962) medium, supplemented with 3% (w/v) sucrose and 0.6% (w/v) agar. The pH of the medium was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. Subculture was done once every 5 wk.
Cryopreservation. Cryopreservation of shoot tips by droplet vitrification was conducted, as described by Zhang et al. (2014). In brief, shoot tips (about 2.5 mm in length) containing five- to six-leaf primordia excised from 5-wk-old in vitro stock shoots were precultured overnight on BM supplemented with 0.5 M sucrose. Precultured shoot tips were treated for 20 min with a loading solution composed of MS medium containing 2 M glycerol and 0.4 M sucrose, followed by dehydration with plant vitrification solution 2 (PVS2, Sakai et al. 1990) for 30 min at 0°C. Afterward, each dehydrated shoot tip was transferred onto 2.5 μL PVS2 carried on sterile aluminum foil strip (0.7 × 2 cm), prior to a direct immerge into liquid nitrogen (LN) for 1 h. Rewarming was performed by removing the aluminum strips from LN and quickly plunging into an unloading solution composed of 1.2 M sucrose in MS for 20 min at room temperature. Cryopreserved shoot tips were post-cultured on a recovery medium composed of BM supplemented with 0.05 mg L−1 gibberellic acid 3 (GA3), for shoot regrowth. The cultures were grown in the dark for the first 3 d and then transferred onto the same fresh recovery medium under light conditions, as described for the in vitro stock cultures. Shoots (≥0.5 cm in length) regenerated directly without any callus formation after 2 mo of culture on the recovery medium. Regenerated shoots were transferred to BM for further growth for 6 mo (subculture every 5 wk), in order to recover and stabilize after cryopreservation.
Root formation. Shoots (longer than 2 cm), which were regenerated from cryopreserved shoot tips and in vitro cultures, were removed from BM, washed thoroughly with tap water to remove the agar, and then rooted in Jiffy-7 peat pellets (Norgro AS, Hamar, Norway) contained in black plastic tray. The cultures were covered with white plastic bags to maintain high humidity and prevent the shoots from wilting and placed in greenhouse conditions under 8-h photoperiod with a supplementary light intensity of 150 μmol m−2 s−1 provided by high-pressure sodium (HPS) lamps (400 W, GAN 4-550, Norway) at a consistent temperature of 22 ± 2°C. The bags were gradually uncovered to reduce the humidity and removed totally after 2 wk of rooting. Data on rooting percentage, number of roots (≥0.5 cm in length), and length of the longest root per shoot were recorded after 3 wk of rooting.
Vegetative and reproductive growth. After 3 wk of rooting, the plants with well-developed roots were transferred into 6-cm pots containing peat (Degernes Torvstrøfabrikk, Degernes, Norway) for vegetative growth, under the same greenhouse conditions, as used for rooting. Parameters including plant height, number of fully opened leaves per plant, and node number and length were measured after 4 wk of growth. The plants were then transferred into 12-cm pots containing peat, for further vegetative growth and flower production, under 18-h photoperiod with a supplementary light intensity of 200 μmol m−2 s−1 at a consistent temperature of 22 ± 2°C. The plants were pruned to maintain eight leaves per plant to induce growth of lateral shoots. Data on vegetative growth and flower production were recorded after 8 and 12 wk of growth, respectively.
Assessment of genetic integrity. Plants regenerated from cryopreserved shoot tips, which had been grown in peat under greenhouse conditions with an 18-h photoperiod for 8 wk, were used for assessment of genetic integrity by ISSR and AFLP markers. Genomic DNA was extracted from 100-mg fresh leaf tissue using a DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s instructions. Purified total DNA was quantified and its quality verified by ultraviolet spectrophotometry.
ISSR analysis. Forty ISSR primers were screened to select suitable primers for assessment of genetic stability in the greenhouse-grown plants. PCR was performed in a 25-μL reaction solution containing 2.5 μL 10 × PCR buffer, 0.2 μL (1 U) Taq polymerase (Roche, Indiana, IN), 0.5 μL dNTP (10 mM), 0.5 μL primer (100 μM), and 1 μL template DNA (100 ng/μL). DNA amplification was performed in a PCR instrument (Bio-Rad, Singapore, Singapore) using the following reaction conditions: initial denaturation step at 94°C for 2 min; followed by 40 cycles at 94°C for 30 s, different annealing temperature (42°C for ISSR-ARG-10, 44°C for ISSR-ARG-11, and 52.5°C for other primers, see Table 4) for 45 s, and 72°C for 2 min; followed by a final extension step at 72°C for 7 min. The PCR products were separated by electrophoresis in 2% (w/v) agarose gel containing 0.1% (w/v) ethidium bromide and visualized under ultraviolet light. The molecular 100-bp and 1-kb DNA ladder (New England BioLabs Inc, Ipswich, UK) were used for estimating the size of the amplified products.
ISSR fingerprints were manually scored for the presence (1) and absence (0) of each band. Bands of equal molecular weight and mobility generated by the same primer were considered to represent the same locus. Both distinct monomorphic bands and polymorphic bands were scored. Electrophoretic DNA bands of low visual intensity that could not be readily distinguished as present or absent were considered ambiguous markers and were not scored.
AFLP analysis. AFLP was performed as described by Elameen et al. (2008), with modifications that used fluorescently labeled primers instead of radioactive ones. Briefly, genomic DNA (300 ng) was double-digested with EcoRI and the MseI isoschizomer Tru1I. Following ligation of the restriction fragments to the adaptors, pre-amplification PCR was carried out with non-selective primers in a total volume of 25 μL, containing 5 μL of five-fold diluted ligation product. Fluorescent primers were labeled, according to Dresler-Nurmi et al. (2000). The EcoRI primers were labeled blue with 5′-6-acrylamide carboxy fluorescein (6-FAM), and MseI primers were labeled yellow 5′-4,7,2,4,5,7 hexachloro-6-carboxy fluorescein (HEX).
The fluorescently labeled PCR products were analyzed using an ABI3730 DNA Analyzer. One microliter of PCR products was added to a loading buffer containing 8.75 μL Hi-Di formamide (Applied Biosystems, Foster City, CA) and 0.25 μL of GeneScan 500 LIZ size standard (Applied Biosystems). The data was collected using the software Data Collection v2.0 (Applied Biosystems), while GeneMapper v4.1 (Applied Biosystems) was used to derive the fragment length of the labeled DNA fragments using the known fragment lengths of the LIZ-labeled marker peaks.
AFLP bands were recorded manually and scored for presence (1) or absence (0). The genetic similarity was estimated with the Dice’s coefficient (Dice 1945) and Jaccard’s coefficient (Jaccard 1908). Both analyses resulted in the same results, and only the results obtained by the Dice’s coefficient are presented. The matrix of similarity data was analyzed using unweighted pair group method with arithmetic mean (UPGMA), as suggested by Sneath and Sokal (1973). UPGMA clustering was also carried out for all the plants tested according to the treatments: cryopreserved and non-cryopreserved (control). These analyses were performed using NTSYSpc software (Rohlf 2000).
Experimental design and statistical analysis of data. All experiments of field performance were organized based on a complete random design. Shoots regenerated from in vitro culture without cryopreservation served as the control. At least ten samples were used in each treatment of three replicates, and each experiment was repeated twice. Data were analyzed by Student’s t test (P ≤ 0.05). Thirty plants recovered from cryopreservation and 30 from in vitro cultures (the control) were randomly selected, from a population of 150 plants following cryopreservation and 200 in vitro culture-derived plants, respectively, and used in the experiments of assessments of genetic integrity. The experiments of ISSR and AFLP were repeated twice to confirm their repeatability.
Results
Root formation. Roots were easily observed on the outside surface of Jiffy-7 after 1 wk of rooting, with a good root system developed after 3 wk of rooting in the shoots regenerated from cryopreserved shoot tips and in vitro cultures (the control; Fig. 1A ). No significant differences were found in rooting percentage between the shoots regenerated from cryopreserved shoot tips (95%) and the control (100%; Table 1). However, root number and length of the longest root were greater in in vitro-derived shoots (14.9 and 4.4 cm, respectively) than in cryo-derived shoots (12.0 and 3.7 cm; Table 1).
Root formation and field performance of plants regenerated from cryopreserved shoot tips and in vitro cultures of Argyranthemum ‘Yellow Empire’. (A) Root formation in shoots regenerated from cryopreserved shoot tips and in vitro cultures after 3 wk of rooting. Morphologies of leaves of plants regenerated from cryopreserved shoot tips and in vitro cultures after 4 wk of vegetative growth under 8-h photoperiod (B) and 12 wk under 18-h photoperiod (C) under greenhouse conditions. (D) Vegetative growth in plants regenerated from cryopreserved shoot tips and in vitro cultures after 8 wk of growth under 18-h photoperiod under greenhouse conditions. Plant growth (E) and morphologies of flowers (F) of plants regenerated from cryopreserved shoot tips and in vitro cultures after 12 wk of growth under 18-h photoperiod under greenhouse conditions.
Vegetative growth. After 4 wk of growth, under 8-h photoperiod, plant height was similar in plants regenerated from cryopreserved shoot tips (9.1 cm) and the control (9.2 cm; Table 2). However, there were significant differences in other parameters including number of fully opened leaves, and node number and length between the two types of plants (Table 2). Morphologies of leaves were identical between cryopreserved and control shoot tips (Fig. 1B ). After 8 wk of growth under 18-h photoperiod, plant height and number, and length of lateral shoots were significantly higher in plants regenerated from control than from cryopreserved shoot tips (Table 2 and Fig. 1D ), but no differences were found in diameter of the main stem (Table 2). After 12 wk of growth under 18-h photoperiod, morphologies of leaves (Fig. 1C ) were identical in the two types of plants. The general appearance of the plants was similar between the two treatments (Fig. 1E ), although plant height and biomass of the plants measured by fresh and dry weight were significantly smaller in cryo-derived plants than in vitro culture-derived ones (Table 3).
Flower production. The number and diameter of flowers were similar in the plants regenerated from cryopreserved shoot tips and in vitro cultures (Tables 2 and 3). Morphologies and color of the flowers were identical between them (Fig. 1F ).
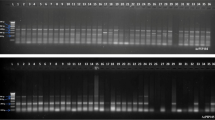
ISSR analysis. Out of the 40 primers tested in the ISSR analysis, ten produced strong and clear reproducible bands (Table 4). These ten primers resulted in 50 clear bands for each plant. The number of bands produced by each primer varied from four to eight (Table 4). In total, 1500 bands were scored across the 30 plants analyzed. No polymorphic bands were detected in all plants regenerated from cryopreserved shoot tips and the control (Table 4 and Fig. 2).
AFLP analysis. AFLP analysis of all 30 plants using five primer combinations (Table 5) resulted in a total number of 151 clear monomorphic bands (Fig. 3). The number of monomorphic bands per primer combination ranged from 26 to 34 bands, with an average of 30.2 monomorphic bands per primer combination. No polymorphic bands were detected across all samples using these five primer combinations. The UPGMA analysis clustered all of the plants tested into one main group (data not shown), indicating no variations between cryo-derived and in vitro culture-derived plants. The matrix of similarity data showed no variations between plants regenerated from cryopreserved shoot tips and the control (data not shown).
AFLP patterns (obtained with GeneMapper software) of plants regenerated from in vitro cultures and cryopreserved shoot tips of Argyranthemum ‘Yellow Empire’ after 8 wk of growth with long-day conditions under greenhouse. AFLP patterns obtained with primer E19 X M15 (A) and primer E19 X M16 (B). Numbers 1, 2, and 3 are plants regenerated from cryopreserved shoot tips and 4 from in vitro cultures (control).
Discussion
Field performance and genetic integrity of Argyranthemum ‘Yellow Empire’ plants derived from cryopreserved shoot tips were compared with those from in vitro cultures (the control) in the present study. For field performance, although some differences were found in root formation and vegetative growth between the two types of the plants, morphologies of the leaves and flowers, and color, number and size of the flowers were identical to each other. For genetic integrity, no polymorphic bands were detected by ISSR and AFLP in the two types of the plants. These results indicate that cryopreservation reduces, to a certain degree, root formation and vegetative growth, but it does not alter morphologies of leaves and flowers, may not cause any genetic alternations, and has no adverse effects on quantity and quality of the flowers, the most economically important trait considered in production of ornamental crops. To the best of our knowledge, this is the first report investigating field performance and genetic integrity in ornamental crops following cryopreservation.
Although information on field performance evaluation of ornamental crops following cryopreservation has been quite limited (Wang and Perl 2006; Kulus and Zalewska 2014), there have been a number of studies on other crops. Harding and Staines (2001) found that Solanum tuberosum plants recovered from cryopreserved shoot tips showed a range of differences in height, tuber weight and size, and leaf morphologies compared to field-propagated plants. Medina et al. (2007) found some differences in fruit quality parameters such as fruit shape, internal flesh color, fruit firmness, and °Brix between strawberry plants (Fragaria × ananassa) derived from cryopreserved shoot tips and in vitro cultures. However, these two types of plants produced similar results in marketable fruit yield, second-class fruit yield, and fruit weight. No adverse effect on agronomic traits appeared to be caused by cryopreservation. Similar results were also reported in other plant species such as Dioscorea floribunda (Ahuja et al. 2002) and Carica papaya (Kaity et al. 2009; Ashmore et al. 2011). These results agree with the present study.
Molecular markers have been widely used for assessment of genetic stability of regenerants derived from cryopreservation (Harding 2004; Wang et al. 2014). Using ISSR for assessment of the genetic stability of plants regenerated from cryopreserved shoot tips of Malus × domestica, Liu et al. (2008) did not detect any polymorphic bands between control and cryopreserved shoots. AFLP analysis revealed no genetic variations in cryopreserved plants of Humulus lupulus (Peredo et al. 2008). Maintenance of the genetic fidelity in the plants recovered from cryopreserved shoot tips was reported in a great number of plant species, such as S. tuberosum (Hirai and Sakai 1999), M. × domestica (Hao et al. 2001), F. × ananassa (Hao et al. 2002), Populus tremula × Populus tremuloides (Jokipii et al. 2004), P. lactiflora (Seo et al. 2007), Dioscorea rotundata (Mandal et al. 2008), Hypericum perforatum (Skyba et al. 2010), and C. papaya (Kaity et al. 2013). These data supported our findings that no polymorphic bands were detected by ISSR and AFLP in the greenhouse-grown plants derived from cryopreserved shoot tips.
Use of more than one DNA amplification technique, to amplify different regions of the genome, provides a better analysis of genetic variation than a single method (Martín et al. 2011). Assessment of genetic stability by RAPD in Chrysanthemum regenerants derived from cryopreserved shoot tips detected a polymorphic rate at 5.8%, while AFLP revealed 40.1% of genetic variations (Martín et al. 2011). The present study employed two molecular techniques: ISSR and AFLP. ISSR uses universal markers; is technically simple, quick to perform, and reproducible; and requires only small amounts of DNA (Bornet and Branchard 2001). AFLP has a higher multiplex ratio than other molecular markers, produces highly reproducible results, and allows direct analysis of variation at loci throughout the whole genome (Powell et al. 1996). Therefore, results of genetic stability assessments in the plants derived from cryopreserved shoot tips of Argyranthemum here should be considered reliable.
There were also some studies in which genetic variations assessed by molecular markers were found in the regenerants from cryopreserved shoot tips of plant species such as Dendranthema grandiflora (Minäno et al. 2009), Rubus (Castillo et al. 2010), Chrysanthemum × morifolium (Martín et al. 2011), and Rabdosia rubescens (Ai et al. 2012). In the case of Rubus (Castillo et al. 2010), when plants showing polymorphic bands were transferred to the field, they exhibited the same AFLP fingerprints as their original mother plants, indicating that there might be a transitory phase of the polymorphism. Similar results were also reported for Abies cephalonica (Aronen et al. 1999) and C. papaya (Kaity et al. 2013). Cryopreservation procedure involves not only freezing in LN but also tissue culture steps for preparing samples before cryopreservation and for plant regeneration after cryopreservation. Tissue culture procedures may also contribute to somaclonal variation (Harding 2004; Wang et al. 2014). Nevertheless, all these data suggest that genetic variation may occur throughout the cryopreservation process and/or the associated tissue culture steps (Harding 2004; Wang et al. 2014), and therefore, the genetic stability of the regenerants following cryopreservation must be assessed.
The present study shows that cryopreservation of Argyranthemum shoot tips does not lead to genetic variation or loss of field performance. Therefore, the droplet vitrification cryopreservation developed here can be considered a promising tool for the long-term storage of Argyranthemum germplasm.
References
Ahuja S, Mandal BB, Dixit S, Srivastava PS (2002) Molecular, phenotypic and biosynthetic stability in Dioscorea floribunda plants derived from cryopreserved shoot tips. Plant Sci 5:971–977
Ai PF, Lu LP, Song JJ (2012) Cryopreservation of in vitro-grown shoot-tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell Tissue Organ Cult 108:381–387
Aronen TS, Krajnakova J, Häggman HM, Ryynänen LA (1999) Genetic fidelity of cryopreserved embrogenic cultures of open-pollinated Abies cephalonica. Plant Sci 142:163–172
Ashmore SE, Drew RA, Kaity A (2011) Storage stability using cryopreservation: a case study in papaya. Acta Horticult 918:125–130
Benson EE (2014) Cryopreservation of phytodiversity: a critical appraisal of theory & practice. Crit Rev Plant Sci 27:141–219
Bornet B, Branchard M (2001) Nonachored inter simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Mol Biol Rep 19:209–215
Brickell C (1999) Encyclopedia of garden plants. The Royal Horticultural Society, London
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol Plant 46:246–256
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Dresler-Nurmi A, Tereffework Z, Kaijalainen S, Lindström K, Hataokka A (2000) Silver stained polyacrylamide gels and fluorescence-based automated capillary electrophoresis for detection of amplified fragment length polymorphism patterns obtained from white-rot fungi in the genus Trametes. J Microbiol Meth 41:161–172
Elameen A, Fjellheim S, Larsen A, Rognli OA, Sundheim L, Msolla S, Masumba E, Klemsdal SS (2008) Analysis of genetic diversity in a sweet potato (Ipomoea batatas L.) germplasm collection from Tanzania as revealed by AFLP. Genet Res Crop Evol 55:397–408
Engelmann F (1997) In vitro conservation methods. In: Callow JA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Oxford, pp 119–161
Hao YJ, Liu QL, Deng XX (2001) Effect of cryopreservation on apple genetic resources at morphological, chromosomal, and molecular levels. Cryobiology 43:46–53
Hao YJ, You CX, Deng XX (2002) Analysis of ploidy and the patterns of amplified fragment length polymorphism and methylation sensitive amplified polymorphism in strawberry plants recovered from cryopreservation. Cryo Letters 23:37–46
Harding K (2004) Genetic intergrity of cryopreserved plant cells: a review. Cryo Letters 25:3–22
Harding K, Staines H (2001) Biometric analysis of phenotypic characters of potato shoot tips recovered from tissue culture, dimethyl sulphoxide treatment and cryopreservation. Cryo Letters 22:255–262
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulation-vitrification. Potato Res 42:153–160
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Jokipii S, Ryynänen L, Kallio PT, Aronen T, Häggman H (2004) A cryopreservation method maintaining the genetic fidelity of a model forest tree, Populus tremula L. × Populus tremuloides Michx. Plant Sci 3:799–806
Kaity A, Ashmore SE, Drew RA (2009) Field performance evaluation and genetic integrity assessment of cryopreserved papaya clone. Plant Cell Rep 28:1421–1430
Kaity A, Drew RA, Ashmore SE (2013) Genetic and epigenetic integrity assessment of acclimatized papaya plants regenerated directly from shoot-tips following short- and long term cryopreservation. Plant Cell Tissue Organ Cult 112:75–86
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species—a review. Sci Hortic 168:88–107
Liu YG, Liu LX, Wang L, Gao AY (2008) Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification. Cryo Letters 29:7–14
Mandal BB, Ahuja-Ghosh S, Srivastava PS (2008) Cryopreservation of Dioscorea rotundata Poir.: a comparative study with two cryogenic procedures and assessment of true-to type of regenerants by RAPD analysis. Cryo Letters 29:399–408
Martín C, Gozález-Benito ME (2005) Survival and genetic stability of Dendranthema grandiflora Tzvelev shoot apices after cryopreservation by vitrification and encapsulation-dehydration. Cryobiology 51:281–289
Martín C, Cervera MT, Gonzalez-Benito ME (2011) Genetic stability analysis of chrysanthemum (Chrysanthemum × morifolium Ramat) after different stages of an encapsulation–dehydration cryopreservation protocol. J Plant Physiol 168:158–166
Medina JJ, Clavero-Ramírez I, González-Benito ME, Gálvez-Farfán J, López-Aranda JM, Soria C (2007) Field performance characterization of strawberry (Fragaria x ananassa Duch.) plants derived from cryopreserved apices. Sci Hortic 113:28–32
Minäno HS, Gonzalez-Benito ME, Martin C (2009) Molecular characterization and analysis of somaclonal variation in chrysanthemum cultivars using RAPD markers. Sci Hortic 122:238–243
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Peredo EL, Arroyo-García R, Reed BM, Revilla MÁ (2008) Genetic and epigenetic stability of cryopreserved and cold-stored hops (Humulus lupulus L.). Cryobiology 57:234–241
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Rohlf FJ (2000) NTSYS-pc, numerical taxonomy and multivariate analysis system, v.2.02. Exeter Software, New York
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Seo MJ, Shin JH, Sohn JK (2007) Cryopreservation of dormant herbaceous peony (Paeonia lactiflora pall.) shoot-tips by desiccation. Cryo Letters 28:207–213
Skyba M, Urbanová M, Kapchina-Toteva V, Košuth J, Harding K, Čellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. Cryo Letters 31:249–260
Sneath PA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco
Tanaka Y, Katsumoto Y, Brugliera F, Mason J (2005) Genetic engineering in floriculture. Plant Cell Tissue Org Cult 80:1–24
Walter E, Erich G, Nils B, Siegmund S (2008) Zander—dictionary of plant names. Eugen Ulmer KG, Germany
Wang QC, Perl A (2006) Cryopreservation in floricultural plants. In: da Silva JT (ed) Floricultral, ornamental and plant biotechnology: advances and topics. Global Science Books, London, pp 523–539
Wang B, Wang RR, Cui ZH, Bi WL, Li JW, Li BQ, Ozudogru EA, Volk GM, Wang QC (2014) Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol Adv 32:583–595
Zhang ZB, Lee Y, Haugslien S, Sivertsen A, Skjeseth G, Clarke JHL, Wang QC, Blystad D-R (2014) Cryotherapy could not eradicate Chrysanthemum stunt viroid from infected Argyranthemum maderense ‘Yellow Empire’. Acta Hortic 1039:201–208
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Zhang, Z., Skjeseth, G., Elameen, A. et al. Field performance evaluation and genetic integrity assessment in Argyranthemum ‘Yellow Empire’ plants recovered from cryopreserved shoot tips. In Vitro Cell.Dev.Biol.-Plant 51, 505–513 (2015). https://doi.org/10.1007/s11627-015-9707-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9707-8