Abstract
In this study, we applied the vitrification (V) cryo-plate protocol for cryopreservation of Clinopodium odorum in vitro-derived shoot tips. We studied the recovery of shoot tips after modification of various parameters of the V cryo-plate protocol, including sucrose concentration in the preconditioning medium, composition of the loading solution (LS) and vitrification solution (VS) and duration of treatment with PVS2 VS, which contained (w/v) 30% glycerol, 15% dimethylsulphoxide, 15% ethylene glycol and 13.7% sucrose. We also compared the efficiency of the V cryo-plate protocol with the dehydration (D) cryo-plate protocol. The optimal conditions determined for the V cryo-plate protocol included a 24-h preconditioning treatment on medium with 0.3 M sucrose; treatment for 20 min at room temperature with LS containing 2.0 M glycerol and 0.4 M sucrose; and dehydration with PVS2 for 60 min at 0°C. Under these conditions, 71.0% recovery of cryopreserved shoot tips was achieved. Only 29.2% regeneration was noted with the D cryo-plate protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clinopodium odorum (Griseb.) Harley is a small deciduous shrub of the family Lamiaceae, which is found in the Americas. In Argentina, small populations of this species, which are characteristic of the Pampa de Achala (Córdoba province), are found at an elevation of 1200 m (Barboza et al. 2009). The fresh herb is used as a flavouring agent for food products, and an infusion of the aerial parts has many medicinal uses (Harley and Paucar 2000; Mahady 2005). This species has become endangered due to habitat degradation, cattle grazing and over-collecting (Goleniowski et al. 2006; Martinez et al. 2006).
Seed is only the means of propagating C. odorum. However, germination of C. odorum seeds is inhibited by the presence of adult plants, thus reducing the possibilities of species dissemination (Cantero and Bianco 1986). A micropropagation protocol has been developed recently by Diaz et al. (2012), which may assist in the safeguarding and augmentation of dwindling natural populations of C. odorum.
Cryopreservation [liquid nitrogen (LN), −196°C] is currently the only technique available to ensure the safe and cost-effective long-term conservation of rare and endangered plant species such as C. odorum (Engelmann 2011). A number of vitrification-based cryopreservation techniques have been developed during the last 20 years. The droplet-vitrification (DV) technique was established in 2005 by Panis et al. (2005). In this technique, explants are treated with a loading solution (LS), then with a highly concentrated vitrification solution (VS), placed in minute droplets of VS on aluminium foils and immersed in LN. For rewarming, aluminium foils with the explants are plunged directly in unloading solution (ULS). The high efficiency of this technique is due to the achievement of very high cooling and warming rates because explants are in direct contact with LN during cooling and with the ULS during warming (Engelmann 2014). The DV technique has been successfully applied to a range of different plant species (Panis et al. 2011).
Very recently, a further improvement to DV has been made with the development of the cryo-plate technique (Yamamoto et al. 2011; Niino et al. 2013). In this technique, explants are placed in the wells of aluminium cryo-plates to which they are made to adhere by encapsulating them in minute droplets of calcium alginate. They are then treated with LS, dehydrated osmotically with a VS (the so-called V cryo-plate protocol) or dehydrated physically by placing them in the air current of a laminar flow cabinet (the so-called D cryo-plate protocol) before immersion in LN. The main advantage of this technique lies with the facilitated manipulation of explants, which adhere to the cryo-plate. The V cryo-plate protocol has been experimented with shoot tips of several species including Dalmatian chrysanthemum (Yamamoto et al. 2011), mint (Yamamoto et al. 2012a), mulberry (Yamamoto et al. 2012b), strawberry (Yamamoto et al. 2012c), carnation (Sekizawa et al. 2011), mat rush (Niino et al. 2013) and with proembryogenic masses of date palm (Salma et al. 2014). There are currently only three reports on the utilization of the D cryo-plate protocol with shoot tips of mat rush (Niino et al. 2013, 2014). persimmon (Matsumoto et al. 2013) and date palm proembryogenic masses (Salma et al. 2014).
In this study, we applied the V cryo-plate protocol to C. odorum in vitro-derived shoot tips. We studied the effect of modification of various steps of the V cryo-plate protocol on recovery of shoot tips and compared its efficiency with the D cryo-plate protocol.
Materials and Methods
Plant material and culture conditions. The C. odorum plants used in this study originated from a batch of in vitro germinated seeds, which had been collected in the Pampa de Achala (Córdoba province, Argentina). Seeds were surface-sterilized with a solution of 70% (v/v) ethanol for 2 min and rinsed three times with sterile distilled water, followed by 15 min in a solution of sodium hypochlorite (NaOCl) 1.5% (v/v) for 15 min and finally rinsed three times with sterile distilled water. For germination, seeds were cultured on 1/2 strength Murashige and Skoog (MS; Murashige and Skoog 1962) medium with 1 mg L−1 indole-3-butyric acid (IBA), 0.1 M sucrose and 7 g L−1 agar. Cultures were maintained at 27 ± 1°C under a 12-h light/12-h dark photoperiod and a light intensity of 50 μmol m−2 s−1. The plantlets obtained from germinated seeds were pooled and multiplied by monthly subcultures on the same culture medium (Fig. 1). One month before cryopreservation experiments, nodal segments with one bud were excised from the in vitro plantlets and cultured in the conditions described above.
Base V cryo-plate cryopreservation protocol. The base V cryo-plate protocol applied to C. odorum shoot tips was derived from the protocol developed by Yamamoto et al. (2011). Apices (around 1 mm long) were dissected from the shoots developed from 1-mo-old nodal segments and cultured overnight on medium with 0.3 M sucrose. The aluminium cryo-plates used (7 × 37 × 0.5 mm, with 10 wells of 1.5 mm diameter and 0.75 mm depth) were provided by Dr. Niino (University of Tsukuba, Japan). Droplets of around 2 μL of 3% (w/v) sodium alginate solution in calcium-free standard medium were poured in the wells of the cryo-plates. Apices were transferred one by one in the wells with a scalpel blade and covered with 2 μL droplets of sodium alginate solution. The calcium chloride solution (0.1 M calcium chloride in medium with 0.3 M sucrose) was poured drop-wise on the section of the cryo-plates until apices were covered. Polymerization was complete after 15 min, and the calcium chloride solution was removed by sucking it gently with a micropipette. Apices on the cryo-plates were treated for 20 min at room temperature with LS containing 2.0 M glycerol and 0.4 M sucrose (Matsumoto et al. 1994). Cryo-plates were removed from LS, blotted dry on filter paper in a Petri dish and dehydrated in PVS2 solution [(w/v) 30% glycerol + 15% ethylene glycol + 13.7% sucrose + 15% dimethylsulphoxide (DMSO) (Sakai et al. 1990)] for 60 min at 0°C. After dehydration, cryo-plates were blotted dry on filter paper in a Petri dish and plunged in uncapped cryotubes previously filled with LN. After 1 h storage in LN, apices were rewarmed by immersing the cryo-plates in 2-mL cryotubes filled with ULS containing 1.2 M sucrose for 15 min. Apices were then detached from the cryo-plates with a scalpel blade and transferred on culture medium with 0.1 M sucrose in the dark for 7 d, then under the light conditions described above.
Modifications of the base V cryo-plate protocol. Various steps of the cryopreservation protocol were modified, and the effect of these modifications on the recovery of shoot tips was studied.
-
Effect of sucrose concentration in preconditioning medium: after dissection, shoot tips were preconditioned overnight on medium containing 0.1, 0.2, 0.3, 0.4, 0.6 or 0.9 M sucrose.
-
Comparison of four types of LS: LS C4 (1.9 M glycerol + 0.5 M sucrose; Kim et al. 2009a), LS C6 (2.2 M glycerol + 0.6 M sucrose; Kim et al. 2009a), LS C7 (2.0 M glycerol + 0.4 M sucrose; Matsumoto et al. 1994) and LS Nii (2.0 M glycerol + 0.6 M sucrose; Niino et al. 2013).
-
Comparison of three types of VS: PVS2 (Sakai et al. 1990) contained (w/v) 30% glycerol, 15% DMSO, 15% ethylene glycol and 13.7% sucrose; PVS3 (Nishizawa et al. 1993) contained (w/v) 50% glycerol and 50% sucrose, applied at room temperature; PVS2 A3 (Kim et al. 2009b) contained (w/v) 37.5% glycerol, 15% DMSO, 15% ethylene glycol and 22.5% sucrose, applied at 0°C.
-
Effect of duration of exposure to PVS2 solution: shoot tips were dehydrated with PVS2 for 0, 10, 20, 30, 40, 50, 60, 90 or 120 min.
D cryo-plate cryopreservation protocol. The D cryo-plate protocol established by Niino et al. (2013) was applied to C. odorum shoot tips. All steps were as described above for the base V cryo-plate protocol except for the dehydration step, which was performed by placing the cryo-plates with the apices under the air current of a laminar air flow cabinet (Sercoflux LP 240, France) for 60, 120 or 180 min at 23°C, 40–50% relative humidity (RH).
Observations and statistical analysis. Experiments were performed once, with three replicates of 10 shoot tips per experimental condition. Regeneration was measured after 3 weeks, by counting the number of shoot tips that had developed in small shoots with new leaves. Statistical analyses were carried out using the R version 3.0.2 free software (R Foundation for Statistical Computing 2013). Survival percentages from data frames containing variables with only two modalities were treated using the Fisher’s exact test. Logistic regression with the generalized linear model (GLM test) was used to evaluate the link between regeneration and experimental conditions (LN exposure, duration). The binomial logistic link function used was f(y) = log[y/(1 − y)]. Significant effects were verified using the χ 2 test. Differences were accepted as significant with a 95% confidence level (P < 0.05).
Results
V cryo-plate protocol.
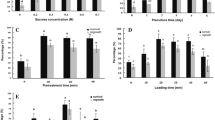
Effect of sucrose concentration in preconditioning medium. In case of control shoot tips, preconditioning with medium containing 0.2–0.4 M sucrose had a positive effect on regeneration of in vitro-derived shoot tips, compared to no preconditioning or preconditioning with lower (0.1 M) or higher sucrose concentrations (0.6 and 0.9 M; Fig. 2). In case of cryopreserved shoot tips, no regeneration was achieved without preconditioning. Regeneration increased progressively in line with increasing sucrose concentrations, reaching a maximum of 43.0% with 0.3 M sucrose, then decreased progressively with higher sucrose concentrations.
Effect of sucrose concentration (M) in the preconditioning medium on regeneration (% ± SD) of non-cryopreserved (−LN) and cryopreserved (+LN) C. odorum shoot tips. Shoot tips were treated with LS C7 for 20 min at room temperature, then with PVS2 vitrification solution for 60 min at 0°C. For each series (−LN, +LN), different letters indicate significant differences between treatments (P < 0.05).
Effect of loading solution. Regeneration of control shoot tips was comparable following treatment with the four LS tested (Fig. 3). After cryopreservation, regeneration was not significantly different following treatment with LS C4 (57.4 %), LS C7 (50.2 %), or LS C6 (36.5 %) and significantly lower with LS Nii.
Effect of treatment with loading solution C4, C6, CNii and C7 on regeneration (% ± SD) of non-cryopreserved (−LN) and cryopreserved (+LN) C. odorum shoot tips. Shoot tips were treated with PVS2 vitrification solution for 60 min at 0°C. For each series (−LN, +LN), different letters indicate significant differences between treatments (P < 0.05). LS C4 (1.9 M glycerol + 0.5 M sucrose; Kim et al. 2009a); LS C6 (2.2 M glycerol + 0.6 M sucrose; Kim et al. 2009a); LS C7 (2.0 M glycerol + 0.4 M sucrose; Matsumoto et al. 1994); and LS Nii (2.0 M glycerol + 0.6 M sucrose; Niino et al. 2013).
Effect of VS. Regeneration of control shoot tips was comparable following treatment with the three VSs tested (Fig. 4). In case of cryopreserved shoot tips, regeneration was not significantly different following treatment the three VSs employed, reaching 36.2% with PVS2, 34.4% with PVS3 and only 22.4% with PVS2 A3.
Effect of treatment with vitrification solutions PVS3, PVS2 A1 and PVS2 A3 on regeneration (% ± SD) of non-cryopreserved (−LN) and cryopreserved (+LN) C. odorum shoot tips. Shoot tips were treated with LS C7 for 20 min at room temperature, then with the different vitrification solutions for 60 min at 0°C. For each series (−LN, +LN), different letters indicate significant differences between treatments (P < 0.05). PVS3 (Nishizawa et al. 1993): (w/v) 50% glycerol and 50% sucrose; PVS2 A1 (Sakai et al. 1990): (w/v) 30% glycerol, 15% DMSO, 15% ethylene glycol and 13.7% sucrose; PVS2 A3 (Kim et al. 2009b): (w/v) 37.5% glycerol, 15% DMSO, 15% ethylene glycol, and 22.5% sucrose.
Effect of PVS2 treatment duration. Regeneration of control shoot tips was 100% for PVS2 treatment durations between 0 and 60 min (Fig. 5). It dropped to 46.7% and to 30.0% after dehydration for 90 and 120 min, respectively. In case of cryopreserved shoot tips, no regeneration was achieved without PVS2 treatment. Regeneration increased from 3.3% after 10 min dehydration to a maximum of 71.0% after 60 min dehydration, then decreased for longer PVS2 treatment durations. Regrowth of cryopreserved shoot tips was slower compared to non-cryopreserved shoot tips (Fig. 6). However, small rooted plantlets could be regenerated from cryopreserved shoot tips 3 weeks after LN exposure.
Effect of duration of treatment (min) with vitrification solution PVS2 on regeneration (% ± SD) of non-cryopreserved (−LN) and cryopreserved (+LN) C. odorum shoot tips. Shoot tips were treated with LS C7 for 20 min at room temperature. For each series (−LN, +LN), different letters indicate significant differences between treatments (P < 0.05).
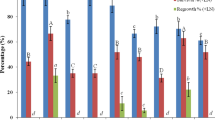
D cryo-plate protocol. In case of control shoot tips, regeneration was 34.6% and 56.7% after dehydration for 60 and 120 min, respectively (Fig. 7). No regeneration was achieved after dehydration for 180 min. Regeneration of cryopreserved shoot tips was 29.2% after 60 min dehydration. It was significantly lower after 120 min dehydration, reaching only 5.5 %.
Effect of dehydration duration (min) on regeneration (% ± SD) of non-cryopreserved (−LN) and cryopreserved (+LN) C. odorum shoot tips. Shoot tips were treated with LS C7 for 20 min at room temperature before dehydration under the laminar air flow for 60, 120 or 180 min at 23°C, 40–50% RH. For each series (−LN, +LN), different letters indicate significant differences between treatments (P < 0.05).
Discussion
The present work represents the first application of cryopreservation to C. odorum shoot tips. The V cryo-plate protocol tested was optimized by studying various parameters, including the sucrose concentration in preconditioning medium, the nature of the LS and VS employed and the duration of treatment with PVS2. It resulted in a maximum of 71% regeneration of cryopreserved shoot tips. The D cryo-plate protocol was also successfully tested but led to only 29% recovery in the experimental conditions employed.
Preconditioning of explants on medium with high sucrose content is necessary in most published cryopreservation protocols (Sakai and Engelmann 2007). In many cases, preconditioning consists of a 24-h culture of explants on medium with 0.3 M sucrose, as with mat rush shoot tips (Niino et al. 2013), but the optimal sucrose concentration and treatment duration can vary depending on the material. The optimal duration of treatment with 0.3 M sucrose was 2 d in the case of carnation shoot tips (Sekizawa et al. 2011) and 5 d in the case of lily shoot tips (Bouman et al. 2003). Coffea sessiliflora shoot tips required a 3–10-day preconditioning treatment with 0.75 M sucrose, while C. racemosa shoot tips required a progressive increase in the sucrose concentration from 0.5 to 1.0 M (Mari et al. 1995). Our experiments, which included preconditioning of C. odorum shoot tips for 24 h with sucrose concentrations comprised between 0.1 and 0.9 M, clearly showed that treatment with 0.3 M sucrose was optimal.
The LS generally employed in vitrification-based cryopreservation protocols comprises 2.0 M glycerol and 0.4 M sucrose (Matsumoto et al. 1994). However, Kim et al. (2009a) showed that only minor changes in the composition of the LS could have a significant impact on recovery of materials sensitive to cytoxicity of VS. These authors showed that incubating chrysanthemum shoot tips in LS C4 containing 1.9 M glycerol + 0.5 M sucrose resulted in 65.3% regeneration after LN exposure, against only 53.9% with LS C7 containing 2.0 M glycerol + 0.4 M sucrose (the original LS solution developed by Matsumoto et al. 1994). Similar results were observed with C. odorum shoot tips with LS C7 being slightly more efficient compared to LS C4, and the other two LSs tested resulting in lower regeneration.
The VS most commonly employed in vitrification-based cryopreservation protocols are PVS2 containing (w/v) 30% glycerol, 15% DMSO, 15% ethylene glycol and 13.7% sucrose (Sakai et al. 1990) and PVS3 containing (w/v) 50% glycerol and 50% sucrose (Nishizawa et al. 1993). PVS2 is a very efficient VS, but it is also very toxic. As PVS3 is considered less toxic than PVS2, it is recommended for cryopreservation of sensitive materials (Engelmann 2011). Recently, Kim et al. (2009b) showed that alternative VS, derived from the original PVS2 and PVS3, could significantly improve the recovery of sensitive materials. These authors showed that cryopreserved chrysanthemum shoot tips produced 30.8% regeneration after treatment with PVS2, 59.8% with PVS2 A3 containing (w/v) 37.5% glycerol, 15% DMSO, 15% ethylene glycol and 22.5% sucrose. Different results were obtained with C. odorum cryopreserved shoot tips since similar regeneration was achieved after treatment with PVS2 and PVS3 and regeneration was lower following treatment with PVS2 A1.
Whatever the VS employed, the duration of treatment, which varies depending on the plant material employed, must be precisely determined because, since the VS used are highly toxic, only slight changes in treatment duration can have a dramatic impact on recovery (Sakai and Engelmann 2007). The optimal PVS2 treatment duration at 0°C was 30 min for blackberry apices and between 10 and 30 min for cherry plum shoot tips (Vujovic et al. 2011). Regeneration of sugarcane apices was optimal after PVS2 treatment for 20–40 min at 0°C (Barraco et al. 2011a), and regeneration of Limonium serotinum shoot tips was highest after PVS2 treatment for 30 min at 0°C (Barraco et al. 2011b). In case of C. odorum shoot tips, the optimal PVS2 treatment duration was 60 min at 0°C. The slightly longer optimal treatment duration noted with C. odorum, compared with the examples quoted above might be due to the presence of the encapsulating alginate matrix, which slows down the dehydration process.
There is currently only one published report on comparison of the V cryo-plate and D cryo-plate techniques. In this work, Niino et al. (2013) showed that the D cryo-plate technique led to higher regeneration compared to the V cryo-plate for cryopreservation of mat rush basal stem buds. These authors hypothesized that the better results obtained with the D cryo-plate were due the fact that this technique avoided the use of the highly toxic PVS2 for dehydration of samples. By contrast, in our experiments, the V cryo-plate was more efficient compared to the D cryo-plate for cryopreservation of C. odorum shoot tips. Results might be improved by modifying the explant size, the volume of the alginate matrix used for encapsulating explants as well as parameters of the preconditioning and loading treatment.
In conclusion, we have shown that the V cryo-plate technique can be efficiently used for long-term conservation of C. odorum germplasm. This technique should be tested with other endangered plant species.
References
Barboza GE, Cantero JJ, Nuñez PA, Ariza Espinar L (2009) Medicinal plants: review and a phytochemical and ethnopharmacological screening of the native Argentine flora, 34th edn. Univ Kurtziana, Nacional de Cordoba, Argentina
Barraco G, Sylvestre I, Engelmann F (2011a) Cryopreservation of sugarcane (Saccharum spp.) shoot tips using encapsulation and droplet-vitrification. Sci Hortic 130:320–324
Barraco G, Sylvestre I, Iapichino G, Engelmann F (2011b) Cryopreservation of Limonium serotinum apical shoots from in vitro plantlets using droplet-vitrification. Sci Hortic 130:309–313
Bouman H, Tiekstra A, Petutschnig E, Homan M, Schreurs R (2003) Cryopreservation of Lilium species and cultivars. Acta Hortic 612:147–154
Cantero JJ, Bianco CA (1986) Las Plantas Vasculares del Suroeste de la Provincia de Cordoba. Catalogo Preliminar de las Especies. Rev Univ Nacional De Rıo Cuarto 6:65–75 (in Spanish)
Diaz MS, Palacio L, Figueroa AC, Goleniowski ME (2012) In vitro propagation of Muña-Muña (Clinopodium odorum (Griseb.) Harley). Biotech Res Intl. doi:10.1155/2012/196583
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol – Plant 47:5–16
Engelmann F (2014) Cryopreservation of clonal crops: a review of key parameters. Acta Hortic 1039:31–39
Goleniowski ME, Bongiovanni GA, Palacio L, Nuñez CO, Cantero JJ (2006) Medicinal plants from the “Sierra de Comechingones”, Argentina. J Ethnopharmacol 107:324–341
Harley RM, Paucar AG (2000) List of species of tropical American Clinopodium (Labiatae), with new combinations. Kew Bull 55:917–927
Kim HH, Lee YG, Ko HC, Park SU, Gwag JG, Cho EG, Engelmann F (2009a) Development of alternative loading solutions in droplet-vitrification procedures. CryoLetters 30:291–299
Kim HH, Lee YG, Shin DJ, Kim T, Cho EG, Engelmann F (2009b) Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 30:320–334
Mahady GB (2005) Medicinal plants for the prevention and treatment of bacterial infections. Curr Pharma Design 11:2405–2427
Mari S, Engelmann F, Chabrillange N, Huet C, Michaux-Ferrière N (1995) Histo-cytological study of coffee (Coffea racemosa and C. sessiliflora) apices of in vitro plantlets during their cryopreservation using the encapsulation-dehydration technique. CryoLetters 16:289–298
Martinez GJ, Planchuelo AM, Fuentes E, Ojeda M (2006) A numeric index to establish conservation priorities for medicinal plants in the Paravachasca Valley, Cordoba, Argentina. Biodiv Conserv 15:2457–2475
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Matsumoto T, Yamamoto S, Fukui K, Niino T (2013) Cryopreservation of persimmon shoot tips using D cryo-plate procedure. In Abstract: 2nd Intl. Symp. on Plant Cryopreservation. Fort Collins, USA, 11–14 August 2013, p 96
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Niino T, Yamamoto S, Fukui K, Castillo Martinez CR, Valle Arizaga M, Matsumoto T, Engelmann F (2013) Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. CryoLetters 34:549–560
Niino T, Wunna WK, Nohara N, Rafique T, Yamamoto S, Fukui K, Valle Arizaga M, Castillo Martinez CR, Matsumoto T, Engelmann F (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotech Rep 31:281–287
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Panis B, Piette B, André E, Van den Houwe I, Swennen R (2011) Droplet vitrification: the first generic cryopreservation protocol for organized plant tissues? Acta Hortic 908:157–163
R Foundation for Statistical Computing (2013) R: a Language and Environment for Statistical Computing. Version 3.0.1 (2013-05-16). http://www.r-project.org. Accessed 24 Oct 2014
Sakai A, Engelmann F (2007) Vitrification, encapsulation–vitrification and droplet–vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Salma M, Fki L, Engelmann-Sylvestre I, Niino T, Engelmann F (2014) Comparison of droplet-vitrification and D-cryoplate for cryopreservation of date palm (Phoenix dactylifera L.) polyembryonic masses. Sci Hortic 179:91–97
Sekizawa K, Yamamoto S, Rafique T, Fukui K, Niino T (2011) Cryopreservation of in vitro-grown shoot tips of carnation (Dianthus caryophyllus L.) by vitrification method using aluminium cryo-plates. Plant Biotech 28:401–405
Vujovic T, Sylvestre I, Ruzic D, Engelmann F (2011) Droplet-vitrification of apical shoot tips of Rubus fruticosus L. and Prunus cerasifera Ehrh. Sci Hortic 130:222–228
Yamamoto S, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 32:256–265
Yamamoto S, Fukui K, Rafique T, Khan NI, Castillo Martinez CR, Sekizawa K, Matsumoto T, Niino T (2012a) Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminium cryo-plates. Plant Genet Res: Charact Util 10:14–19
Yamamoto S, Rafique T, Fukui K, Sekizawa K, Koyama A, Ichihashi T, Niino T (2012b) Development of an effective cryopreservation protocol using aluminium cryo-plates for in vitro-grown shoot tips of mulberries (Morus spp.) originated from the tropics and subtropics. Sanshi-Konchu Biotechnol 81:57–62 (in Japanese)
Yamamoto S, Rafique T, Fukui K, Sekizawa K, Niino T (2012c) V-Cryo-plate procedure as an effective protocol for cryobanks. Case study of mint cryopreservation. CryoLetters 33:12–23
Acknowledgements
This work has been performed in the framework of the AMCRYO project, funded by Agropolis Fondation (project no. 1202–007). Dr. Maria Ester Goleniowski (CEPROCOR, Cordoba, Argentina) introduced the plant material in vitro and performed the initial multiplication of cultures. We thank Lionel Feuillassier for his assistance in performing the statistical analysis of results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John Forster
Rights and permissions
About this article
Cite this article
Engelmann-Sylvestre, I., Engelmann, F. Cryopreservation of in vitro-grown shoot tips of Clinopodium odorum using aluminium cryo-plates. In Vitro Cell.Dev.Biol.-Plant 51, 185–191 (2015). https://doi.org/10.1007/s11627-015-9668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9668-y